Preparation of Pd-Loaded Hierarchical FAU Membranes and Testing in Acetophenone Hydrogenation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preliminary Remarks
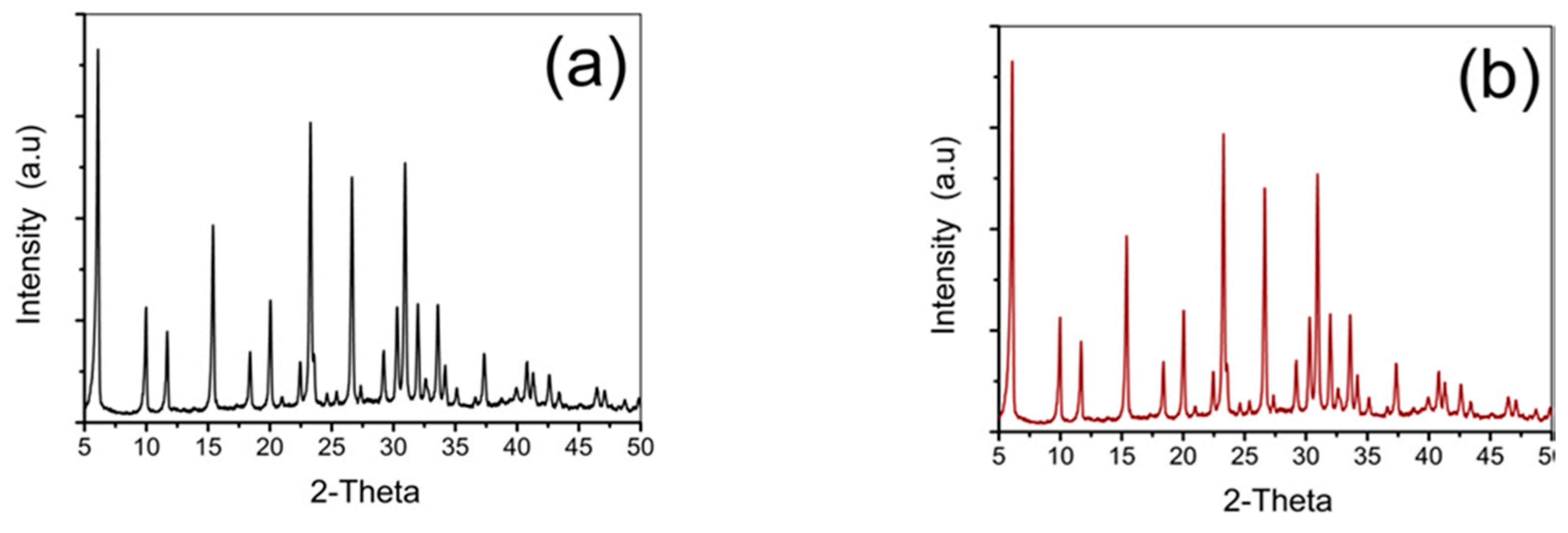
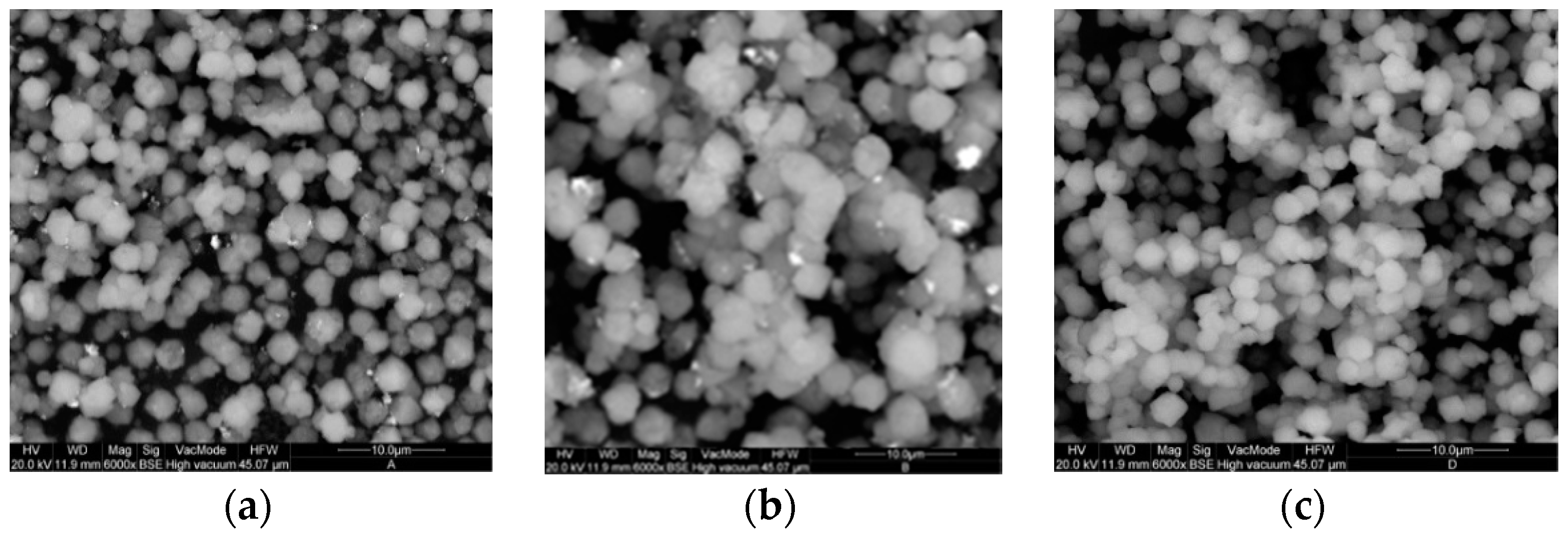
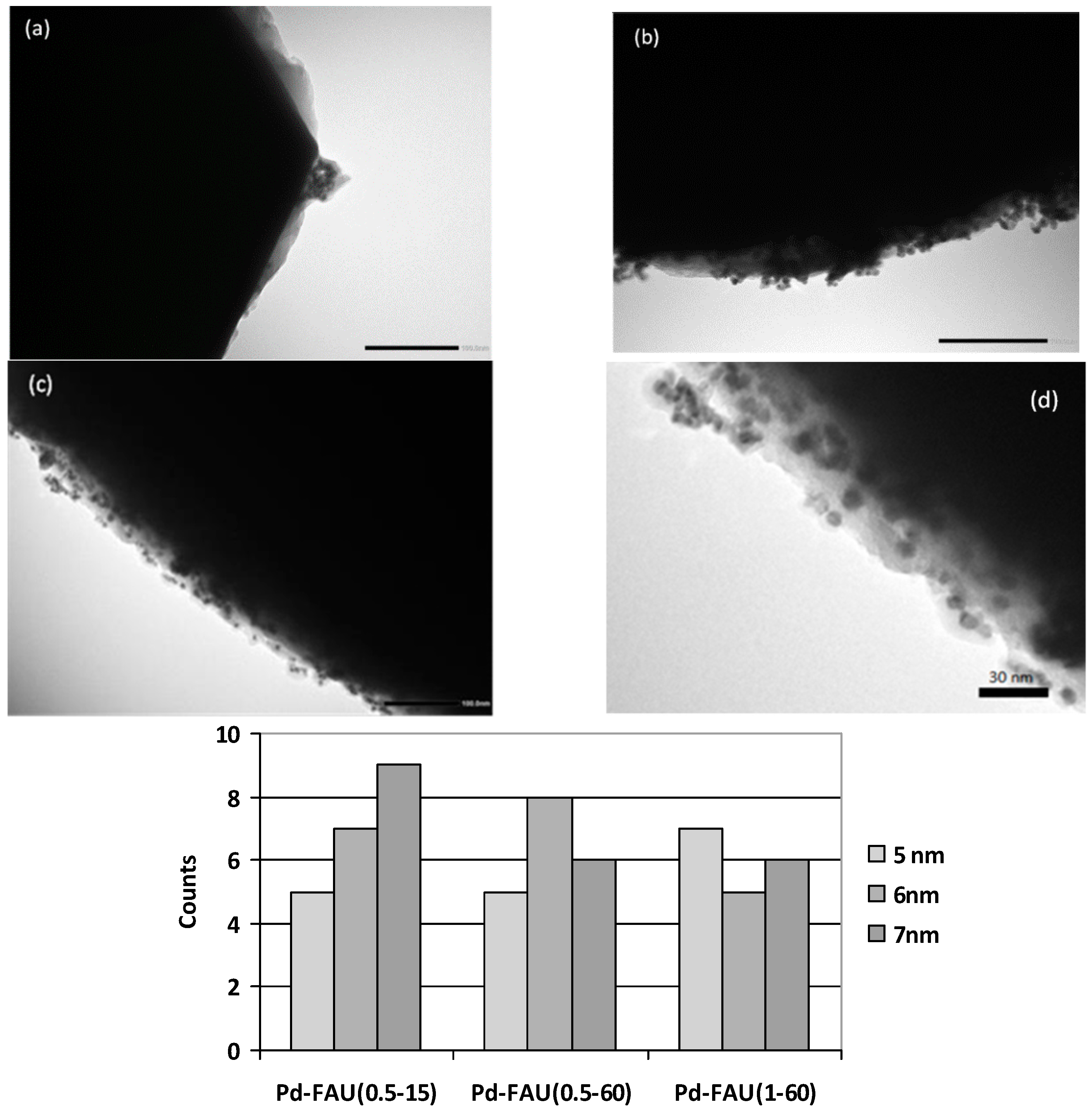
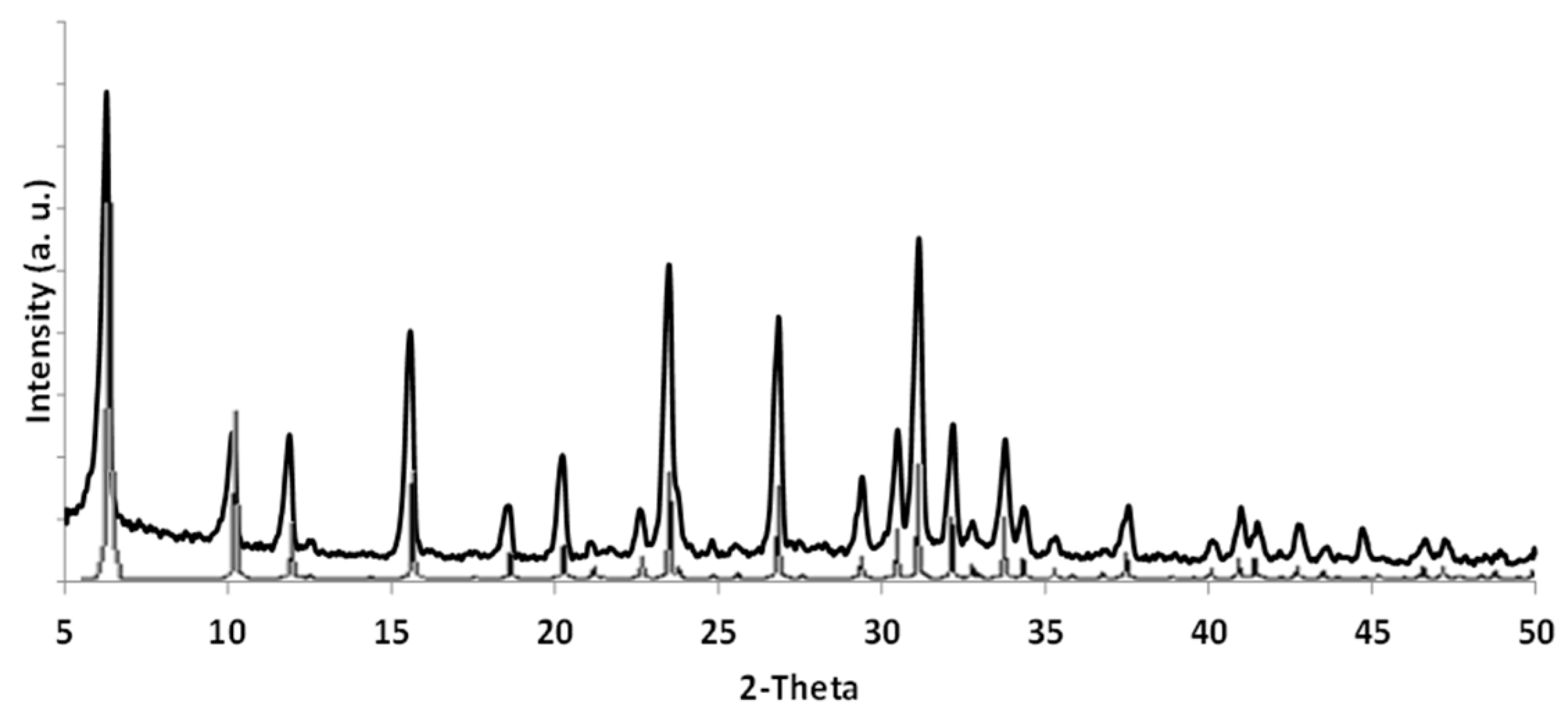
2.2. Pd-FAU Crystals: Effect of Pd Content and Exchange Temperature
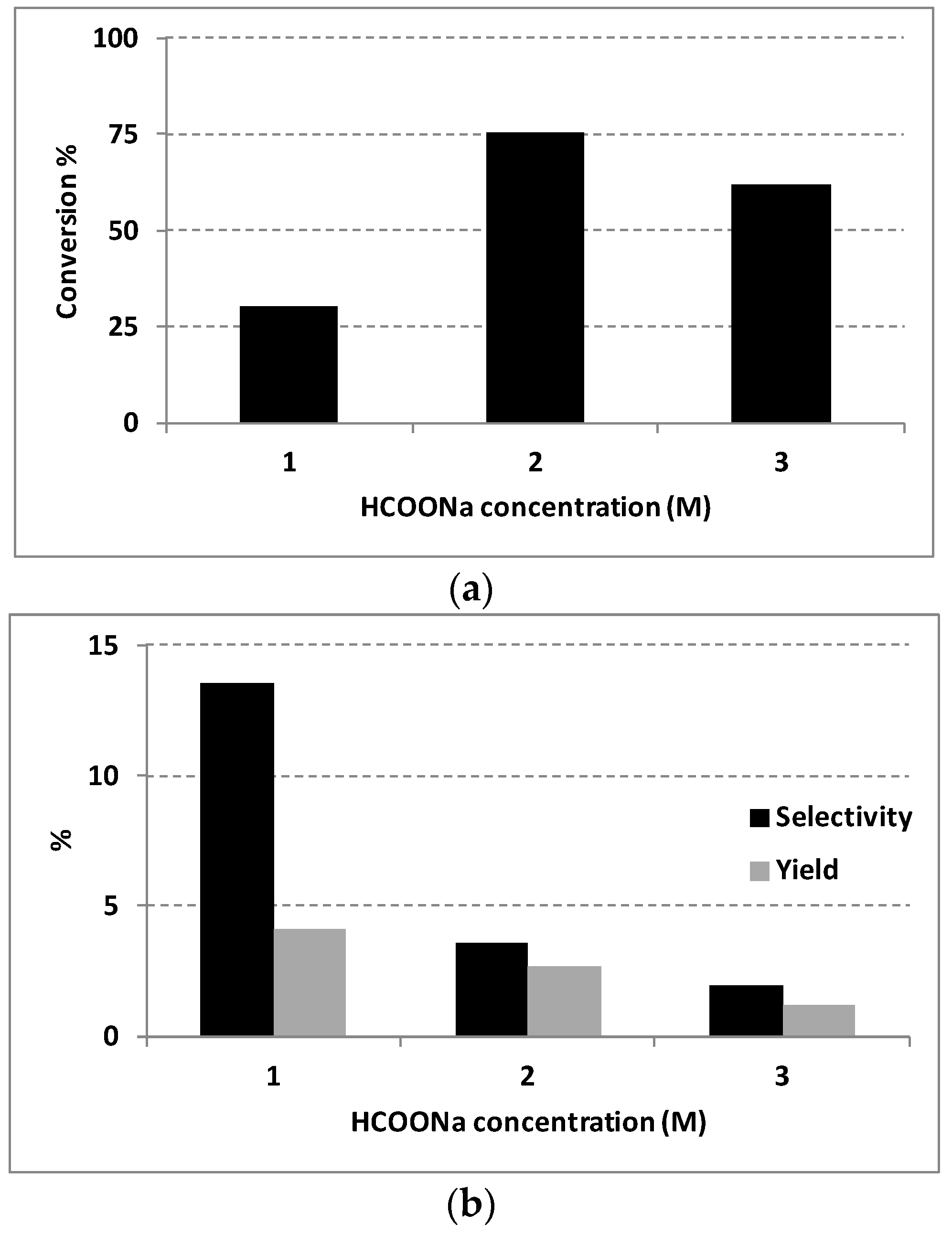
2.3. Influence of the Solvent and the Hydrogen Donor
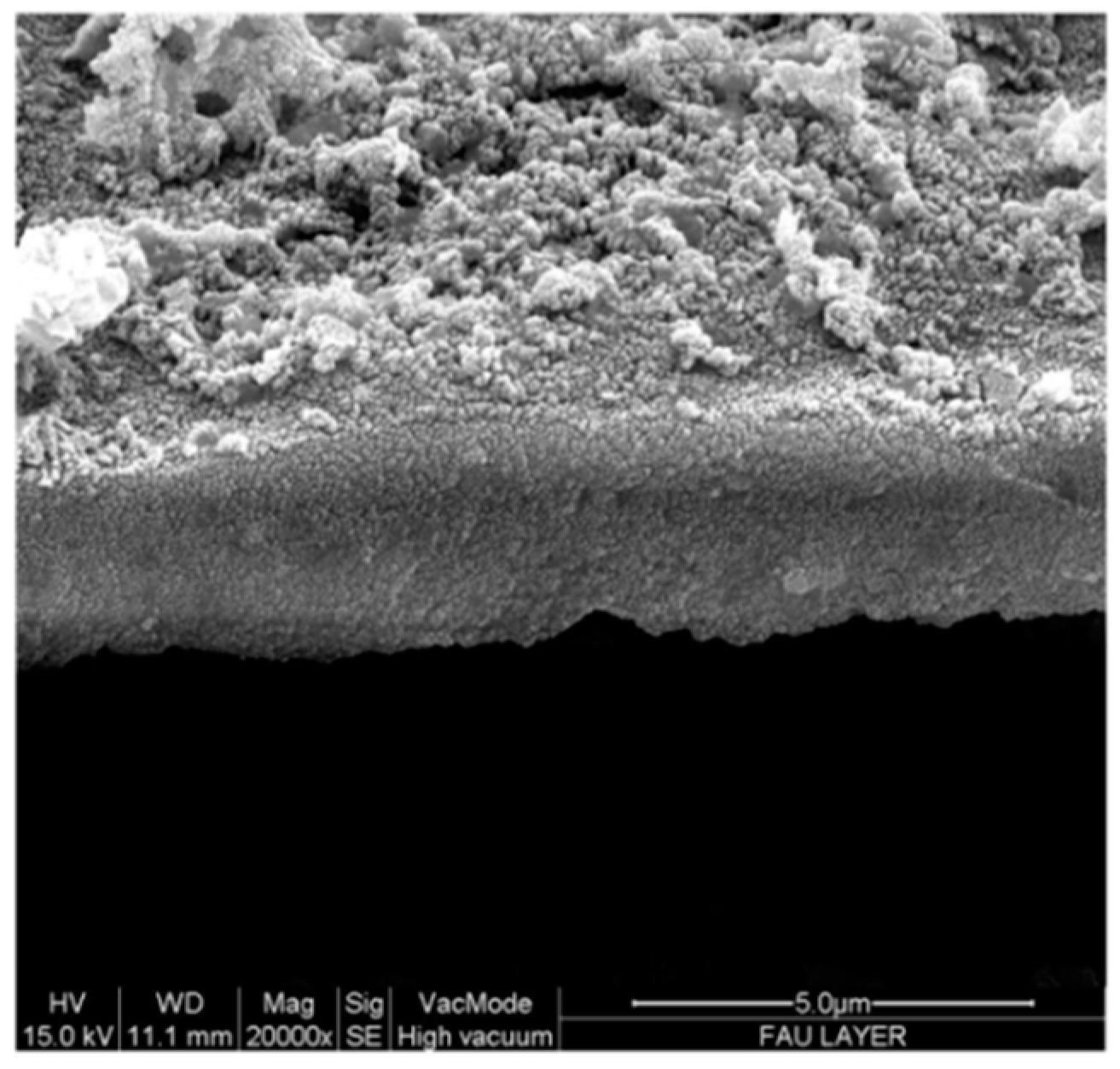
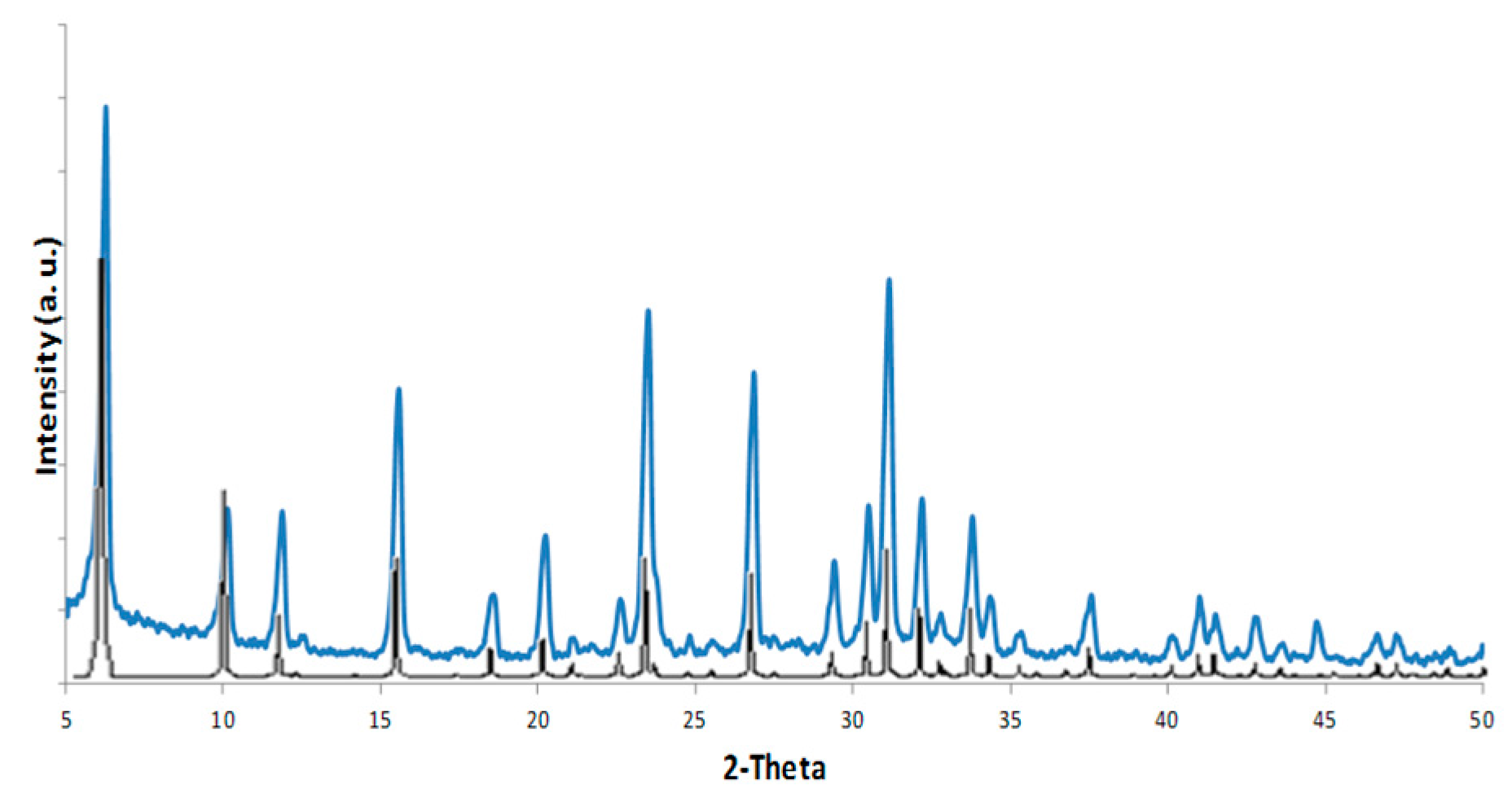
2.4. Pd-FAU Membrane: Synthesis, Characterization and Catalytic Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Nanozeolite-Based Membrane
3.3. Preparation of Pd-Loaded FAU Crystals and Membrane
3.4. Characterization
3.5. Activity Tests and Product Analysis
3.5.1. Procedure of Catalytic Tests Using Pd-FAU, Pd, and FAU Catalysts
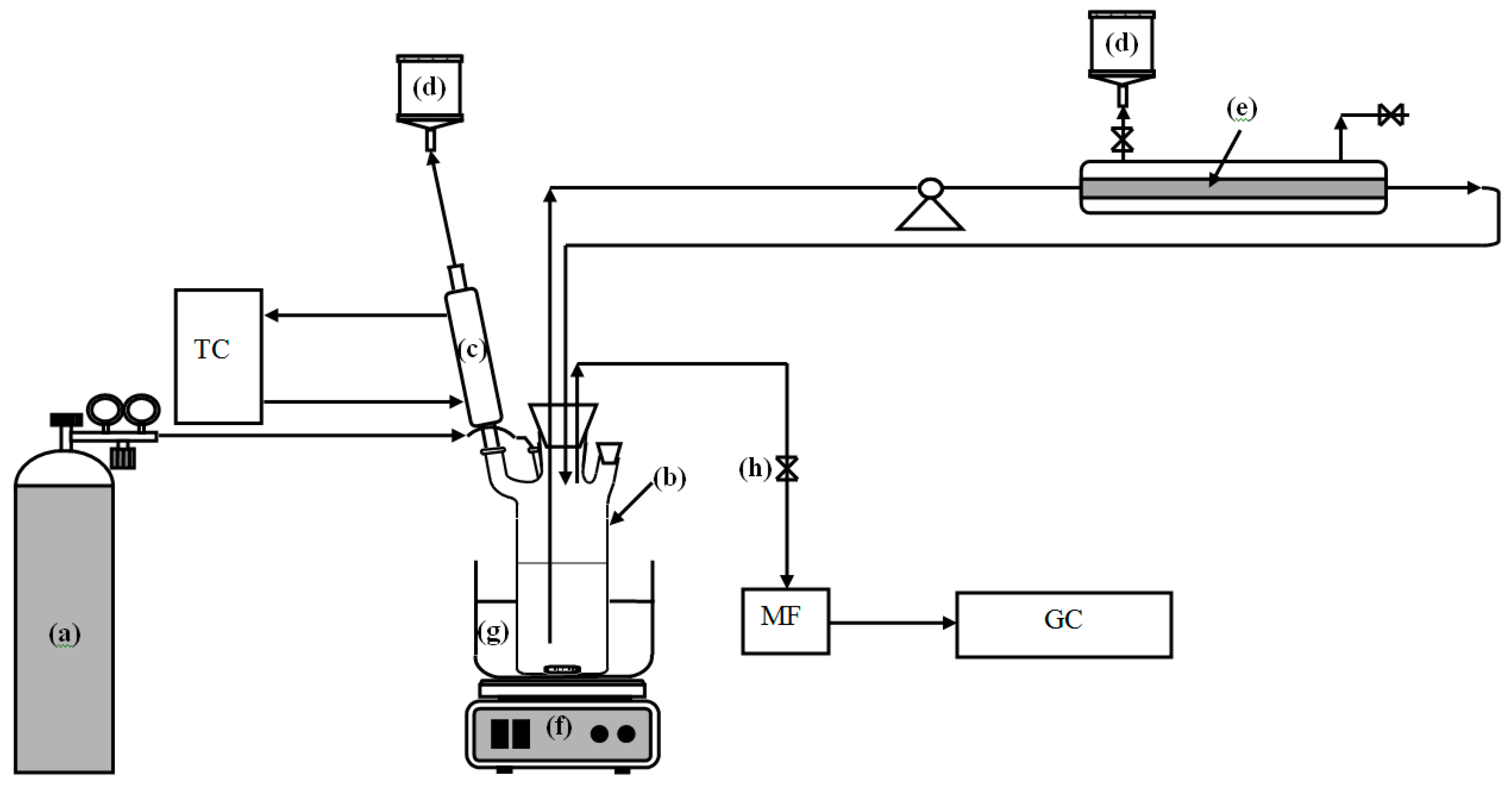
3.5.2. Procedure of Catalytic Tests Using Pd-FAU Membrane
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Magano, J.; Dunetz, J.R. Large-Scale Carbonyl Reductions in the Pharmaceutical Industry. Org. Process. Res. Dev. 2012, 16, 1156–1184. [Google Scholar] [CrossRef]
- Klabunovskii, E.; Smith, G.V.; Zsigmond, A. Heterogeneous Enantioselective Hydrogenation—Theory and Practice; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Smith, G.V.; Notheisz, F. Heterogeneous Catalysis in Organic Chemistry; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Nishimura, S. Heterogeneous Catalytic Hydrogenations for Organic Synthesis; John Wiley & Sons Inc.: New York, NY, USA, 2001. [Google Scholar]
- Clapham, S.E.; Hadzovic, A.; Morris, R.H. Mechanisms of the H2-Hydrogenation and Transfer Hydrogenation of Polar Bonds Catalyzed by Ruthenium Hydride Complexes. Coord. Chem. Rev. 2004, 248, 2201–2237. [Google Scholar] [CrossRef]
- Casey, C.P.; Guan, H. An Efficient and Chemoselective Iron Catalyst for the Hydrogenation of Ketones. J. Am. Chem. Soc. 2007, 129, 5816–5817. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Sun, X.; Zhang, X. Highly Enantioselective Hydrogenation of α-Keto Esters Catalyzed by Ru-Tunephos Complexes. Synlett 2006, 1169–1172. [Google Scholar] [CrossRef]
- Ager, D.J.; de Vries, A.H.M.; de Vries, J.G. Asymmetric homogeneous hydrogenations at scale. Chem. Soc. Rev. 2012, 41, 3340–3380. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Wan, B.; Wu, F.; Lu, S. First example of asymmetric transfer hydrogenation in water induced by a chiral amino alcohol hydrochloride. Tetrahedron Lett. 2005, 46, 7341–7344. [Google Scholar] [CrossRef]
- Cha, J.S. Recent Developments In Meerwein-Ponndorf-Verley and Related Reactions for the Reduction of Organic Functional Groups Using Aluminum, Boron, and Other Metal Reagents: A Review. Org. Process. Res. Dev. 2006, 10, 1032–1053. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Li, X.; Zanotti-Gerosa, A.; Hancock, F.; Vinci, D.; Ruan, J.; Xiao, J. On Water and in Air: Fast and Highly Chemoselective Transfer Hydrogenation of Aldehydes with Iridium Catalysts. Angew. Chem. Int. Ed. 2006, 45, 6717–6722. [Google Scholar] [CrossRef] [PubMed]
- Soltani, O.; Ariger, M.A.; Vázquez-Villa, H.; Carreira, E.M. Transfer Hydrogenation in Water: Enantioselective, Catalytic Reduction of α-Cyano and α-Nitro Substituted Acetophenones. Org. Lett. 2010, 12, 2893–2895. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.G.; Do, D.T.; Johnson, J.S. Asymmetric Synthesis of anti-β-Amino-α-Hydroxy Esters via Dynamic Kinetic Resolution of β-Amino-α-Keto Esters. Org. Lett. 2013, 15, 2446–2449. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, S.I.; Nakano, N.; Saitoh, T. Reduction of Carbonyl Compounds by Lanthanide Metal/2-Propanol: In-situ Generation of Samarium Isopropyloxide for Stereoselective Meerwein−Ponndorf−Verley Reduction. Eur. J. Org. Chem. 2004, 13, 2863–2867. [Google Scholar] [CrossRef]
- Alonso, F.; Riente, P.; Reinoso, F.R.; Martínez, J.R.; Escribano, A.S.; Yus, M. A Highly Reusable Carbon-Supported Platinum Catalyst for the Hydrogen-Transfer Reduction of Ketones. ChemCatChem 2009, 1, 75–77. [Google Scholar] [CrossRef]
- Yu, J.Q.; Wu, H.C.; Ramarao, C.; Spencer, J.B.; Ley, S.V. Transfer hydrogenation using recyclable polyurea-encapsulated palladium: Efficient and chemoselective reduction of aryl ketones. Chem. Commun. 2003, 678–679. [Google Scholar] [CrossRef]
- Shimura, K.; Shimizu, K.I. Transfer hydrogenation of ketones by ceria-supported Ni catalysts. Green Chem. 2012, 14, 2983–2985. [Google Scholar] [CrossRef]
- Matharu, D.S.; Morris, D.J.; Clarkson, G.J.; Wills, M. An outstanding catalyst for asymmetric transfer hydrogenation in aqueous solution and formic acid/triethylamine. Chem. Commun. 2006, 3232–3234. [Google Scholar] [CrossRef] [PubMed]
- Cortez, N.A.; Aguirre, G.; Parra-Hake, M.; Somanathan, R. Water-soluble chiral monosulfonamide-cyclohexane-1,2-diamine-RhCp∗ complex and its application in the asymmetric transfer hydrogenation (ATH) of ketones. Tetrahedron Lett. 2007, 48, 4335–4338. [Google Scholar] [CrossRef]
- Cortez, N.A.; Aguirre, G.; Parra-Hake, M.; Somanathan, R. Ruthenium(II) and rhodium(III) catalyzed asymmetric transfer hydrogenation (ATH) of acetophenone in isopropanol and in aqueous sodium formate using new chiral substituted aromatic monosulfonamide ligands derived from (1R,2R)-diaminocyclohexane. Tetrahedron Asymmetry 2008, 19, 1304–1309. [Google Scholar] [CrossRef]
- Fontananova, E.; Drioli, E.; Giorno, L. Catalytic Membranes and Membrane Reactors. In Comprehensive Membrane Science and Engineering; Drioli, E., Giorno, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 109–133. [Google Scholar]
- Molinari, R.; Argurio, P.; Poerio, T. Vanadium(III) and Vanadium(IV) catalysts in a membrane reactor for benzene hydroxylation to phenol and study of membrane material resistance. Appl. Catal. A-Gen. 2012, 437–438, 131–138. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, T.; Zhang, X.; Yeung, K.L. Continuous flow ZIF-8/NaA composite membrane microreactor for efficient Knoevenagel condensation. Catal. Commun. 2015, 68, 93–96. [Google Scholar] [CrossRef]
- Chen, R.Z.; Jiang, Y.G.; Xing, W.H.; Jin, W.Q. Fabrication and Catalytic Properties of Palladium Nanoparticles Deposited on a Silanized Asymmetric Ceramic Support. Ind. Eng. Chem. Res. 2011, 50, 4405–4411. [Google Scholar] [CrossRef]
- Molinari, R.; Caruso, A.; Argurio, P.; Poerio, T. Degradation of the drugs Gemfibrozil and Tamoxifen in pressurized and de-pressurized membrane photoreactors using suspended polycrystalline TiO2 as catalyst. J. Membr. Sci. 2008, 319, 54–63. [Google Scholar] [CrossRef]
- Molinari, R.; Argurio, P.; Lavorato, C. Review on Reduction and Partial Oxidation of Organics. In Photocatalytic (Membrane) Reactors. Curr. Org. Chem. 2013, 17, 2516–2537. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Photocatalytic reduction of acetophenone in membrane reactors under UV and visible light using TiO2 and Pd/TiO2 catalysts. Chem. Eng. J. 2015, 274, 307–316. [Google Scholar] [CrossRef]
- Molinari, R.; Argurio, P.; Poerio, T. Vanadyl acetylacetonate filled PVDF membranes as the core of a liquid phase continuous process for pure phenol production from benzene. J. Membr. Sci. 2015, 476, 490–499. [Google Scholar] [CrossRef]
- Van Donk, S.; Janssen, A.; Bitter, J.; de Jong, K. Generation, Characterization, and Impact of Mesopores in Zeolite Catalysts. Catal. Rev. 2003, 45, 297–319. [Google Scholar] [CrossRef]
- Fan, W.; Snyder, M.A.; Kumar, S.; Lee, P.S.; Yoo, W.C.; McComick, A.V.; Penn, P.L.; Stein, A.; Tsapatsis, M. Hierarchical nanofabrication of microporous crystals with ordered mesoporosity. Nat. Mater. 2008, 7, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Wang, L.; Yin, C.; Lin, K.; Di, Y.; Li, J.; Xu, R.; Su, D.; Schlogl, R.; Yokoi, T.; et al. Catalytic Properties of Hierarchical Mesoporous Zeolites Templated with a Mixture of Small Organic Ammonium Salts and Mesoscale Cationic Polymers. Angew. Chem. Int. Ed. 2006, 118, 3162–3165. [Google Scholar] [CrossRef]
- Yang, X.Y.; Tian, G.; Chen, L.H.; Li, Y.; Rooke, J.C.; Wei, Y.X.; Liu, Z.M.; Deng, Z.; van Tendeloo, G.; Su, B.L. Well-Organized Zeolite Nanocrystal Aggregates with Interconnected Hierarchically Micro–Meso–Macropore Systems Showing Enhanced Catalytic Performance. Chem. Eur. J. 2011, 17, 14987–14995. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; van Tendeloo, G.; Xiao, F.; Su, B.L. One-Pot Synthesis of Catalytically Stable and Active Nanoreactors: Encapsulation of Size-ControlledNanoparticles within a Hierarchically Macroporous Core@Ordered Mesoporous Shell System. Adv. Mater. 2009, 21, 1368–1372. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Vantomme, A.; Yu, J.; van Tendeloo, G.; Su, B. Chemistry of Trimethyl Aluminum: A Spontaneous Route to Thermally STable 3D Crystalline Macroporous Alumina Foams with a Hierarchy of Pore Sizes. Chem. Mater. 2010, 22, 3251–3258. [Google Scholar] [CrossRef]
- Mastropietro, T.F.; Molinari, R.; Argurio, P.; Curcio, E.; Drioli, E.; Poerio, T. Synthesis of Nay-Type Nanozeolites and Their Assembling into Microporous Membranes. Chem. Eng. Trans. 2015, 32, 715–720. [Google Scholar]
- Bertero, N.M.; Apesteguia, C.R.; Marchi, A.J. Catalytic and kinetic study of the liquid-phase hydrogenation of acetophenone over Cu/SiO2 catalyst. Appl. Catal. A Gen. 2008, 349, 100–109. [Google Scholar] [CrossRef]
- Surburg, H.; Panten, J. Common Fragrances and Flavour Materials: Preparation, Properties and Uses, 5th ed.; Wiley-VCH: Weinheim, Germany, 2006; p. 108. [Google Scholar]
- Casagrande, M.; Storaro, L.; Talon, A.; Lenarda, M.; Frattini, R.; Castellon, E.R.; Torres, P.M. Liquid phase acetophenone hydrogenation on Ru/Cr/B catalysts supported on silica. J. Mol. Catal. A Chem. 2002, 188, 133–139. [Google Scholar] [CrossRef]
- Rylander, P. Hydrogenation Methods; Academic Press Inc.: London, UK, 1985; p. 66. [Google Scholar]
- Gao, F.; Allian, A.D.; Zhang, H.; Cheng, S.; Garland, M. Chemical and kinetic study of acetophenone hydrogenation over Pt/Al2O3: Application of BTEM and other multivariate techniques to quantitative on-line FTIR measurements. J. Catal. 2006, 241, 189–199. [Google Scholar] [CrossRef]
- Lenarda, M.; Casagrande, M.; Moretti, E.; Storaro, L.; Frattini, R.; Polizzi, S. Selective catalytic low pressure hydrogenation of acetophenone on Pd/ZnO/ZnAl2O4. Catal. Lett. 2007, 114, 79–84. [Google Scholar] [CrossRef]
- Wang, Y.; Su, N.; Ye, L.; Ren, Y.; Chen, X.; Du, Y.; Li, Z.; Yue, B.; Tsang, S.C.E. Tuning enantioselectivity in asymmetric hydrogenation of acetophenone and its derivatives via confinement effect over free-standing mesoporous palladium network catalysts. J. Catal. 2014, 313, 113–126. [Google Scholar] [CrossRef]
- Facchetti, G.; Gandolfi, R.; Fusè, M.; Zerla, D.; Cesarotti, E.; Pellizzoni, M.; Rimoldi, I. Simple 1,3-diamines and their application as ligands in ruthenium(II) catalysts for asymmetric transfer hydrogenation of aryl ketones. New J. Chem. 2015, 39, 3792–3800. [Google Scholar] [CrossRef]
- Liu, W.P.; Yuan, M.L.; Yang, X.H.; Li, K.; Xie, J.H.; Zhou, Q.L. Efficient asymmetric transfer hydrogenation of ketones in ethanol with chiral iridium complexes of spiroPAP ligands as catalysts. Chem. Commun. 2015, 51, 6123–6125. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.I.; Nishiyama, H. Recent topics of transfer hydrogenation. Tetrahedron Lett. 2014, 55, 3133–3146. [Google Scholar] [CrossRef]
- Bertero, N.M.; Trasarti, A.F.; Apesteguía, C.R.; Marchi, A.J. Solvent effect in the liquid-phase hydrogenation of acetophenone over Ni/SiO2: A comprehensive study of the phenomenon. Appl. Catal. A Gen. 2011, 394, 228–238. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Martin, N.; Vlachos, D.G. Effect of hydrogen donor on liquid phase catalytic transfer hydrogenation of furfural over a Ru/RuO2/C catalyst. J. Mol. Catal. A Chem. 2014, 392, 223–228. [Google Scholar] [CrossRef]
- Wang, D.; Deraedt, C.; Ruiz, J.; Astruc, D. Sodium hydroxide-catalyzed transfer hydrogenation of carbonyl compounds and nitroarenes using ethanol or isopropanol as both solvent and hydrogen donor. J. Mol. Catal. A Chem. 2015, 400, 14–21. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Y.; Lu, Q.; Meng, X.; Xiaohua, W.; Guo, Y.; Wang, Y.; Liu, X.; Zhang, Z. Highly selective asymmetry transfer hydrogenation of prochiral acetophenone catalyzed by palladium-chitosan on silica. Catal. Lett. 2005, 100, 213–217. [Google Scholar] [CrossRef]
- Cejka, J.; Mintova, S. Perspectives of Micro/Mesoporous Composites in Catalysis. Catal. Rev. 2007, 49, 457–509. [Google Scholar] [CrossRef]
- Mastropietro, T.F.; Drioli, E.; Poerio, T. Low temperature synthesis of nanosized NaY zeolite crystals from organic-free gel by using supported seeds. RSC Adv. 2014, 4, 21951–21957. [Google Scholar] [CrossRef]
- Mastropietro, T.F.; Brunetti, A.; Zito, P.; Poerio, T.; Richter, H.; Weyd, M.; Wöhner, S.; Drioli, E.; Barbieri, G. Study of the Separation Properties of FAU Membranes Constituted by Hierarchically Assembled Nanozeolites. Sep. Purif. Technol. 2015, 156, 321–327. [Google Scholar] [CrossRef]
- Long, J.; Zhou, Y.; Li, Y.W. Transfer hydrogenation of unsaturated bonds in the absence of base additives catalyzed by a cobalt-based heterogeneous catalyst. Chem. Commun. 2015, 51, 2331–2334. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi Kantam, M.; Rao, B.P.C.; Choudary, B.M.; Sreedhara, B. Selective Transfer Hydrogenation of Carbonyl Compounds by Ruthenium Nanoclusters Supported on Alkali-Exchanged Zeolite Beta. Adv. Synth. Catal. 2006, 348, 1970–1976. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Y.; Jin, F.M.; Zhou, X.F.; Kishita, A.; Tohji, K. Hydrogen-Transfer Reduction of Ketones into Corresponding Alcohols Using Formic Acid as a Hydrogen Donor without a Metal Catalyst in High-Temperature Water. Ind. Eng. Chem. Res. 2010, 49, 6255–6259. [Google Scholar] [CrossRef]
- Nemeth, J.; Kiss, A.; Hell, Z. Palladium-catalysed transfer hydrogenation of aromatic nitro compounds—An unusual chain elongation. Tetrahedron Lett. 2003, 54, 6094–6096. [Google Scholar] [CrossRef]
- Sample Availability: Not available.

| Microcrystalline FAU | Pd-FAU(0.5-15) | Pd-FAU(0.5-60) | Pd-FAU(1-60) | |
|---|---|---|---|---|
| BET Surface Area (m2/g) | 616 | 751 | 727 | 267 |
| t-plot External Surface Area (m2/g) | 51 | 73 | 67 | 127 |
| t-plot Micropore Surface Area (m2/g) | 566 | 678 | 660 | 140 |
| t-plot micropore volume (cm3/g) | 0.23 | 0.27 | 0.26 | 0.06 |
| Adsorption average pore width (4V/A by BET) (nm) | 1.7 | 1.8 | 1.9 | 2.5 |
| Catalyst | Conversion % | Selectivity % | Yield % |
|---|---|---|---|
| Pd-FAU(0.5-15) | 13.08 | 0.52 | 0.07 |
| Pd-FAU(0.5-60) | 15.27 | 2.60 | 0.40 |
| Pd-FAU(1-60) | 10.36 | 1.25 | 0.13 |
| Hydrogen Donor | Solvent | Conversion % | Selectivity % | Yield % |
|---|---|---|---|---|
| HCOONa 2M | Water | 75.43 | 3.57 | 2.69 |
| 2-propanol | 2-propanol | 15.27 | 2.60 | 0.40 |
| Ethanol | Ethanol | 32.05 | 0.35 | 0.11 |
| Catalyst | Catalyst Amount | Conversion % | Selectivity % | Yield % | Productivity based on Pd content (mgPE gPd−1·h−1) |
|---|---|---|---|---|---|
| Pd-FAU(0.5-60) | 0.125 g Pd content 1.5% (1.88 mg) | 30.37 | 13.56 | 4.12 | 144.6 |
| Pd | 3.75 mg | 40.40 | 8.00 | 3.23 | 56.8 |
| FAU | 0.125 g | 51.41 | 0.26 | 0.13 | / |
| Catalyst (*) | Productivity (mg gcat−1·h−1) | Productivity Based on Pd Content (mgPE gPd−1·h−1) |
|---|---|---|
| 0.125 g of Pd-FAU(0.5-60) | 2.2 | 144.6 |
| 0.0125 g of Pd-FAU on membrane | 11.0 | 731.3 |
| Pd-FAU(0.5-60) | Pd-FAU Membrane | |
|---|---|---|
| BET Surface Area (m2/g) | 727 | 473 |
| t-plot External Surface Area (m2/g) | 67 | 229 |
| t-plot Micropore Surface Area (m2/g) | 660 | 243 |
| BJH Adsorption cumulative surface area of mesopores (m2/g) | - | 219 |
| t-plot micropore volume (cm3/g) | 0.26 | 0.19 |
| BJH Adsorption cumulative volume of mesopores (cm3/g) | - | 0.43 |
| Adsorption average pore width (4 V/A by BET) (nm) | 1.9 | 4.8 |
| BJH Adsorption average pore diameter (4 V/A) (nm) | - | 7.9 |
| Pd-FAU Supported Membrane | Yield % | Conversion % | Selectivity % | Productivity (mgPE gcat−1·h−1) |
|---|---|---|---|---|
| Run 1 | 0.35 | 62.06 | 0.56 | 11.0 |
| Run 2 | 0.35 | 30.99 | 1.11 | 10.8 |
| Run 3 | 0.27 | 35.54 | 0.77 | 8.6 |
| Pd-FAU Membrane | Yield (%) | Conversion (%) | Selectivity (%) | Productivity (mgPE gcat−1·h−1) |
|---|---|---|---|---|
| Batch reactor | 0.35 | 62.06 | 0.56 | 11.0 |
| Tangential-flow reactor | 0.69 | 93.90 | 0.75 | 22.0 |
| Catalyst | Catalyst Amount [g] | AP [M] | HD a [M] | T [°C] | C [%] | S [%] | Y [%] | RT b [h] |
|---|---|---|---|---|---|---|---|---|
| Pd-FAU membrane | 0.0125 Pd content 1.4% (1.75 mg) | 0.026 | HCOONa [1] | 60 | 93.90 | 0.75 | 0.96 | 24 |
| Pd–CS–SiO2 [53] | 0.1 Pd content 3.2%(3.2 mg) | 3 × 10−3 | HCOONH4, [8 × 10−3] (in ethanol) | 80 | - | - | 1.2 | 24 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinari, R.; Lavorato, C.; Mastropietro, T.F.; Argurio, P.; Drioli, E.; Poerio, T. Preparation of Pd-Loaded Hierarchical FAU Membranes and Testing in Acetophenone Hydrogenation. Molecules 2016, 21, 394. https://doi.org/10.3390/molecules21030394
Molinari R, Lavorato C, Mastropietro TF, Argurio P, Drioli E, Poerio T. Preparation of Pd-Loaded Hierarchical FAU Membranes and Testing in Acetophenone Hydrogenation. Molecules. 2016; 21(3):394. https://doi.org/10.3390/molecules21030394
Chicago/Turabian StyleMolinari, Raffaele, Cristina Lavorato, Teresa F. Mastropietro, Pietro Argurio, Enrico Drioli, and Teresa Poerio. 2016. "Preparation of Pd-Loaded Hierarchical FAU Membranes and Testing in Acetophenone Hydrogenation" Molecules 21, no. 3: 394. https://doi.org/10.3390/molecules21030394
APA StyleMolinari, R., Lavorato, C., Mastropietro, T. F., Argurio, P., Drioli, E., & Poerio, T. (2016). Preparation of Pd-Loaded Hierarchical FAU Membranes and Testing in Acetophenone Hydrogenation. Molecules, 21(3), 394. https://doi.org/10.3390/molecules21030394