Highly Efficient Synthesis of an Emerging Lipophilic Antioxidant: 2-Ethylhexyl Ferulate
Abstract
:1. Introduction
2. Results and Discussion
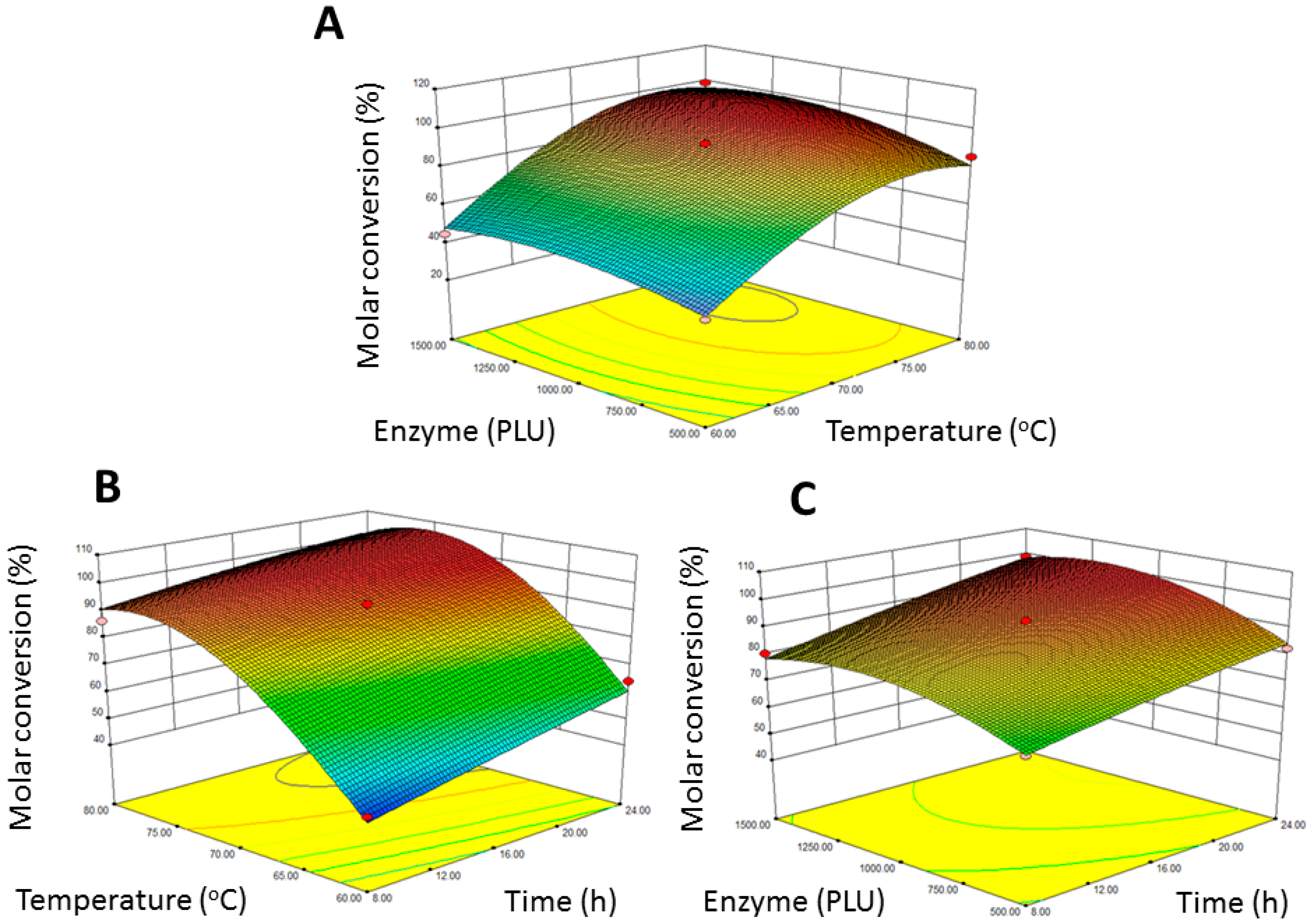
2.1. RSM Model
| Y = −968.26667 + 3.60052X1 + 26.28208X2 + 0.039783X3 − 0.027812X1X2 + 0.0003X1X3 + |
| 0.0004X2X3 − 0.028971X12 − 0.17154X22 − 0.0000305167X32 |
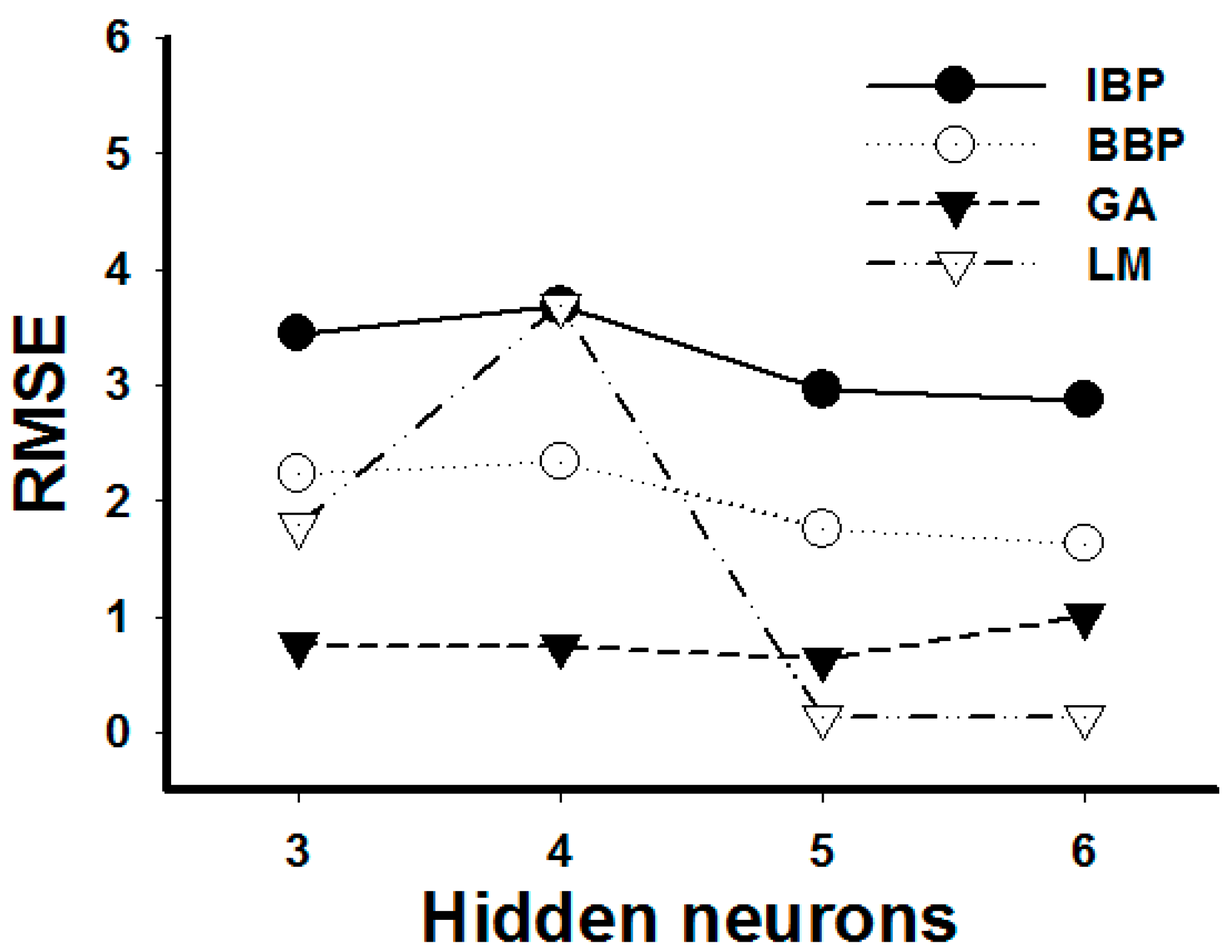
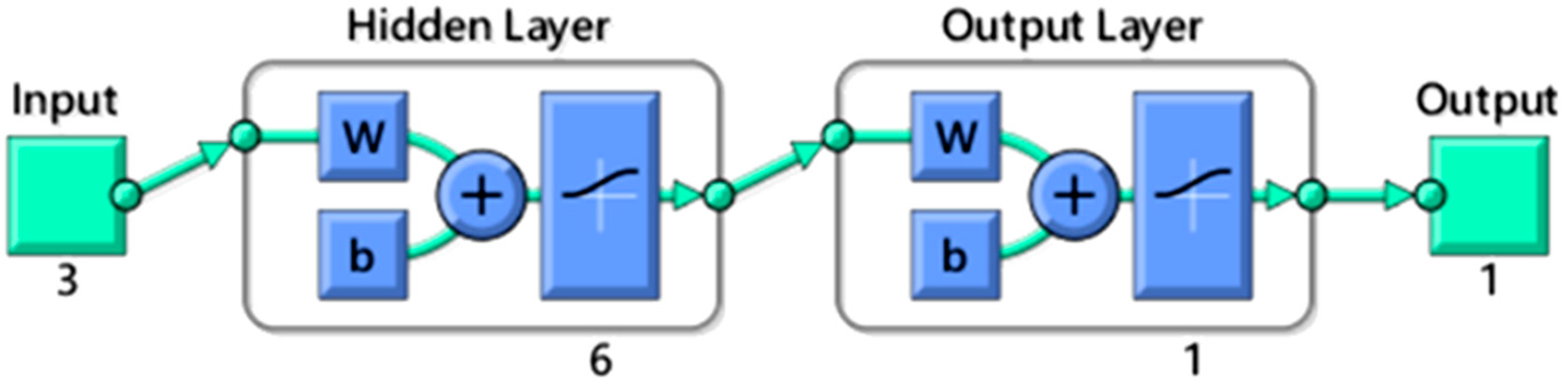
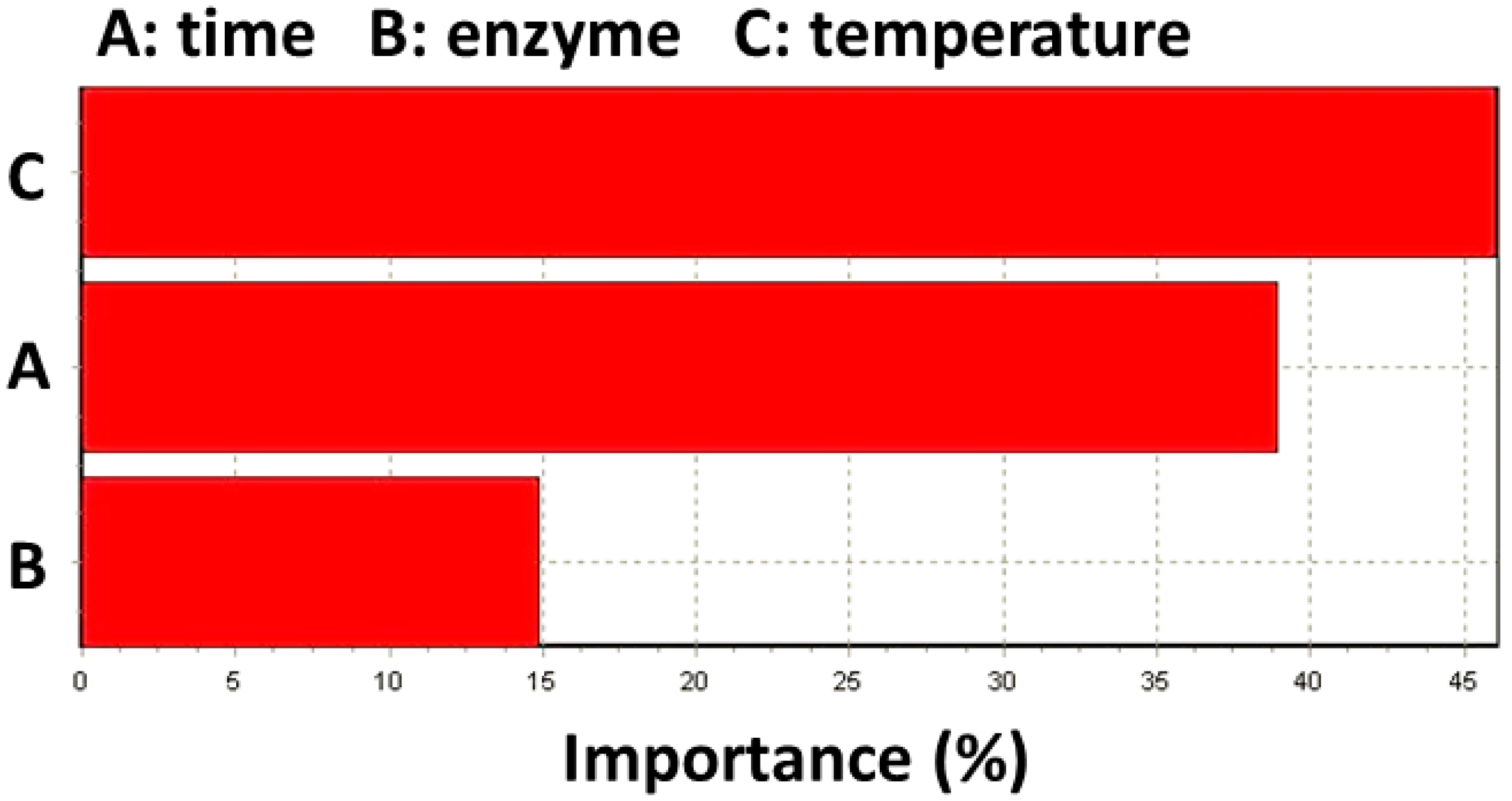
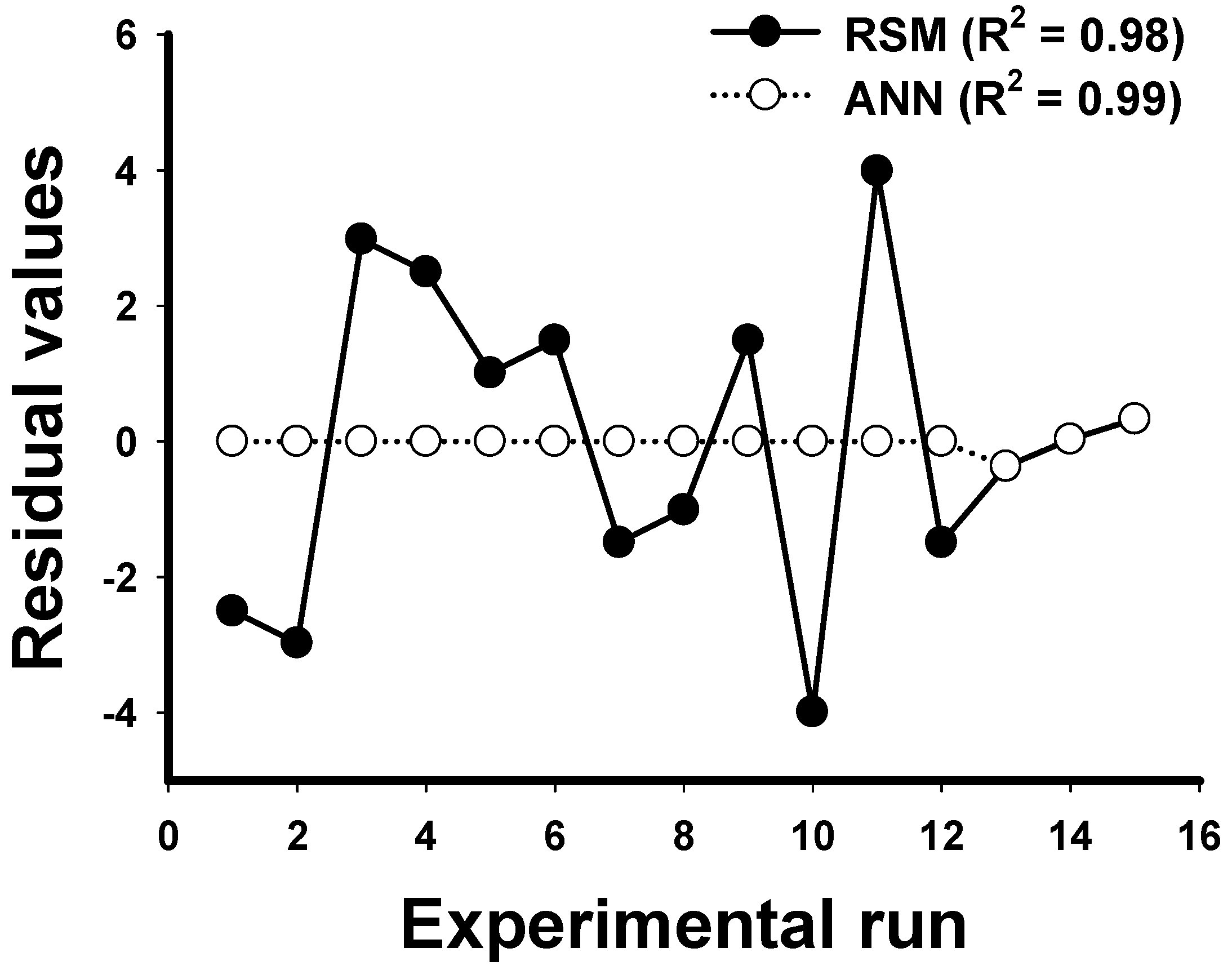
2.2. ANN Model
2.3. Comparison between RSM and ANN and Optimization for Experimental Condition
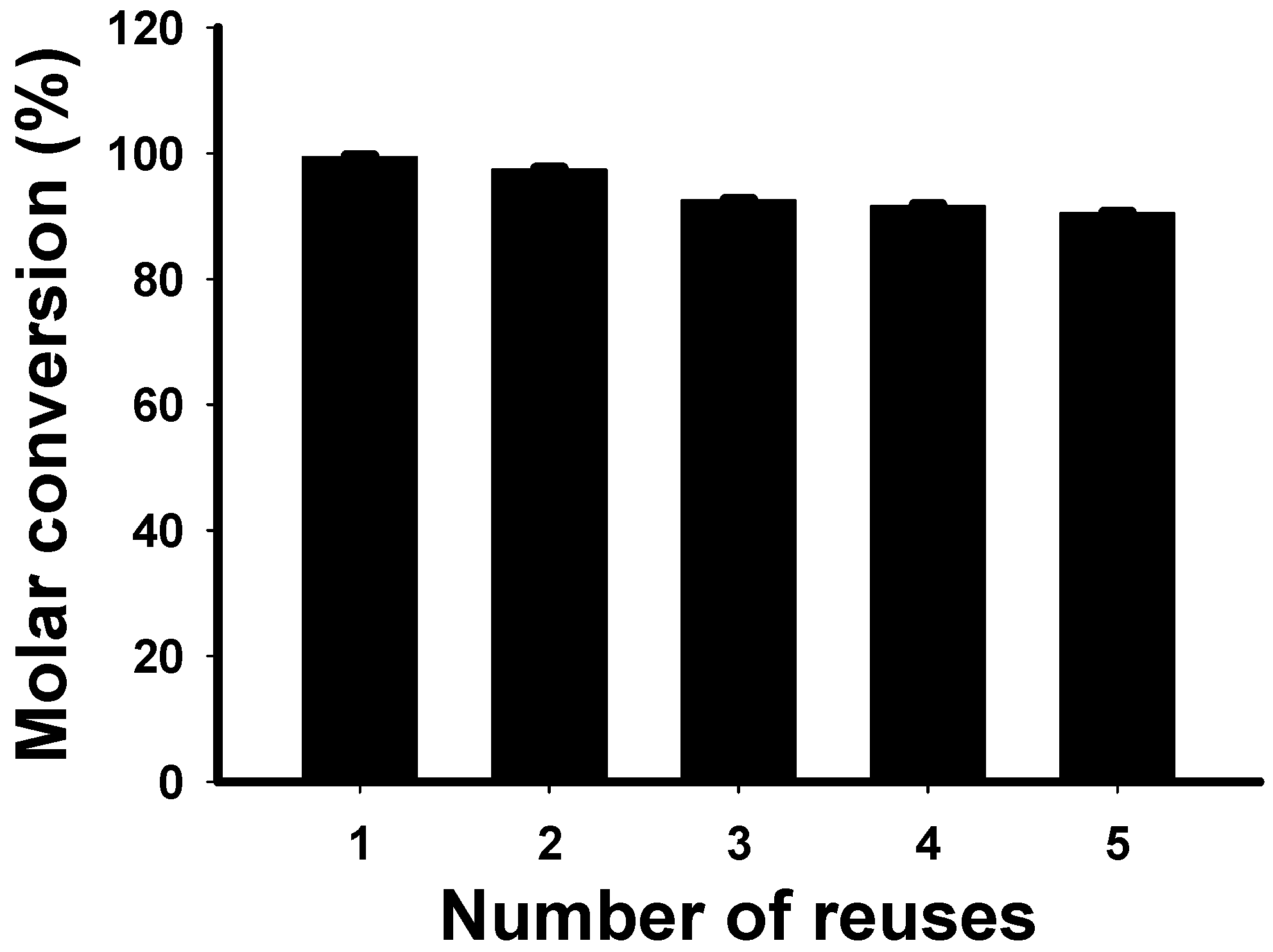
2.4. Evaluation of Enzyme Reusability
3. Experimental Section
3.1. Materials
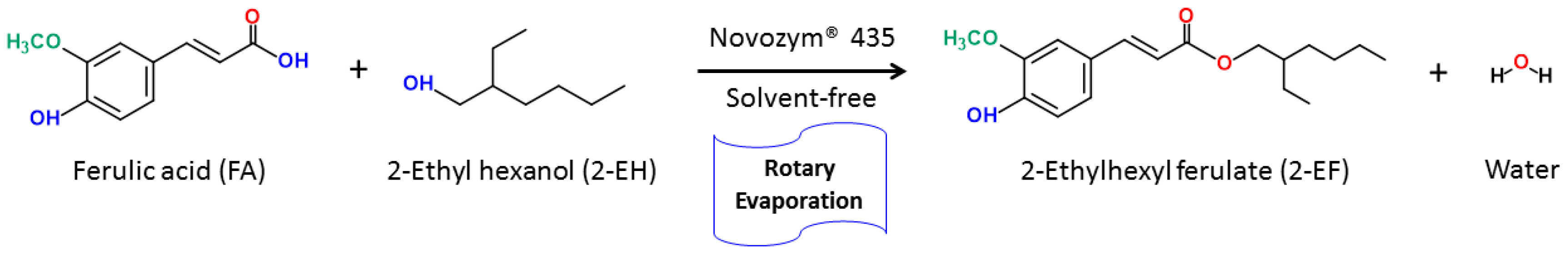
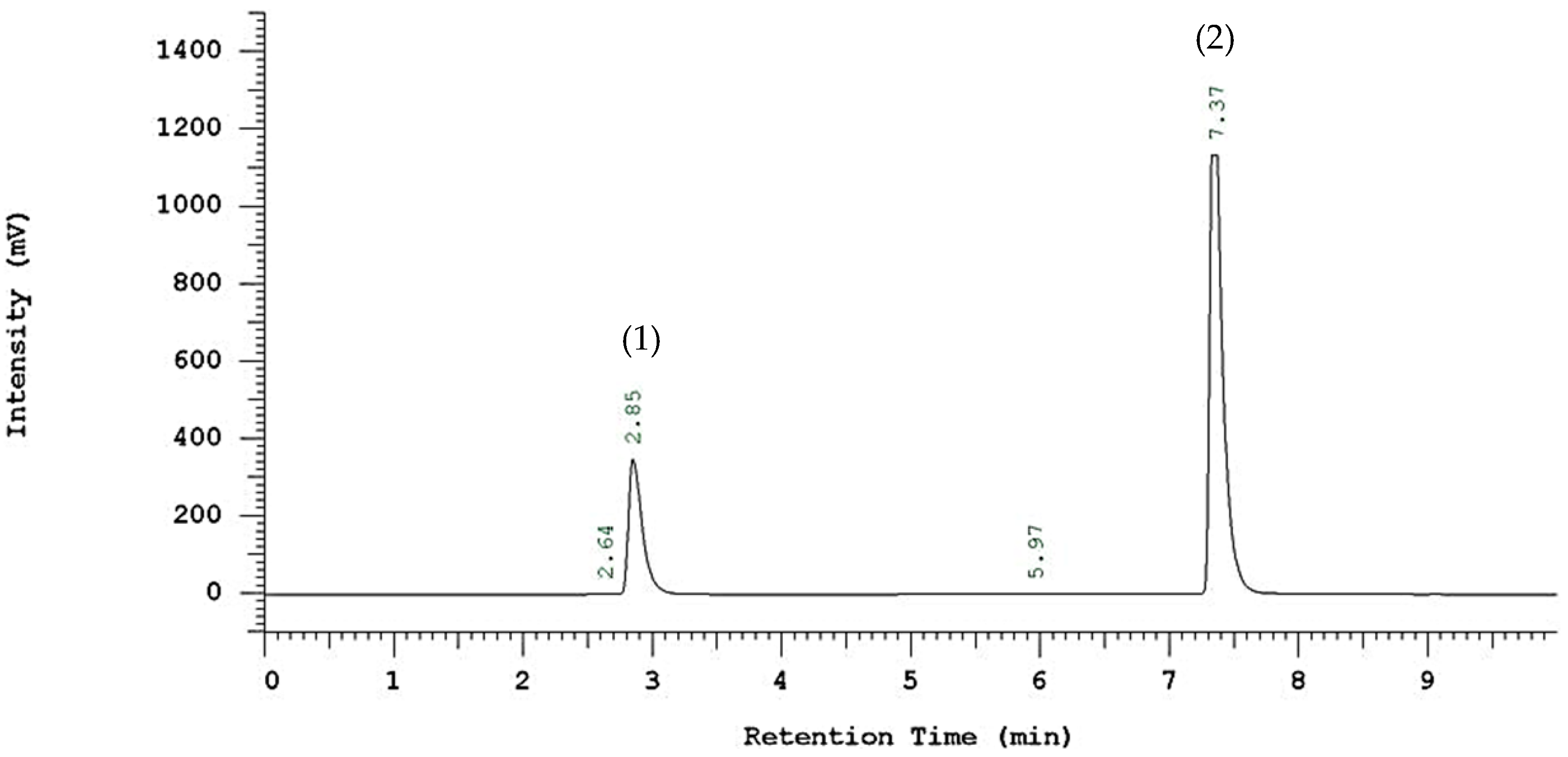
3.2. Enzymatic 2-ethylhexyl Ferulate Synthesis
3.3. Box-Behnken Design
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, J.; Wen, X.Y.; Lu, J.F.; Kan, J.; Jin, C.H. Free radical mediated grafting of chitosan with caffeic and ferulic acids: Structures and antioxidant activity. Int. J. Biol. Macromol. 2014, 65, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Choo, W.S.; Birch, E.J. Radical scavenging activity of lipophilized products from lipase-catalyzed transesterification of triolein with cinnamic and ferulic acids. Lipids 2009, 44, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, S.; Kurokawa, T.; Nakata, C.; Saito, Y.; Oikawa, S.; Kobayashi, M.; Hirano, T.; Iseki, K. In vitro and in vivo antioxidant properties of ferulic acid: A comparative study with other natural oxidation inhibitors. Food Chem. 2009, 114, 466–471. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Guyot, B.; Bosquette, B.; Pina, M.; Graille, J. Esterification of phenolic acids from green coffee with an immobilized lipase from Candida antarctica in solvent-free medium. Biotechnol. Lett. 1997, 19, 529–532. [Google Scholar] [CrossRef]
- Stamatis, H.; Sereti, V.; Kolisis, F.N. Studies on the enzymatic synthesis of lipophilic derivatives of natural antioxidants. J. Am. Oil Chem. Soc. 1999, 76, 1505–1510. [Google Scholar] [CrossRef]
- Vosmann, K.; Weitkamp, P.; Weber, N. Solvent-free lipase-catalyzed preparation of long-chain alkyl phenylpropanoates and phenylpropyl alkanoates. J. Agric. Food Chem. 2006, 54, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Stamatis, H.; Sereti, V.; Kolisis, F.N. Enzymatic synthesis of hydrophilic and hydrophobic derivatives of natural phenolic acids in organic media. J. Mol. Catal. B Enzym. 2001, 11, 323–328. [Google Scholar] [CrossRef]
- Chigorimbo-Murefu, N.T.L.; Riva, S.; Burton, S.G. Lipase-catalysed synthesis of esters of ferulic acid with natural compounds and evaluation of their antioxidant properties. J. Mol. Catal. B Enzym. 2009, 56, 277–282. [Google Scholar] [CrossRef]
- Laszlo, J.A.; Compton, D.L. Enzymatic glycerolysis and transesterification of vegetable oil for enhanced production of feruloylated glycerols. J. Am. Oil Chem. Soc. 2006, 83, 765–770. [Google Scholar] [CrossRef]
- Sun, S.; Shan, L.; Jin, Q.; Liu, Y.; Wang, X. Solvent-free synthesis of glyceryl ferulate using a commercial microbial lipase. Biotechnol. Lett. 2007, 29, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Nazare, A.C.; de Faria, C.M.; Chiari, B.G.; Petronio, M.S.; Regasini, L.O.; Silva, D.H.; Correa, M.A.; Isaac, V.L.; da Fonseca, L.M.; Ximenes, V.F. Ethyl ferulate, a component with anti-inflammatory properties for emulsion-based creams. Molecules 2014, 19, 8124–8139. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.V.; Gomes, B.S.; Neto, B.S.; Ferreira, A.R.; de Sousa, D.P.; E Martins, M.D.; Oliveira, F.A. Ferulic acid ethyl ester diminished Complete Freund’s Adjuvant-induced incapacitation through antioxidant and anti-inflammatory activity. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 389, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.C.; Oliveira, G.L.; Machado, K.C.; Islam, M.T.; Junior, A.L.; de Sousa, D.P.; Freitas, R.M. Anticonvulsant and behavioral effects observed in mice following treatment with an ester derivative of ferulic acid: Isopentyl ferulate. Chem. Biol. Interact. 2015, 242, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Shirai, A.; Kajiura, M.; Omasa, T. Synergistic photobactericidal activity based on ultraviolet-A irradiation and ferulic acid derivatives. Photochem. Photobiol. 2015, 91, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Z.; Diao, X.J.; Yang, W.H.; Li, F.; He, G.W.; Gong, G.Q.; Xu, Y.G. Design, synthesis and antithrombotic evaluation of novel dabigatran prodrugs containing methyl ferulate. Bioorg. Med. Chem. Lett. 2013, 23, 2089–2092. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Lee, Y.M.; Hsu, C.H.; Leu, S.Y.; Chiang, H.Y.; Yen, M.H.; Cheng, P.Y. The effect of ferulic acid ethyl ester on leptin-induced proliferation and migration of aortic smooth muscle cells. Exp. Mol. Med. 2015, 47, e180. [Google Scholar] [CrossRef] [PubMed]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, S.; Piana, C.; Setti, L.; Hochkoeppler, A.; Pifferi, P.G.; Williamson, G.; Faulds, C.B. Synthesis of pentylferulate by a feruloyl esterase from Aspergillus niger using water-in-oil microemulsions. Biotechnol. Lett. 2001, 23, 325–330. [Google Scholar] [CrossRef]
- Sun, S.; Song, F.; Bi, Y.; Yang, G.; Liu, W. Solvent-free enzymatic transesterification of ethyl ferulate and monostearin: Optimized by response surface methodology. J. Biotechnol. 2012, 164, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Kimura, Y.; Kadota, M.; Tsuno, T.; Adachi, S. Continuous synthesis of alkyl ferulate by immobilized Candida antarctica lipase at high temperature. Biotechnol. Lett. 2006, 28, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Compton, D.L.; Laszlo, J.A.; Berhow, M.A. Lipase-catalyzed synthesis of ferulate esters. J. Am. Oil Chem. Soc. 2000, 77, 513–519. [Google Scholar] [CrossRef]
- Sun, S.; Zhu, S.; Bi, Y. Solvent-free enzymatic synthesis of feruloylated structured lipids by the transesterification of ethyl ferulate with castor oil. Food Chem. 2014, 158, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Su, D.; Huang, Y.; Wang, Y.; Li, Y.H. Ultrasound-assisted aqueous two-phase system for extraction and enrichment of Zanthoxylum armatum lignans. Molecules 2015, 20, 15273–15286. [Google Scholar] [CrossRef] [PubMed]
- Rafigh, S.M.; Yazdi, A.V.; Vossoughi, M.; Safekordi, A.A.; Ardjmand, M. Optimization of culture medium and modeling of curdlan production from Paenibacillus polymyxa by RSM and ANN. Int. J. Biol. Macromol. 2014, 70, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Maran, J.P.; Priya, B. Comparison of response surface methodology and artificial neural network approach towards efficient ultrasound-assisted biodiesel production from muskmelon oil. Ultrason. Sonochem. 2015, 23, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.C.; Li, Y.; Twu, Y.K.; Shieh, C.J. High efficient synthesis of enzymatic 2-ethylhexyl ferulate at solvent-free and reduced pressure evaporation system. J. Mater. Sci. Chem. Eng. 2015, 3, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Wu, D.; Zhang, Q.A.; Sun, D.W. Ultrasound-assisted extraction of phenolics from wine lees: Modeling, optimization and stability of extracts during storage. Ultrason. Sonochem. 2014, 21, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of 2-ethylhexyl ferulate are available from the authors.
| Treatment No. a | Experimental Variables | Observed Molar Conversion (%) | ||
|---|---|---|---|---|
| X1 (h) | X2 (PLU) | X3 (°C) | ||
| 1 | 16 | 500 | 80 | 85.7 ± 3.4 |
| 2 | 16 | 1000 | 70 | 92.4 ± 3.1 |
| 3 | 16 | 1000 | 70 | 92.0 ± 2.8 |
| 4 | 16 | 1000 | 70 | 91.7 ± 2.3 |
| 5 | 8 | 500 | 70 | 68.7 ± 4.1 |
| 6 | 24 | 500 | 70 | 82.3 ± 3.2 |
| 7 | 8 | 1500 | 70 | 80.4 ± 2.1 |
| 8 | 24 | 1500 | 70 | 98.8 ± 1.6 |
| 9 | 16 | 500 | 60 | 39.8 ± 2.7 |
| 10 | 8 | 1000 | 60 | 42.7 ± 3.3 |
| 11 | 16 | 1500 | 60 | 44.8 ± 4.2 |
| 12 | 16 | 1500 | 80 | 98.9 ± 2.5 |
| 13 | 24 | 1000 | 60 | 64.2 ± 2.1 |
| 14 | 8 | 1000 | 80 | 86.3 ± 2.8 |
| 15 | 24 | 1000 | 80 | 98.7 ± 1.9 |
| Source | Sum of Squares | Degree of Freedom | Prob > F a |
|---|---|---|---|
| Model | 22.50 | 9 | 0.0005 |
| Linear | |||
| X1 | 1.87 | 1 | 0.0033 |
| X2 | 0.85 | 1 | 0.0614 |
| X3 | 14.72 | 1 | <0.0001 |
| Interaction | |||
| X1X2 | 0.01 | 1 | 0.7297 |
| X1X3 | 0.17 | 1 | 0.1762 |
| X2X3 | 0.02 | 1 | 0.5973 |
| Square | |||
| X12 | 0.02 | 1 | 0.6545 |
| X22 | 0.79 | 1 | 0.0188 |
| X32 | 4.33 | 1 | 0.0005 |
| Lack of fit | 15.20 | 3 | 0.09 |
| Pure error | 0.99 | 2 | |
| Total error | 16.19 | 5 |
| Run | Independent Variable | Molar Conversion (%) | ||||
|---|---|---|---|---|---|---|
| X1 (h) | X2 (PLU) | X3 (°C) | Experimental | RSM Predicted | ANN Predicted | |
| 1 | 14 | 1250 | 75 | 93.2 | 98.8 | 95.0 |
| 2 | 15 | 750 | 65 | 76.8 | 71.2 | 73.5 |
| 3 | 10 | 850 | 75 | 74.6 | 90 | 85.3 |
| 4 | 9 | 1400 | 65 | 65.4 | 65.1 | 62.2 |
| 5 | 20 | 950 | 60 | 51.7 | 56.9 | 56.3 |
| AAD | 10.046 | 9.430 | ||||
| RMSE | 7.322 | 5.152 | ||||
| Model | Optimal Condition | Relative Conversion (%) | |||
|---|---|---|---|---|---|
| X1 (h) | X2 (PLU) | X3 (°C) | Experimental Value | Predicted Value | |
| RSM | 23 | 1422 | 71 | 99.43 | 102.74 |
| ANN | 24 | 1485 | 78 | 99.74 | 99.82 |
| Parameters | Symbol | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Reaction time (h) | X1 | 8 | 16 | 24 |
| Enzyme amount (PLU) a | X2 | 500 | 1000 | 1500 |
| Reaction temperature (°C) | X3 | 60 | 70 | 80 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.-C.; Li, Y.; Kuo, C.-H.; Twu, Y.-K.; Shieh, C.-J. Highly Efficient Synthesis of an Emerging Lipophilic Antioxidant: 2-Ethylhexyl Ferulate. Molecules 2016, 21, 478. https://doi.org/10.3390/molecules21040478
Huang K-C, Li Y, Kuo C-H, Twu Y-K, Shieh C-J. Highly Efficient Synthesis of an Emerging Lipophilic Antioxidant: 2-Ethylhexyl Ferulate. Molecules. 2016; 21(4):478. https://doi.org/10.3390/molecules21040478
Chicago/Turabian StyleHuang, Kuo-Chuan, Ying Li, Chia-Hung Kuo, Yawo-Kuo Twu, and Chwen-Jen Shieh. 2016. "Highly Efficient Synthesis of an Emerging Lipophilic Antioxidant: 2-Ethylhexyl Ferulate" Molecules 21, no. 4: 478. https://doi.org/10.3390/molecules21040478
APA StyleHuang, K.-C., Li, Y., Kuo, C.-H., Twu, Y.-K., & Shieh, C.-J. (2016). Highly Efficient Synthesis of an Emerging Lipophilic Antioxidant: 2-Ethylhexyl Ferulate. Molecules, 21(4), 478. https://doi.org/10.3390/molecules21040478









