PTR-MS Characterization of VOCs Associated with Commercial Aromatic Bakery Yeasts of Wine and Beer Origin
Abstract
:1. Introduction
2. Results
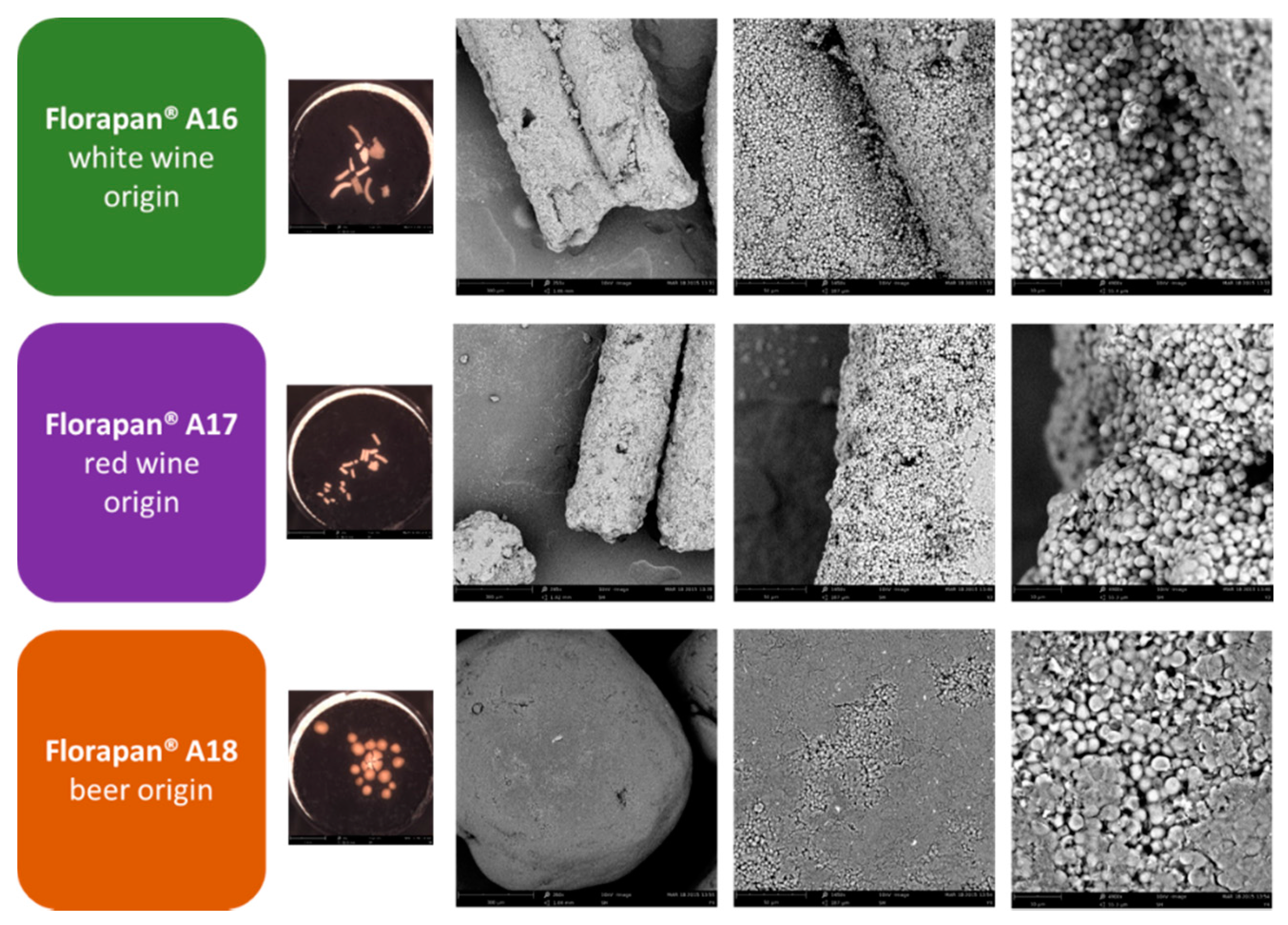
2.1. Commercial Aromatic Strains, SEM Visualization of Active Dry Yeasts, and Automated Monitoring of the Leavening Process
2.2. Ethanol Monitoring and Fermentative Performances
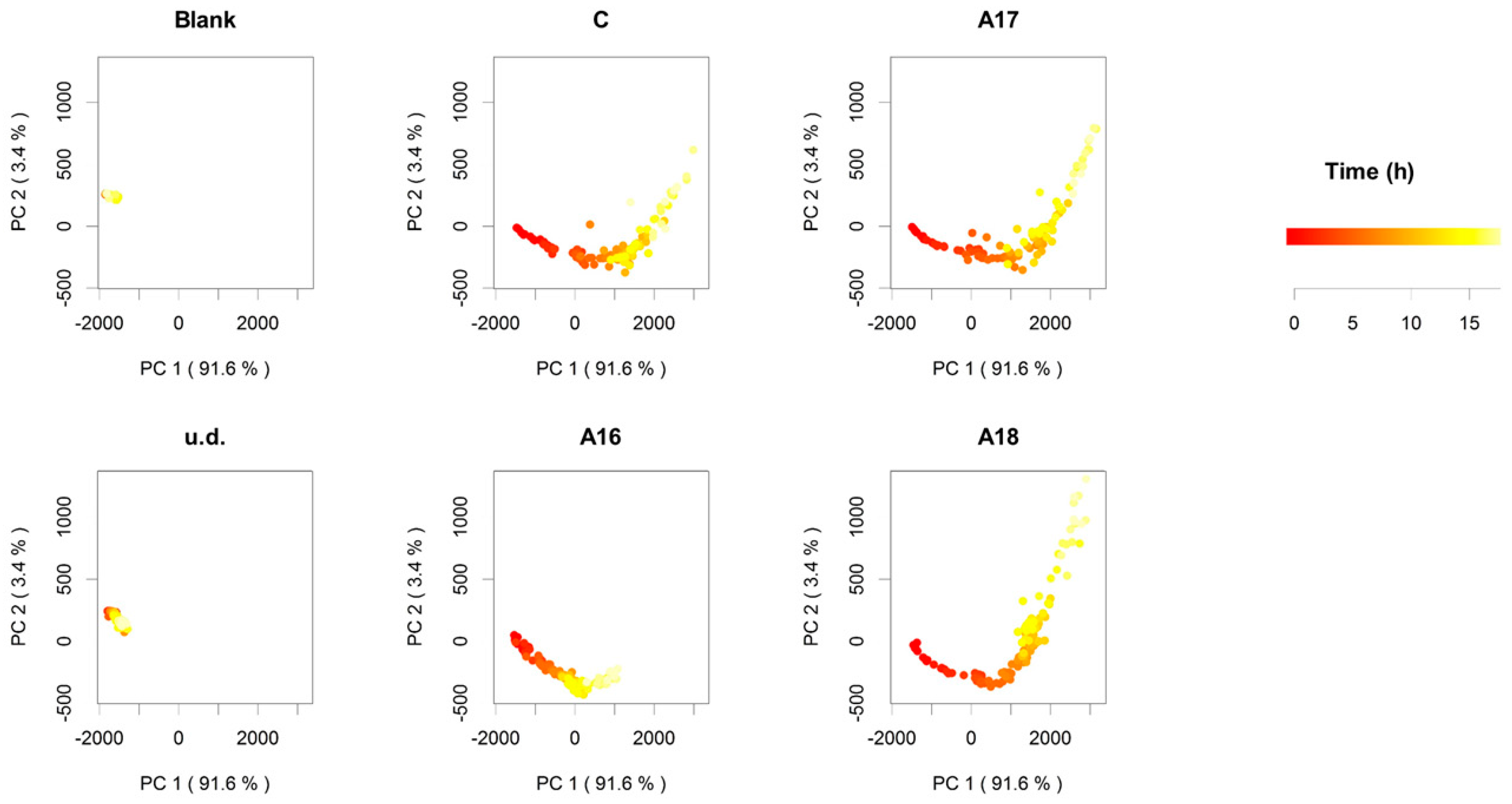
2.3. Volatile Profiles and Aromatic Properties
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. SEM
4.3. Yeast Count
4.4. PTR-ToF-MS Analysis
4.5. Data Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CFU | colony forming unit |
| DIMS | direct-injection mass spectrometry |
| GC | gas chromatography |
| PCA | principal component analysis |
| PEEK | polyether ether ketone |
| PTR-MS | proton-transfer-reaction mass spectrometry |
| PTR-TOF-MS | proton-transfer-reaction time-of-flight mass spectrometry |
| SEM | scanning electron microscopy |
| VOC | volatile organic compound |
References
- Birch, A.N.; Petersen, M.A.; Arneborg, N.; Hansen, Å.S. Influence of commercial baker’s yeasts on bread aroma profiles. Food Res. Int. 2013, 52, 160–166. [Google Scholar] [CrossRef]
- Aprea, E.; Biasioli, F.; Gasperi, F. Volatile compounds of raspberry fruit: From analytical methods to biological role and sensory impact. Molecules 2015, 20, 2445–2474. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Lasekan, O.; Naidu, K.R.M.; Lasekan, S. Headspace Solid-Phase Microextraction Gas Chromatography-Mass Spectrometry and Gas Chromatography-Olfactometry analysis of volatile compounds in pineapple breads. Molecules 2012, 17, 13795–13812. [Google Scholar] [CrossRef] [PubMed]
- Birch, A.N.; Petersen, M.A.; Hansen, Å.S. REVIEW: Aroma of wheat bread crumb. Cereal Chem. J. 2013, 91, 105–114. [Google Scholar] [CrossRef]
- Makhoul, S.; Romano, A.; Capozzi, V.; Spano, G.; Aprea, E.; Cappellin, L.; Benozzi, E.; Scampicchio, M.; Märk, T.D.; Gasperi, F.; et al. Volatile compound production during the bread-making process: Effect of flour, yeast and their interaction. Food Bioprocess Technol. 2015, 8, 1925–1937. [Google Scholar] [CrossRef]
- Pico, J.; Bernal, J.; Gómez, M. Wheat bread aroma compounds in crumb and crust: A review. Food Res. Int. 2015, 75, 200–215. [Google Scholar] [CrossRef]
- Steensels, J.; Meersman, E.; Snoek, T.; Saels, V.; Verstrepen, K.J. Large-scale selection and breeding to generate industrial yeasts with superior aroma production. Appl. Environ. Microbiol. 2014, 80, 6965–6975. [Google Scholar] [CrossRef] [PubMed]
- Randez-Gil, F.; Córcoles-Sáez, I.; Prieto, J.A. Genetic and phenotypic characteristics of baker’s yeast: relevance to baking. Annu. Rev. Food Sci. Technol. 2013, 4, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Makhoul, S.; Romano, A.; Cappellin, L.; Spano, G.; Capozzi, V.; Benozzi, E.; Märk, T.D.; Aprea, E.; Gasperi, F.; El-Nakat, H.; et al. Proton-transfer-reaction mass spectrometry for the study of the production of volatile compounds by bakery yeast starters. J. Mass Spectrom. 2014, 49, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Carrau, F.; Gaggero, C.; Aguilar, P.S. Yeast diversity and native vigor for flavor phenotypes. Trends Biotechnol. 2015, 33, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Gélinas, P. Inventions on baker’s yeast strains and specialty ingredients. Recent Pat. Food Nutr. Agric. 2009, 1, 104–132. [Google Scholar] [CrossRef] [PubMed]
- Mantzouridou, F.; Paraskevopoulou, A. Volatile bio-ester production from orange pulp-containing medium using Saccharomyces cerevisiae. Food Bioprocess Technol. 2012, 6, 3326–3334. [Google Scholar] [CrossRef]
- Lambert, J.L.; Le-Bail, A.; Zuniga, R.; Van-Haesendonck, I.; Vnzeveren, E.; Petit, C.; Rosell, M.C.; Collar, C.; Curic, D.; Colic-Baric, I.; et al. The attitudes of European consumers toward innovation in bread; interest of the consumers toward selected quality attributes. J. Sens. Stud. 2009, 24, 204–219. [Google Scholar] [CrossRef]
- Hugenholtz, J. Traditional biotechnology for new foods and beverages. Curr. Opin. Biotechnol. 2013, 24, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Biasioli, F.; Yeretzian, C.; Märk, T.D.; Dewulf, J.; van Langenhove, H. Direct-injection mass spectrometry adds the time dimension to (B)VOC analysis. TrAC Trends Anal. Chem. 2011, 30, 1003–1017. [Google Scholar] [CrossRef]
- Lindinger, W.; Hansel, A. Analysis of trace gases at ppb levels by proton transfer reaction mass spectrometry (PTR-MS). Plasma Sources Sci. Technol. 1997, 6. [Google Scholar] [CrossRef]
- Tasin, M.; Cappellin, L.; Biasioli, F. Fast direct injection mass-spectrometric characterization of stimuli for insect electrophysiology by Proton Transfer Reaction-Time of Flight Mass-Spectrometry (PTR-ToF-MS). Sensors 2012, 12, 4091–4104. [Google Scholar] [CrossRef] [PubMed]
- Karl, T.; Yeretzian, C.; Jordan, A.; Lindinger, W. Dynamic measurements of partition coefficients using proton-transfer-reaction mass spectrometry (PTR–MS). Int. J. Mass Spectrom. 2003, 223–224, 383–395. [Google Scholar] [CrossRef]
- Sulzer, P.; Edtbauer, A.; Hartungen, E.; Jürschik, S.; Jordan, A.; Hanel, G.; Feil, S.; Jaksch, S.; Märk, L.; Märk, T.D. From conventional proton-transfer-reaction mass spectrometry (PTR-MS) to universal trace gas analysis. Int. J. Mass Spectrom. 2012, 321–322, 66–70. [Google Scholar] [CrossRef]
- Jordan, A.; Haidacher, S.; Hanel, G.; Hartungen, E.; Herbig, J.; Märk, L.; Schottkowsky, R.; Seehauser, H.; Sulzer, P.; Märk, T.D. An online ultra-high sensitivity Proton-transfer-reaction mass-spectrometer combined with switchable reagent ion capability (PTR + SRI − MS). Int. J. Mass Spectrom. 2009, 286, 32–38. [Google Scholar] [CrossRef]
- Cappellin, L.; Loreto, F.; Aprea, E.; Romano, A.; del Pulgar, J.S.; Gasperi, F.; Biasioli, F. PTR-MS in Italy: A multipurpose sensor with applications in environmental, agri-food and health science. Sensors 2013, 13, 11923–11955. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.B.; Alewijn, M.; Boerrigter-Eenling, R.; van Ruth, S.M. Compositional signatures of conventional, free range, and organic pork meat using fingerprint techniques. Foods 2015, 4, 359–375. [Google Scholar] [CrossRef]
- Soukoulis, C.; Biasioli, F.; Aprea, E.; Schuhfried, E.; Cappellin, L.; Märk, T.D.; Gasperi, F. PTR-TOF-MS analysis for influence of milk base supplementation on texture and headspace concentration of endogenous volatile compounds in yogurt. Food Bioprocess Technol. 2011, 5, 2085–2097. [Google Scholar] [CrossRef]
- Mesurolle, J.; Saint-Eve, A.; Déléris, I.; Souchon, I. Impact of fruit piece structure in yogurts on the dynamics of aroma release and sensory perception. Molecules 2013, 18, 6035–6056. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Capozzi, V.; Spano, G.; Biasioli, F. Proton transfer reaction–mass spectrometry: online and rapid determination of volatile organic compounds of microbial origin. Appl. Microbiol. Biotechnol. 2015, 99, 3787–3795. [Google Scholar] [CrossRef] [PubMed]
- Benozzi, E.; Romano, A.; Capozzi, V.; Makhoul, S.; Cappellin, L.; Khomenko, I.; Aprea, E.; Scampicchio, M.; Spano, G.; Märk, T.D.; et al. Monitoring of lactic fermentation driven by different starter cultures via direct injection mass spectrometric analysis of flavour-related volatile compounds. Food Res. Int. 2015, 76, 682–688. [Google Scholar] [CrossRef]
- Capozzi, V.; Makhoul, S.; Romano, A.; Cappellin, L.; Spano, G.; Scampicchio, M.; Biasioli, F. Monitoring of microbial volatile organic compounds in traditional fermented foods: The importance of tailored approaches to optimize VOCs contribute for consumer acceptance. In Fermented Foods: Sources, Consumption and Health Benefits, 1st ed.; Morton, A.E., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2015; pp. 147–158. [Google Scholar]
- Onishi, M.; Inoue, M.; Araki, T.; Iwabuchi, H.; Sagara, Y. A PTR-MS-based protocol for simulating bread aroma during mastication. Food Bioprocess Technol. 2010, 5, 1228–1237. [Google Scholar] [CrossRef]
- Pico, J.; Gómez, M.; Bernal, J.; Bernal, J.L. Analytical methods for volatile compounds in wheat bread. J. Chromatogr. A 2016, 1428, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Aslankoohi, E.; Zhu, B.; Rezaei, M.N.; Voordeckers, K.; de Maeyer, D.; Marchal, K.; Dornez, E.; Courtin, C.M.; Verstrepen, K.J. Dynamics of the Saccharomyces cerevisiae transcriptome during bread dough fermentation. Appl. Environ. Microbiol. 2013, 79, 7325–7333. [Google Scholar] [CrossRef] [PubMed]
- Aromatic Yeasts. Available online: http://www.lallemandbaking.com/our-products/bakery_cultures/aromatic-yeasts/ (accessed on 21 December 2015).
- Aprea, E.; Biasioli, F.; Märk, T.D.; Gasperi, F. PTR-MS study of esters in water and water/ethanol solutions: Fragmentation patterns and partition coefficients. Int. J. Mass Spectrom. 2007, 262, 114–121. [Google Scholar] [CrossRef]
- Romano, A.; Fischer, L.; Herbig, J.; Campbell-Sills, H.; Coulon, J.; Lucas, P.; Cappellin, L.; Biasioli, F. Wine analysis by FastGC proton-transfer reaction-time-of-flight-mass spectrometry. Int. J. Mass Spectrom. 2014, 369, 81–86. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Feron, G.; Ayed, C.; Qannari, E.M.; Courcoux, P.; Laboure, H.; Guichard, E. Understanding aroma release from model cheeses by a statistical multiblock approach on oral processing. PLoS ONE 2014, 9, e93113. [Google Scholar] [CrossRef] [PubMed]
- Negoias, S.; Visschers, R.; Boelrijk, A.; Hummel, T. New ways to understand aroma perception. Food Chem. 2008, 108, 1247–1254. [Google Scholar] [CrossRef]
- Hummel, T. Retronasal perception of odors. Chem. Biodivers. 2008, 5, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Bojanowski, V.; Hummel, T. Retronasal perception of odors. Physiol. Behav. 2012, 107, 484–487. [Google Scholar] [CrossRef] [PubMed]
- El Hadi, M.A.M.; Zhang, F.-J.; Wu, F.-F.; Zhou, C.-H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed]
- Yener, S.; Romano, A.; Cappellin, L.; Märk, T. D.; Sánchez Del Pulgar, J.; Gasperi, F.; Navarini, L.; Biasioli, F. PTR-ToF-MS characterisation of roasted coffees (C. arabica) from different geographic origins. J Mass Spectrom. 2014, 49, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Croissant, A.E.; Watson, D.M.; Drake, M.A. Application of sensory and instrumental volatile analyses to dairy products. Annu. Rev. Food Sci. Technol. 2011, 2, 395–421. [Google Scholar] [CrossRef] [PubMed]
- Farneti, B.; Khomenko, I.; Cappellin, L.; Ting, V.; Romano, A.; Biasioli, F.; Costa, G.; Costa, F. Comprehensive VOC profiling of an apple germplasm collection by PTR-ToF-MS. Metabolomics 2014, 11, 838–850. [Google Scholar] [CrossRef]
- Capozzi, V.; Menga, V.; Digesu, A.M.; de Vita, P.; van Sinderen, D.; Cattivelli, L.; Fares, C.; Spano, G. Biotechnological production of vitamin B2-enriched bread and pasta. J. Agric. Food Chem. 2011, 59, 8013–8020. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; De Angelis, M.; Di Cagno, R.; Pinto, D.; Siragusa, S.; Rizzello, C.G.; Gobbetti, M. Robustness of Lactobacillus plantarum starters during daily propagation of wheat flour sourdough type I. Food Microbiol. 2010, 27, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Russo, P.; Fragasso, M.; de Vita, P.; Fiocco, D.; Spano, G. Biotechnology and pasta-making: Lactic Acid bacteria as a new driver of innovation. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Capozzi, V.; Arena, M.P.; Spadaccino, G.; Dueñas, M.T.; López, P.; Fiocco, D.; Spano, G. Riboflavin-overproducing strains of Lactobacillus fermentum for riboflavin-enriched bread. Appl. Microbiol. Biotechnol. 2014, 98, 3691–3700. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; van Kerrebroeck, S.; Harth, H.; Huys, G.; Daniel, H.-M.; Weckx, S. Microbial ecology of sourdough fermentations: diverse or uniform? Food Microbiol. 2014, 37, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, D.; Naes, T.; Bauer, F.F. Linking gene regulation and the exo-metabolome: A comparative transcriptomics approach to identify genes that impact on the production of volatile aroma compounds in yeast. BMC Genom. 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.C.; Shaffer, C.E.H.; Bennett, G.N. Microbial formation of esters. Appl. Microbiol. Biotechnol. 2009, 85, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: the soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Mouret, J.R.; Perez, M.; Angenieux, M.; Nicolle, P.; Farines, V.; Sablayrolles, J.M. Online-based kinetic analysis of higher alcohol and ester synthesis during winemaking fermentations. Food Bioprocess Technol. 2013, 7, 1235–1245. [Google Scholar] [CrossRef]
- Alves, Z.; Melo, A.; Figueiredo, A.R.; Coimbra, M.A.; Gomes, A.C.; Rocha, S.M. Exploring the Saccharomyces cerevisiae volatile metabolome: indigenous versus commercial strains. PLoS ONE 2015, 10, e0143641. [Google Scholar] [CrossRef] [PubMed]
- Hiralal, L.; Olaniran, A.O.; Pillay, B. Aroma-active ester profile of ale beer produced under different fermentation and nutritional conditions. J. Biosci. Bioeng. 2014, 117, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Mouret, J.R.; Cadiere, A.; Aguera, E.; Rollero, S.; Ortiz-Julien, A.; Sablayrolles, J.M.; Dequin, S. Dynamics and quantitative analysis of the synthesis of fermentative aromas by an evolved wine strain of Saccharomyces cerevisiae. Yeast 2015, 32, 257–269. [Google Scholar] [PubMed]
- Romano, P.; Suzzi, G. Acetoin production in Saccharomyces cerevisiae wine yeasts. FEMS Microbiol. Lett. 1993, 108, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Suzzi, G.; Mortimer, R.; Polsinelli, M. Production of high levels of acetoin in Saccharomyces cerevisiae wine yeasts is a recessive trait. J. Appl. Bacteriol. 1995, 78, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lu, J.R. Strategies for enhancing fermentative production of acetoin: A review. Biotechnol. Adv. 2014, 32, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Granchi, L.; Caruso, M.; Borra, G.; Palla, G.; Fiore, C.; Ganucci, D.; Caligiani, A.; Brandolini, V. The species-specific ratios of 2,3-butanediol and acetoin isomers as a tool to evaluate wine yeast performance. Int. J. Food Microbiol. 2003, 86, 163–168. [Google Scholar] [CrossRef]
- González, E.; Fernández, M.R.; Marco, D.; Calam, E.; Sumoy, L.; Parés, X.; Dequin, S.; Biosca, J.A. Role of Saccharomyces cerevisiae oxidoreductases Bdh1p and Ara1p in the metabolism of acetoin and 2,3-butanediol. Appl. Environ. Microbiol. 2010, 76, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Nedović, V.; Gibson, B.; Mantzouridou, T.F.; Bugarski, B.; Djordjević, V.; Kalušević, A.; Paraskevopoulou, A.; Sandell, M.; Šmogrovičová, D.; Yilmaztekin, M. Aroma formation by immobilized yeast cells in fermentation processes. Yeast 2015, 32, 173–216. [Google Scholar] [CrossRef] [PubMed]
- Bloem, A.; Sanchez, I.; Dequin, S.; Camarasa, C. Metabolic impact of redox cofactor perturbations on the formation of aroma compounds in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2015, 82, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Cappellin, L.; Biasioli, F.; Fabris, A.; Schuhfried, E.; Soukoulis, C.; Märk, T. D.; Gasperi, F. Improved mass accuracy in PTR-TOF-MS: Another step towards better compound identification in PTR-MS. Int. J. Mass Spectrom. 2010, 290, 60–63. [Google Scholar] [CrossRef]
- Cappellin, L.; Biasioli, F.; Granitto, P.M.; Schuhfried, E.; Soukoulis, C.; Costa, F.; Märk, T.D.; Gasperi, F. On data analysis in PTR-TOF-MS: From raw spectra to data mining. Sens. Actuators B Chem. 2011, 155, 183–190. [Google Scholar] [CrossRef]
- Lindinger, W.; Hansel, A.; Jordan, A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research. Int. J. Mass Spectrom. Ion Process. 1998, 173, 191–241. [Google Scholar] [CrossRef]
- Cappellin, L.; Karl, T.; Probst, M.; Ismailova, O.; Winkler, P.M.; Soukoulis, C.; Aprea, E.; Märk, T.D.; Gasperi, F.; Biasioli, F. On quantitative determination of volatile organic compound concentrations using Proton Transfer Reaction Time-of-Flight Mass Spectrometry. Environ. Sci. Technol. 2012, 46, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.
| m/z | Cycle | C(ppbV) | A16(ppbV) | A17(ppbV) | A18(ppbV) |
|---|---|---|---|---|---|
| 65.058 | 10 | 44 ± 6 b | 10 ± 1 a | 49 ± 5 b | 45 ± 4 b |
| 65.058 | 15 | 73 ± 9 b | 19 ± 2 a | 85 ± 13 b | 74 ± 8 b |
| 65.058 | 20 | 66 ± 13 b | 20 ± 4 a | 79 ± 31 b | 69 ± 12 b |
| 65.058 | 24 | 119 ± 12 b | 54 ± 6 a | 144 ± 7 c | 142 ± 10 c |
| 75.080 | 10 | 155 ± 29 b | 18 ± 2 a | 181 ± 29 b | 155 ± 19 b |
| 75.080 | 15 | 277 ± 50 b | 37 ± 6 a | 341 ± 63 b | 287 ± 33 b |
| 75.080 | 20 | 252 ± 65 b | 41 ± 9 a | 332 ± 166 b | 270 ± 62 b |
| 75.080 | 24 | 512 ± 60 b | 133 ± 25 a | 704 ± 92 c | 637 ± 55 b |
| m/z | Cycle | C(ppbV) | A16(ppbV) | A17(ppbV) | A18(ppbV) |
|---|---|---|---|---|---|
| 89.060 | 10 | 173 ± 58 b | 52 ± 3 a | 157 ± 17 b | 270 ± 37 c |
| 89.060 | 15 | 314 ± 53 b | 91 ± 14 a | 478 ± 78 c | 644 ± 88 d |
| 89.060 | 20 | 396 ± 77 b | 108 ± 15 a | 625 ± 168 c | 947 ± 137 d |
| 89.060 | 24 | 661 ± 110 b | 225 ± 20 a | 1261 ± 102 c | 1855 ± 182 d |
| 103.075 | 10 | 1.7 ± 0.5 b | 0.9 ± 0.2 a | 1.8 ± 0.1 b | 2.8 ± 0.3 c |
| 103.075 | 15 | 2.2 ± 0.4 b | 1.5 ± 0.3 a | 3.0 ± 0.4 c | 3.7 ± 0.4 c |
| 103.075 | 20 | 2.4 ± 0.4 a, b | 1.6 ± 0.4 a | 3.0 ± 0.5 b | 4.3 ± 0.6 c |
| 103.075 | 24 | 3.2 ± 0.7 a | 3.0 ± 0.7 a | 5.1 ± 0.6 b | 6.7 ± 0.6 c |
| 117.093 | 10 | 2.1 ± 0.8 b | 0.9 ± 0.1 a | 2.7 ± 0.2 b, c | 3.1 ± 0.4 c |
| 117.093 | 15 | 3.0 ± 0.6 b | 1.4 ± 0.2 a | 6.0 ± 1.3 c | 4.9 ± 0.4 c |
| 117.093 | 20 | 3.3 ± 0.5 a | 1.6 ± 0.3 a | 6.1 ± 1.7 b | 6.1 ± 0.9 b |
| 117.093 | 24 | 4.9 ± 0.6 a | 3.2 ± 0.3 a | 11 ± 1 b | 11 ± 1 b |
| m/z | C (ppbV) | A16 (ppbV) | A17 (ppbV) | A18 (ppbV) |
|---|---|---|---|---|
| 39.022 | 184.6 | 216.0 | 209.1 | 78.8 |
| 41.038 | 98.8 | 109.3 | 113.8 | 66.4 |
| 43.956 | 0.4 | 0.2 | 0.6 | 0.9 |
| 57.070 | 340.6 | 213.9 | 58.8 | 417.9 |
| 61.028 | 30.1 | 190.3 | 334.1 | 49.2 |
| 71.085 | 255.5 | 123.5 | 344.1 | 319.4 |
| 89.059 | 264.7 | 117.4 | 635.8 | 908.1 |
| 103.075 | 0.8 | 1.4 | 2.1 | 2.4 |
| 117.093 | 1.6 | 1.6 | 5.2 | 5.1 |
| m/z | Cycle | A16/C (before normalization) | A16/C (after normalization) |
|---|---|---|---|
| 89.059 | 10 | 0.300 | 2.027 |
| 89.059 | 15 | 0.291 | 1.791 |
| 89.059 | 20 | 0.273 | 1.431 |
| 89.059 | 24 | 0.342 | 1.150 |
| 103.075 | 10 | 0.529 | 3.582 |
| 103.075 | 15 | 0.682 | 4.196 |
| 103.075 | 20 | 0.667 | 3.487 |
| 103.075 | 24 | 0.937 | 3.156 |
| 117.093 | 10 | 0.429 | 2.900 |
| 117.093 | 15 | 0.467 | 2.872 |
| 117.093 | 20 | 0.485 | 2.536 |
| 117.093 | 24 | 0.653 | 2.198 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capozzi, V.; Makhoul, S.; Aprea, E.; Romano, A.; Cappellin, L.; Sanchez Jimena, A.; Spano, G.; Gasperi, F.; Scampicchio, M.; Biasioli, F. PTR-MS Characterization of VOCs Associated with Commercial Aromatic Bakery Yeasts of Wine and Beer Origin. Molecules 2016, 21, 483. https://doi.org/10.3390/molecules21040483
Capozzi V, Makhoul S, Aprea E, Romano A, Cappellin L, Sanchez Jimena A, Spano G, Gasperi F, Scampicchio M, Biasioli F. PTR-MS Characterization of VOCs Associated with Commercial Aromatic Bakery Yeasts of Wine and Beer Origin. Molecules. 2016; 21(4):483. https://doi.org/10.3390/molecules21040483
Chicago/Turabian StyleCapozzi, Vittorio, Salim Makhoul, Eugenio Aprea, Andrea Romano, Luca Cappellin, Ana Sanchez Jimena, Giuseppe Spano, Flavia Gasperi, Matteo Scampicchio, and Franco Biasioli. 2016. "PTR-MS Characterization of VOCs Associated with Commercial Aromatic Bakery Yeasts of Wine and Beer Origin" Molecules 21, no. 4: 483. https://doi.org/10.3390/molecules21040483
APA StyleCapozzi, V., Makhoul, S., Aprea, E., Romano, A., Cappellin, L., Sanchez Jimena, A., Spano, G., Gasperi, F., Scampicchio, M., & Biasioli, F. (2016). PTR-MS Characterization of VOCs Associated with Commercial Aromatic Bakery Yeasts of Wine and Beer Origin. Molecules, 21(4), 483. https://doi.org/10.3390/molecules21040483











