Coenzyme Q and Its Role in the Dietary Therapy against Aging
Abstract
:1. Coenzyme Q: Structure, Localization and Forms
2. Physiological Roles of CoQ
2.1. Mitochondrial CoQ Is an Essential Factor for Cell Bioenergetics
2.2. CoQ Acts as Lipid-Soluble Antioxidant in All Biological Membranes
2.3. CoQ Collaborates in Redox State Regulation by Plasma Membrane Redox System Activity
2.4. CoQ Participates in Cell Signaling by Plasma Membrane Redox System
- Reactive oxygen species (ROS): As previously stated, NAD(P)H dehydrogenases found in plasma membrane, cytochrome b5-reductase and other NAD(P)H dehydrogenases found in plasma membrane, reduce CoQ in a one-electron mechanism yielding semiquinone forms [58]. These CoQ forms have pro-oxidant activity of generating O2·− or H2O2 that would act as second messengers on cell signaling mechanisms. Consequently, these would modulate different cell responses affecting cell growth and differentiation processes [59].
- Voltage-dependent anion channel (VDAC) proteins: As in mitochondria, plasma membrane also contains proteins belonging to the VDAC protein family. One of the components of this family, VDAC1, can function as NADH-ferricyanide reductase, an activity associated to the plasma membrane redox system [61]. Taken into consideration that CoQ is involved in the regulation of VDAC/permeability transition pore in mitochondria [62], López-Lluch et al. [6] have suggested a putative relationship of the activity of CoQ in plasma membrane and cell signaling linked to VDAC1 to be considered in future research.
- NAD+-dependent deacetylases: This group of proteins implicated in genetic expression, such as sirtuins, could be affected and regulated in some manner by the activity of NADH-dependent reductase in plasma membrane [63]. It has been proposed that the variations in activity of CoQ-dependent NADH oxidoreductases in the different biological membranes could also regulate sirtuins because of the effects on redox state [6].
- Mg2+-dependent neutral sphingomyelinase: It has been reported that CoQ-dependent plasma membrane redox system is involved in inhibition of Mg2+-dependent neutral sphingomyelinase after oxidative stress [64,65,66]. This is an integral plasma membrane protein involved in the release of ceramide from plasma membrane sphingomyelin and participates in cell signaling, apoptosis, and the modulation of cell responses [6,67].
2.5. CoQ Exerts Anti-Inflammatory Effects through Its Antioxidant Activity
2.6. Mitochondrial CoQ Prevents Events Leading to Programmed Cell Death
- permeability transition pores inhibitors, like CoQ0 ,CoQ2, and decylubiquinone.
- permeability transition pores inducers, like idebenone 2,3-dimethoxy-5-methyl-6-(10-hydroxydecyl)-1,4-benzoquinone).
- permeability transition pores-inactive quinones, which counteract the effects of both inhibitors and inducers, such as CoQ1.
2.7. CoQ Present in Lysosomal Membrane Participates in pH Maintenance
3. Endogenous and Exogenous Sources of CoQ
3.1. CoQ Biosynthesis
3.2. Dietary CoQ Has Shown to Increase CoQ Levels in Different Body Compartments
4. Evidence for CoQ as Anti-Aging Compound
4.1. CoQ Levels Are Affected by Aging
4.2. Several Age-Related Pathologies Are Associated with Low Levels of CoQ
4.3. Aging, Development and Lifespan Are Associated to Changes in CoQ Bionsynthesis
5. Studies on Dietary Therapies with CoQ on Aging
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CoQ | Coenzyme Q |
| CoQH | partially reduced coenzyme Q |
| CoQH2 | fully reduced coenzyme Q |
| Cytb5R | NADH: cytochrome b5 reductase |
| FAD+ | reduced flavine adenine dinucleotide |
| FADH2 | reduced flavine adenine dinucleotide |
| farnesyl-PP | farnesyl-pyrophosphate(isopentenyl-PP) |
| HMG-CoA | 3-hydroxy-3-metylglutaryl-CoA |
| LDL | low-density lipoprotein |
| LOO | lipid hydroperoxyl radical |
| LOOH | lipid hydroperoxyde |
| LPS | lipopolysaccharide |
| MAPKs | mitogen-activated protein kinases |
| mPTP | mitochondrial permeability transition pore |
| mtDNA | mitochondrial DNA |
| mtETC | mitochondrial electron transport chain |
| MUFA | monounsarturated fatty acid |
| NQO1 | NAD(P)H-quinone oxidoreductase 1 |
| pHB | para-hydroxybenzoate |
| PUFA | polyunsarturated fatty acids |
| ROS | Reactive oxygen species |
| SFA | saturated fatty acids |
| SIRT1 | sirtuin1 |
| VDAC | Voltage-dependent anion channelNuclear factor κB (NF-κB) |
| VLDL | very low-density lipoprotein |
| α-tp | α-tocopherol |
| α-tp*− | α-tocopheryl anion radical |
References
- Cluis, C.P.; Burja, A.M.; Martin, V.J. Current prospects for the production of coenzyme Q10 in microbes. J. Trends Biotechnol. 2007, 25, 514–521. [Google Scholar]
- Prakash, S.; Sunitha, J.; Hans, M. Role of coenzyme Q10 as an antioxidant and bioenergizer in periodontal diseases. Indian J. Pharmacol. 2010, 42, 334. [Google Scholar] [CrossRef] [PubMed]
- Varela-López, A.; Bullón, P.; Giampieri, F.; Quiles, J.L. Non-Nutrient, Naturally Occurring Phenolic Compounds with Antioxidant Activity for the Prevention and Treatment of Periodontal Diseases. Antioxidants 2015, 4, 447–481. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Ferri, E.; Gorini, A.; Villa, R.F.; Rodriguez Huertas, J.F.; Fiorella, P.; Genova, M.L.; Lenaz, G.; Marchetti, M. Natural distribution and occurrence of coenzyme Q homologues. Membr. Biochem. 1990, 9, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Lenaz, G.; Fato, R.; Di Bernardo, S.; Jarreta, D.; Costa, A.; Genova, M.L.; Parenti Castelli, G. Localization and mobility of coenzyme Q in lipid bilayers and membranes. Biofactors 1999, 9, 87–93. [Google Scholar] [CrossRef] [PubMed]
- López-Lluch, G.; Rodríguez-Aguilera, J.C.; Santos-Ocaña, C.; Navas, P. Is coenzyme Q a key factor in aging? Mech. Ageing Dev. 2010, 131, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Samorì, B.; Lenaz, G.; Battino, M.; Marconi, G.; Domini, I. On coenzyme Q orientation in membranes: A linear dichroism study of ubiquinones in a model bilayer. J. Membr. Biol. 1992, 128, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Fato, R.; Battino, M.; Castelli, G.P.; Lenaz, G. Measurement of the lateral diffusion coefficients of ubiquinones in lipid vesicles by fluorescence quenching of 12-(9-anthroyl) stearate. FEBS Lett. 1985, 179, 238–242. [Google Scholar] [CrossRef]
- Lenaz, G.; Samori, B.; Fato, R.; Battino, M.; Castelli, G.P.; Domini, I. Localization and preferred orientations of ubiquinone homologs in model bilayers. Biochem. Cell Biol. 1992, 70, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Crane, F.L. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Turunen, M.; Olsson, J.; Dallner, G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta 2004, 1660, 171–199. [Google Scholar] [CrossRef] [PubMed]
- Gaby, A.R. The role of coenzyme Q10 in clinical medicine: Part I. Altern. Med. Rev. 1996, 1, 11–17. [Google Scholar]
- Littarru, G.P.; Tiano, L. Bioenergetic and antioxidant properties of coenzyme Q10: Recent developments. Mol. Biotechnol. 2007, 37, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Bhagavan, H.N.; Chopra, R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic. Res. 2006, 40, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Matsura, T.; Yamada, K.; Kawasaki, T. Difference in antioxidant activity between reduced coenzyme Q9 and reduced coenzyme Q10 in the cell: Studies with isolated rat and guinea pig hepatocytes treated with a water-soluble radical initiator. Biochim. Biophys. Acta 1992, 1123, 30–315. [Google Scholar] [CrossRef]
- James, A.M.; Smith, R.A.J.; Murphy, M.P. Antioxidant and prooxidant properties of mitochondrial Coenzyme Q. Arch. Biochem. Biophys. 2004, 423, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Fato, R.; Battino, M.; Degli Esposti, M.; Parenti Castelli, G.; Lenaz, G. Determination of partition and lateral diffusion coefficients of ubiquinones by fluorescence quenching of n-(9-anthroyloxy) stearic acids in phospholipid vesicles and mitochondrial membranes. Biochemistry 1986, 25, 3378–3390. [Google Scholar] [CrossRef] [PubMed]
- Genova, M.L.; Lenaz, G. New developments on the functions of coenzyme Q in mitochondria. Biofactors 2011, 37, 330–354. [Google Scholar] [CrossRef] [PubMed]
- Bentinger, M.; Tekle, M.; Dallner, G. Coenzyme Q–biosynthesis and functions. Biochem. Biophys. Res. Commun. 2010, 396, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Lass, A.; Forster, M.J.; Sohal, R.S. Effects of coenzyme Q10 and alpha-tocopherol administration on their tissue levels in the mouse: Elevation of mitochondrial alpha-tocopherol by coenzyme Q10. Free Radic. Biol. Med. 1999, 26, 1375–1382. [Google Scholar] [CrossRef]
- Lass, A.; Sohal, R.S. Effects of coenzyme Q10 and alphatocopherol administration on their tissue levels in the mouse: Elevation of mitochondrial alpha-tocopherol by coenzyme Q10. FASEB J. 2000, 14, 87–94. [Google Scholar] [PubMed]
- Dallner, G.; Sindelar, P.J. Regulation of ubiquinone metabolism. Free Radic. Biol. Med. 2000, 29, 285–294. [Google Scholar] [CrossRef]
- Sohal, R.S.; Forster, M.J. Coenzyme Q, oxidative stress and aging. Mitochondrion 2007, 7, S103–S111. [Google Scholar] [CrossRef] [PubMed]
- Ernster, L.; Dallner, G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta 1995, 1271, 195–204. [Google Scholar] [CrossRef]
- Mitchell, P. Protonmotive redox mechanism of the cytochrome b-c1 complex in the respiratory chain: Protonmotive ubiquinone cycle. FEBS Lett. 1975, 56, 1–6. [Google Scholar] [CrossRef]
- Bentinger, M.; Brismar, K.; Dallner, G. The antioxidant role of coenzyme Q. Mitochondrion 2007, 7, S41–S50. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Fato, R.; Parenti-Castelli, G.; Lenaz, G. Coenzyme Q can control the efficiency of oxidative phosphorylation. Int. J. Tissue React. 1989, 12, 137–144. [Google Scholar]
- Rauchová, H.; Battino, M.; Fato, R.; Lenaz, G.; Drahota, Z. Coenzyme Q-pool function in glycerol-3-phosphate oxidation in hamster brown adipose tissue mitochondria. J. Bioenerg. Biomembr. 1992, 24, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef] [PubMed]
- Santos-Ocaña, C.; Do, T.Q.; Padilla, S.; Navas, P.; Clarke, C.F.J. Uptake of exogenous coenzyme Q and transport to mitochondria is required for bc1 complex stability in yeast coq mutants. Biol. Chem. 2002, 277, 10973–10981. [Google Scholar] [CrossRef] [PubMed]
- Larm, J.A.; Vaillant, F.; Linnane, A.W.; Lawen, A.J. Up-regulation of the plasma membrane oxidoreductase as a prerequisite for the viability of human Namalwa rho 0 cells. Biol. Chem. 1994, 269, 30097–30100. [Google Scholar]
- Ernster, L. Lipid peroxidation in biological membranes: Mechanisms and implications. In Active Oxygens, Lipid Peroxides, and Antioxidants; Yagi, K., Ed.; CRC Press: Boca Raton, FA, USA, 1993; pp. 1–38. [Google Scholar]
- Crane, F.L.; Navas, P. The diversity of coenzyme Q function. Mol. Aspects Med. 1997, 18, S1–S6. [Google Scholar] [CrossRef]
- Gómez-Díaz, C.; Rodríguez-Aguilera, J.C.; Barroso, M.P.; Villalba, J.M.; Navarro, F.; Crane, F.L.; Navas, P. Antioxidant ascorbate is stabilized by NADH-coenzyme Q10 reductase in the plasma membrane. J. Bioenerg. Biomembr. 1997, 29, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Santos-Ocaña, C.; Villalba, J.M.; Córdoba, F.; Padilla, S.; Crane, F.L.; Clarke, C.F.; Navas, P.J. Genetic evidence for coenzyme Q requirement in plasma membrane electron transport. Bioenerg. Biomembr. 1998, 30, 465–475. [Google Scholar] [CrossRef]
- Bello, R.I.; Kagan, V.E.; Tyurin, V.; Navarro, F.; Alcaín, F.J.; Villalba, J.M. Regeneration of lipophil antioxidants by NAD(P)H:quinone oxidoreductase 1. Protoplasma 2003, 221, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.; Serbinova, E.; Packer, L. Antioxidant effects of ubiquinones in microsomes and mitochondria are mediated by tocopherol recycling. Biochem. Biophys. Res. Commun. 1990, 169, 851–857. [Google Scholar] [CrossRef]
- Bindoli, A.; Valente, M.; Cavallini, L. Inhibition of lipid peroxidation by alpha tocopherolquinone and alpha tocopherolhydroquinone. Biochem. Int. 1985, 10, 753–761. [Google Scholar] [PubMed]
- Frei, B.; Kim, M.C.; Ames, B.N. Ubiquinol-10 is an effective lipid-soluble antioxidant at physiological concentrations. Proc. Natl. Acad. Sci. USA 1990, 87, 4879–4883. [Google Scholar] [CrossRef] [PubMed]
- Navarro, F.; Navas, P.; Burgess, J.R.; Bello, R.I.; de Cabo, R.; Arroyo, A.; Villalba, J.M. Vitamin E and selenium deficiency induces expression of the ubiquinone-dependent antioxidant system at the plasma membrane. FASEB J. 1998, 12, 1665–1673. [Google Scholar] [PubMed]
- Arroyo, A.; Navarro, F.; Navas, P.; Villalba, J.M. Ubiquinol regeneration by plasma membrane ubiquinone reductase. Protoplasma 1998, 205, 107–113. [Google Scholar] [CrossRef]
- Beyer, R.E.; Segura-Aguilar, J.; di Bernardo, S.; Cavazzoni, M.; Fato, R.; Fiorentini, D.; Galli, M.C.; Setti, M.; Landi, L.; Lenaz, G. The role of DT-diaphorase in the maintenance of the reduced antioxidant form of coenzyme Q in membrane systems. Proc. Natl. Acad. Sci. USA 1996, 93, 2528–2532. [Google Scholar] [CrossRef] [PubMed]
- Rushmore, T.H.; Morton, M.R.; Pickett, C.B.J. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. Biol. Chem. 1991, 266, 11632–11639. [Google Scholar]
- Takahashi, T.; Yamaguchi, T.; Shitashige, M.; Okamoto, T.; Kishi, T. Reduction of ubiquinone in membrane lipids by rat liver cytosol and its involvement in the cellular defence system against lipid peroxidation. Biochem. J. 1995, 309, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Takahashi, T.; Usui, A.; Hashizume, N.; Okamoto, T. Cytosolic NADPH-UQ reductase, the enzyme responsible for cellular ubiquinone redox cycle as an endogenous antioxidant in the rat liver. Biofactors 1999, 9, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Hohda, T.; Sugimoto, N.; Mizobuchi, S.; Okamoto, T.; Mori, K.; Kishi, T. Antioxidant roles of cellular ubiquinone and related redox cycles: Potentiated resistance of rat hepatocytes having stimulated NADPH-dependent ubiquinone reductase against hydrogen peroxide toxicity. Biol. Pharm. Bull. 1999, 22, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Okuno, M.; Okamoto, T.; Kishi, T. NADPH-dependent coenzyme Q reductase is the main enzyme responsible for the reduction of non-mitochondrial CoQ in cells. Biofactors 2008, 32, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Bowry, V.W.; Frei, B. Ubiquinol-10 protects human low density lipoprotein more efficient against lipid peroxidation than does alpha-tocopherol. Proc. Natl. Acad. Sci. USA 1991, 88, 1646–1650. [Google Scholar] [CrossRef] [PubMed]
- De Grey, A.D. A proposed mechanism for the lowering of mitochondrial electron leak by caloric restriction. Mitochondrion 2001, 1, 129–139. [Google Scholar] [CrossRef]
- Gómez-Díaz, C.; Villalba, J.M.; Pérez-Vicente, R.; Crane, F.L.; Navas, P. Ascorbate stabilization is stimulated in rho(0)HL-60 cells by CoQ10 increase at the plasma membrane. Biochem. Biophys. Res. Commun. 1997, 234, 79–81. [Google Scholar] [CrossRef]
- Hyun, D.-H.; Emerson, S.S.; Jo, D.-G.; Mattson, M.P.; de Cabo, R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc. Natl. Acad. Sci. USA 2006, 103, 19908–19912. [Google Scholar] [CrossRef] [PubMed]
- Hyun, D.-H.; Hunt, N.D.; Emerson, S.S.; Hernandez, J.O.; Mattson, M.P.; de Cabo, R. Up-regulation of plasma membrane-associated redox activities in neuronal cells lacking functional mitochondria. J. Neurochem. 2007, 100, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Quesada, J.M.; Lopez-LLuch, G.; Buron, M.I.; Alcain, F.J.; Borrego, F.; Velde, J.P.; Blanco, I.; Bouillon, R.; Navas, P. Ascorbate increases the 1,25 dihydroxyvitamin D3-induced monocytic differentiation of HL-60 cells. Calcif. Tissue Int. 1996, 59, 277–282. [Google Scholar] [CrossRef] [PubMed]
- López-Lluch, G.; Burón, M.I.; Alcaín, F.J.; Quesada, J.M.; Navas, P. Redox regulation of cAMP levels by ascorbate in 1,25-dihydroxy-vitamin D3-induced differentiation of HL-60 cells. Biochem. J. 1998, 331, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Sun, I.L.; Sun, E.E.; Crane, F.L. Stimulation of serum-free cell proliferation by coenzyme Q. Biochem. Biophys. Res. Commun. 1992, 189, 8–13. [Google Scholar] [CrossRef]
- Brightman, A.O.; Wang, J.; Miu, R.K.; Sun, I.L.; Barr, R.; Crane, F.L.; Morré, D.J. A growth factor- and hormone-stimulated NADH oxidase from rat liver plasma membrane. Biochim. Biophys. Acta 1992, 1105, 109–117. [Google Scholar] [CrossRef]
- López-Lluch, G.; Blázquez, M.V.; Pérez-Vicente, R.; Macho, A.; Burón, M.I.; Alcaín, F.J.; Muñoz, E.; Navas, P. Cellular redox state and activating protein-1 are involved in ascorbate effect on calcitriol-induced differentiation. Protoplasma 2001, 217, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Villalba, J.M.; Navarro, F.; Córdoba, F.; Serrano, A.; Arroyo, A.; Crane, F.L.; Navas, P. Coenzyme Q reductase from liver plasma membrane: Purification and role in trans-plasma-membrane electron transport. Proc. Natl. Acad. Sci. USA 1995, 92, 4887–4891. [Google Scholar] [CrossRef] [PubMed]
- Linnane, A.W.; Kios, M.; Vitetta, L. Coenzyme Q(10)–its role as a prooxidant in the formation of superoxide anion/hydrogen peroxide and the regulation of the metabolome. Mitochondrion 2007, 7, S51–S61. [Google Scholar] [CrossRef] [PubMed]
- Crane, F.L.; Sun, I.L.; Crowe, R.A.; Alcain, F.J.; Löw, H. Coenzyme Q10, plasma membrane oxidase and growth control. Mol. Aspects Med. 1994, 15, S1–S11. [Google Scholar] [CrossRef]
- Baker, M.A.; Lane, D.J.R.; Ly, J.D.; De Pinto, V.; Lawen, A.J. VDAC1 is a transplasma membrane NADH-ferricyanide reductase. Biol. Chem. 2004, 279, 4811–4819. [Google Scholar] [CrossRef] [PubMed]
- Papucci, L.; Schiavone, N.; Witort, E.; Donnini, M.; Lapucci, A.; Tempestini, A.; Formigli, L.; Zecchi-Orlandini, S.; Orlandini, G.; Carella, G.; et al. Coenzyme Q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J. Biol. Chem. 2003, 278, 28220–28228. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Brachmann, C.B.; Celic, I.; Kenna, M.A.; Muhammad, S.; Starai, V.J.; Avalos, J.L.; Escalante-Semerena, J.C.; Grubmeyer, C.; Wolberger, C.; et al. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc. Natl. Acad. Sci. USA 2000, 97, 6658–6663. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ayala, D.J.; Martín, S.F.; Barroso, M.P.; Gómez-Díaz, C.; Villalba, J.M.; Rodríguez-Aguilera, J.C.; López-Lluch, G.; Navas, P. Coenzyme Q protects cells against serum withdrawal-induced apoptosis by inhibition of ceramide release and caspase-3 activation. Antioxid. Redox. Signal. 2000, 2, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Martín, S.F.; Gómez-Díaz, C.; Bello, R.I.; Navas, P.; Villalba, J.M. Inhibition of neutral Mg2+-dependent sphingomyelinase by ubiquinol-mediated plasma membrane electron transport. Protoplasma 2003, 221, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Villalba, J.M.; Navas, P. Plasma membrane redox system in the control of stress-induced apoptosis. Antioxid. Redox. Signal. 2000, 2, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Han, H.; Rollins, S.; Cleveland, T.J. Molecular cloning, characterization, and expression of a novel human neutral sphingomyelinase. Biol. Chem. 1999, 274, 37407–37412. [Google Scholar] [CrossRef]
- Schmelzer, C.; Lindner, I.; Vock, C.; Fujii, K.; Döring, F. Functional connections and pathways of coenzyme Q10-inducible genes: An in-silico study. IUBMB Life 2007, 59, 628–633. [Google Scholar] [PubMed]
- Kaltschmidt, B.; Sparna, T.; Kaltschmidt, C. Activation of NF-kappa B by reactive oxygen intermediates in the nervous system. Antioxid. Redox. Signal. 1999, 1, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, C.; Döring, F. Identification of LPS-inducible genes downregulated by ubiquinone in human THP-1 monocytes. Biofactors 2010, 36, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Naderi, J.; Somayajulu-Nitu, M.; Mukerji, A.; Sharda, P.; Sikorska, M.; Borowy-Borowski, H.; Antonsson, B.; Pandey, S. Water-soluble formulation of Coenzyme Q10 inhibits Bax-induced destabilization of mitochondria in mammalian cells. Apoptosis 2006, 11, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, T.; Otani, H.; Nakao, Y.; Hattori, R.; Osako, M.; Imamura, H.; Das, D.K. Dual involvement of coenzyme Q10 in redox signaling and inhibition of death signaling in the rat heart mitochondria. Antioxid. Redox. Signal. 2001, 3, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P.; Forte, M. The mitochondrial permeability transition pore. Novartis Found Symp. 2007, 287, 157–164; discussion 164–169. [Google Scholar] [PubMed]
- Devun, F.; Walter, L.; Belliere, J.; Cottet-Rousselle, C.; Leverve, X.; Fontaine, E. Ubiquinone analogs: A mitochondrial permeability transition poredependent pathway to selective cell death. PLoS ONE 2010, 5, e11792. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, E.; Ichas, F.; Bernardi, P.J. A ubiquinone-binding site regulates the mitochondrial permeability transition pore. Biol. Chem. 1998, 273, 25734–25740. [Google Scholar] [CrossRef]
- Walter, L.; Nogueira, V.; Leverve, X.; Heitz, M.P.; Bernardi, P.; Fontaine, E. Three classes of ubiquinone analogs regulate the mitochondrial permeability transition pore through a common site. J. Biol. Chem. 2000, 275, 29521–29527. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.M.; Seiça, R.; Oliveira, P.J.; Coxito, P.M.; Moreno, A.J.; Palmeira, C.M.; Santos, M.S. Diabetes induces metabolic adaptations in rat liver mitochondria: Role of coenzyme Q and cardiolipin contents. Biochim. Biophys. Acta 2003, 1639, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zou, L.-Y.; Cao, C.-M.; Yang, E.S. Coenzyme Q10 protects SHSY5Y neuronal cells from beta amyloid toxicity and oxygen-glucose deprivation by inhibiting the opening of the mitochondrial permeability transition pore. Biofactors 2005, 25, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Moreno-Fernández, A.M.; Gomez-Skarmeta, J.L.; de Miguel, M.; Garrido-Maraver, J.; Oropesa-Avila, M.; Rodríguez-Hernández, A.; Navas, P.; Sánchez-Alcázar, J.A. Coenzyme Q10 and alpha-tocopherol protect against amitriptyline toxicity. Toxicol. Appl. Pharmacol. 2009, 235, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Gille, L.; Nohl, H. The existence of a lysosomal redox chain and the role of ubiquinone. Biochem. Biophys. 2000, 375, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Crane, F.L. The evolution of coenzyme Q. Biofactors 2008, 32, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Grünler, J.; Ericsson, J.; Dallner, G. Branch-point reactions in the biosynthesis of cholesterol, dolichol, ubiquinone and prenylated proteins. Biochim. Biophys. Acta 1994, 1212, 259–277. [Google Scholar] [CrossRef]
- Villalba, J.M.; Parrado, C.; Santos-Gonzalez, M.; Alcain, F.J. Therapeutic use of coenzyme Q10 and coenzyme Q10-related compounds and formulations. Expert Opin. Investig. Drugs 2010, 19, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Tran, U.C.; Clarke, C.F. Endogenous synthesis of coenzyme Q in eukaryotes. Mitochondrion 2007, 7, S62–S71. [Google Scholar] [CrossRef] [PubMed]
- Forsgren, M.; Attersand, A.; Lake, S.; Grunler, J.; Swiezewska, E.; Dallner, G.; Climent, I. Isolation and functional expression of human COQ2, a gene encoding a polyprenyl transferase involved in the synthesis of CoQ. Biochem. J. 2004, 382, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Quinzii, C.M.; DiMauro, S.; Hirano, M. Human coenzyme Q10 deficiency. Neurochem. Res. 2007, 32, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ayala, D.J.M.; Brea-Calvo, G.; López-Lluch, G.; Navas, P. Coenzyme Q distribution in HL-60 human cells depends on the endomembrane system. Biochim. Biophys. Acta 2005, 1713, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Aberg, F.; Appelkvist, E.L.; Dallner, G.; Ernster, L. Distribution and redox state of ubiquinones in rat and human tissues. Biochem. Biophys. 1992, 295, 230–234. [Google Scholar] [CrossRef]
- Runquist, M.; Parmryd, I.; Thelin, A.; Chojnacki, T.; Dallner, G.J. Distribution of branch point prenyltransferases in regions of bovine brain. Neurochem 1995, 65, 2299–2306. [Google Scholar] [CrossRef]
- Molyneux, S.L.; Florkowski, C.M.; Lever, M.; George, P.M. Biological variation of coenzyme Q10. Clin. Chem. 2005, 51, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Elmberger, P.G.; Kal`en, A.; Appelkvist, E.L.; Dallner, G. In vitro and in vivo synthesis of dolichol and other main mevalonate products in various organs of the rat. Eur. J. Biochem. 1987, 168, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Elmberger, P.G.; Edlund, C.; Kristensson, K.; Dallner, G. Rates of cholesterol, ubiquinone, dolichol and dolichyl-P biosynthesis in rat brain slices. FEBS Lett. 1990, 269, 15–18. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. Regulation of the mevalonate pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. Lipid Res. 1980, 21, 505–517. [Google Scholar]
- Bentinger, M.; Tekle, M.; Brismar, K.; Chojnacki, T.; Swiezewska, E.; Dallner, G.J. Polyisoprenoid epoxides stimulate the biosynthesis of coenzyme Q and inhibit cholesterol synthesis. Biol. Chem. 2008, 283, 14645–14653. [Google Scholar] [CrossRef] [PubMed]
- Aberg, F.; Zhang, Y.; Teclebrhan, H.; Appelkvist, E.L.; Dallner, G. Increases in tissue levels of ubiquinone in association with peroxisome proliferation. Chem. Biol. Interact. 1996, 99, 205–218. [Google Scholar] [CrossRef]
- Brea-Calvo, G.; Rodríguez-Hernández, A.; Fernández-Ayala, D.J.M.; Navas, P.; Sánchez-Alcázar, J.A. Chemotherapy induces an increase in coenzyme Q10 levels in cancer cell lines. Free Radic. Biol. Med. 2006, 40, 1293–1302. [Google Scholar] [PubMed]
- Brea-Calvo, G.; Siendones, E.; Sánchez-Alcázar, J.A.; de Cabo, R.; Navas, P. Cell survival from chemotherapy depends on NF-kappaB transcriptional upregulation of coenzyme Q biosynthesis. PLoS ONE 2009, 4, e5301. [Google Scholar] [CrossRef] [PubMed]
- Reahal, S.; Wrigglesworth, J. Tissue concentrations of coenzyme Q10 in the rat following its oral and intraperitoneal administration. Drug Metab. Dispos. 1992, 20, 423–427. [Google Scholar] [PubMed]
- Zhang, Y.; Aberg, F.; Appelkvist, E.L.; Dallner, G.; Ernster, L. Uptake of dietary coenzyme Q supplement is limited in rats. J. Nutr. 1995, 125, 446–453. [Google Scholar] [PubMed]
- Zhang, Y.; Turunen, M.; Appelkvist, E.L. Restricted uptake of dietary coenzyme Q is in contrast to the unrestricted uptake of alpha-tocopherol into rat organs and cells. J. Nutr. 1996, 126, 2089–2097. [Google Scholar] [PubMed]
- Turunen, M.; Appelkvist, E.L.; Sindelar, P.; Dallner, G. Blood concentration of coenzyme Q(10) increases in rats when esterified forms are administered. J. Nutr. 1999, 129, 2113–2118. [Google Scholar] [PubMed]
- Bentinger, M.; Dallner, G.; Chojnacki, T.; Swiezewska, E. Distribution and breakdown of labeled coenzyme Q10 in rat. Free Radic. Biol. Med. 2003, 34, 563–575. [Google Scholar] [CrossRef]
- Bhagavan, H.N.; Chopra, R.K.; Craft, N.E.; Chitchumroonchokchai, C.; Failla, M.L. Assessment of coenzyme Q10 absorption using an in vitro digestion-Caco-2 cell model. Int. J. Pharm. 2007, 333, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Malm, C.; Tonkonogi, M.; Ekblom, B.; Sjödin, B.; Sahlin, K. Effect of Q10 supplementation on tissue Q10 levels and adenine nucleotide catabolism during high-intensity exercise. Int. J. Sport Nutr. 1999, 9, 166–180. [Google Scholar] [PubMed]
- Watts, G.F.; Playford, D.A.; Croft, K.D.; Ward, N.C.; Mori, T.A.; Burke, V. Coenzyme Q(10) improves endothelial dysfunction of the brachial artery in Type II diabetes mellitus. Diabetologia 2002, 45, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; Hahn, A. Plasma ubiquinone status and response to six-month supplementation combined with multivitamins in healthy elderly women–results of a randomized, double-blind, placebo-controlled study. Int. J. Vitam. Nutr. Res. 2003, 73, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Hosoe, K.; Kitano, M.; Kishida, H.; Kubo, H.; Fujii, K.; Kitahara, M. Study on safety and bioavailability of ubiquinol (Kaneka QH) after single and 4-week multiple oral administration to healthy volunteers. Regul. Toxicol. Pharmacol. 2007, 47, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ikematsu, H.; Nakamura, K.; Harashima, S.-I.; Fujii, K.; Fukutomi, N. Safety assessment of coenzyme Q10 (Kaneka Q10) in healthy subjects: A double-blind, randomized, placebo-controlled trial. Regul. Toxicol. Pharmacol. 2006, 44, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.T.; Yang, L.; Browne, S.; Baik, M.; Beal, M.F. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc. Natl. Acad. Sci. USA 1998, 95, 8892–8897. [Google Scholar] [CrossRef] [PubMed]
- Kwong, L.K.; Kamzalov, S.; Rebrin, I.; Bayne, A.-C.V.; Jana, C.K.; Morris, P.; Forster, M.J.; Sohal, R.S. Effects of coenzyme Q(10) administration on its tissue concentrations, mitochondrial oxidant generation, and oxidative stress in the rat. Free Radic. Biol. Med. 2002, 33, 627–638. [Google Scholar] [CrossRef]
- The Parkinson Study Group QE3 Investigators. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease. JAMA Neurol. 2014, 71, 543–552. [Google Scholar]
- Kaufmann, P.; Thompson, J.L.P.; Levy, G.; Buchsbaum, S.J.; Krivickas, L.S.; Katz, J.; Rollins, Y.; Barohn, R.J.; Jackson, C.E.; Tiryaki, E.; et al. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann. Neurol. 2009, 66, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Huang, Y.C.; Chen, S.J.; Lin, P.T. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with coronary artery disease. Nutrition 2012, 28, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Bargossi, A.M.; Grossi, G.; Fiorella, P.L.; Gaddi, A.; di Giulio, R.; Battino, M. Exogenous CoQ10 supplementation prevents plasma ubiquinone reduction induced by HMG-CoA reductase inhibitors. Mol. Aspects Med. 1994, 15, s187–s193. [Google Scholar] [CrossRef]
- Bargossi, A.M.; Battino, M.; Gaddi, A.; Fiorella, P.L.; Grossi, G.; Barozzi, G.; Di Giulio, R.; Descovich, G.; Sassi, S.; Genova, M.L. Exogenous CoQ10 preserves plasma ubiquinone levels in patients treated with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Int. J. Clin. Lab. Res. 1994, 24, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-K.; Pugh, T.D.; Klopp, R.G.; Edwards, J.; Allison, D.B.; Weindruch, R.; Prolla, T.A. The impact of alpha-lipoic acid, coenzyme Q10 and caloric restriction on life span and gene expression patterns in mice. Free Radic. Biol. Med. 2004, 36, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.J.; Quiles, J.L.; Huertas, J.R.; Mataix, J. Coenzyme Q10 protects from aging-related oxidative stress and improves mitochondrial function in heart of rats fed a polyunsaturated fatty acid (PUFA)-rich diet. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Kamzalov, S.; Sumien, N.; Ferguson, M.; Rebrin, I.; Heinrich, K.R.; Forster, M.J. Effect of coenzyme Q10 intake on endogenous coenzyme Q content, mitochondrial electron transport chain, antioxidative defenses, and life span of mice. Free Radic. Biol. Med. 2006, 40, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Lass, A.; Kwong, L.; Sohal, R.S. Mitochondrial coenzyme Q content and aging. Biofactors 1999, 9, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Kamzalov, S.; Sumien, N.; Forster, M.J.; Sohal, R.S. Coenzyme Q intake elevates the mitochondrial and tissue levels of Coenzyme Q and alpha-tocopherol in young mice. J. Nutr. 2003, 133, 3175–3180. [Google Scholar] [PubMed]
- Huertas, J.R.; Martinez-Velasco, E.; Ibánñez, S.; López-Frias, M.; Ochoa, J.J.; Quiles, J.; Parenti Castelli, G.; Mataix, J.; Lenaz, G. Virgin olive oil and coenzyme Q10 protect heart mitochondria from peroxidative damage during aging. Biofactors 1999, 9, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Ochoa, J.J.; Huertas, J.R.; Mataix, J. Coenzyme Q supplementation protects from age-related DNA double-strand breaks and increases lifespan in rats fed on a PUFA-rich diet. Exp. Gerontol. 2004, 39, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, A.; Itagaki, S.; Kurokawa, T.; Kobayashi, M.; Hirano, T.; Iseki, K. Improvement in intestinal coenzyme q10 absorption by food intake. Yakugaku Zasshi 2007, 127, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Mataix, J.; Mañas, M.; Quiles, J.; Battino, M.; Cassinello, M.; Lopez-Frias, M.; Huertas, J.R. Coenzyme Q content depends upon oxidative stress and dietary fat unsaturation. Mol. Aspect Med. 1997, 18, 129–135. [Google Scholar] [CrossRef]
- Ramirez-Tortosa, M.C.; Granados, S.; Ramirez-Tortosa, C.L.; Ochoa, J.J.; Camacho, P.; García-Valdós, L.; Battino, M.; Quiles, J.L. Oxidative stress status in liver mitochondria and lymphocyte DNA damage of atherosclerotic rabbits supplemented with water soluble coenzyme Q10. Biofactors 2008, 32, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, T.; Fujii, K.; Funahashi, I.; Fukotomi, M.; Hosoe, K. Safety assesment of coenzyme Q10 (CoQ). Biofactors 2008, 32, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Sumien, N.; Heinrich, K.R.; Shetty, R.A.; Sohal, R.S.; Foster, M.J. Prolonged intake of coenzyme Q10 impairs cognitive functions in mice. J. Nutr. 2009, 139, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- Alho, H.; Lonnrot, K. Coenzyme Q supplementation and longevity. In Coenzyme Q: Molecular Mechanisms in Health and Disease, 1st ed.; Kagan, V.E., Quinn, P.J., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 371–380. [Google Scholar]
- Onur, S.; Niklowitz, P.; Fischer, A.; Metges, C.C.; Grune, T.; Menke, T.; Rimbach, G.; Döring, F. A comparative study into alterations of coenzyme Q redox status in ageing pigs, mice, and worms. Biofactors 2014, 40, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Kamzalov, S.; Sohal, R.S. Effect of age and caloric restriction on coenzyme Q and alpha-tocopherol levels in the rat. Exp. Gerontol. 2004, 39, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Appelkvist, E.L.; Kristensson, K.; Dallner, G. The lipid compositions of different regions of rat brain during development and aging. Neurobiol. Aging 1996, 17, 869–875. [Google Scholar] [CrossRef]
- Battino, M.; Gorini, A.; Villa, R.F.; Genova, M.L.; Bovina, C.; Sassi, S.; Littarru, G.P.; Lenaz, G. Coenzyme Q content in synaptic and non-synaptic mitochondria from different brain regions in the ageing rat. Mech. Ageing Dev. 1995, 78, 173–187. [Google Scholar] [CrossRef]
- Kalén, A.; Appelkvist, E.L.; Dallner, G. Age-related changes in the lipid compositions of rat and human tissues. Lipids 1989, 24, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Beyer, R.E.; Burnett, B.A.; Cartwright, K.J.; Edington, D.W.; Falzon, M.J.; Kreitman, K.R.; Kuhn, T.W.; Ramp, B.J.; Rhee, S.Y.; Rosenwasser, M.J. Tissue coenzyme Q (ubiquinone) and protein concentrations over the life span of the laboratory rat. Mech. Ageing Dev. 1985, 32, 267–281. [Google Scholar] [CrossRef]
- Söderberg, M.; Edlund, C.; Kristensson, K.; Dallner, G. Lipid compositions of different regions of the human brain during aging. J. Neurochem. 1990, 54, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Edlund, C.; Söderberg, M.; Kristensson, K.; Dallner, G. Ubiquinone, dolichol, and cholesterol metabolism in aging and Alzheimer’s disease. Biochem. Cell Biol. 1992, 70, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Quinn, P.J. Coenzyme Q: Molecular Mechanisms in Health and Disease, 1st ed.; CRC Press: Boca Ratón, FL, USA, 2000; p. 408. [Google Scholar]
- Witting, P.K.; Pettersson, K.; Letters, J.; Stocker, R. Anti-atherogenic effect of coenzyme Q10 in apolipoprotein E gene knockout mice. Free Radic. Biol. Med. 2000, 29, 295–305. [Google Scholar] [CrossRef]
- Ayaz, M.; Tuncer, S.; Okudan, N.; Gokbel, H. Coenzyme Q(10) and alphalipoic acid supplementation in diabetic rats: Conduction velocity distributions. Methods Find Exp. Clin. Pharmacol. 2008, 30, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Chew, G.T.; Watts, G.F.; Davis, T.M.E.; Stuckey, B.G.A.; Beilin, L.J.; Thompson, P.L.; Burke, V.; Currie, P. Hemodynamic effects of fenofibrate and coenzyme Q10 in type 2 diabetic subjects with left ventricular diastolic dysfunction. J. Diabetes Care 2008, 31, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Nunes, E.; Gomes, A.; Santos, M.S.; Proença, T.; Martins, M.I.; Seiça, R.M. Supplementation of coenzyme Q10 and alpha-tocopherol lowers glycated hemoglobin level and lipid peroxidation in pancreas of diabetic rats. Nutr. Res. 2008, 28, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.A.; de Bustos, F.; Ortiz, S.; Del Ser, T.; Seijo, M.; Benito-Léon, J.; Oliva, J.M.; Pérez, S.; Manzanares, J. Serum levels of coenzyme Q in patients with Lewy body disease. J. Neural Transm. 2002, 109, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Sohmiya, M.; Tanaka, M.; Suzuki, Y.; Tanino, Y.; Okamoto, K.; Yamamoto, Y. An increase of oxidized coenzyme Q-10 occurs in the plasma of sporadic ALS patients. J. Neurol. Sci. 2005, 228, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Isobe, C.; Abe, T.; Terayama, Y. Levels of reduced and oxidized coenzyme Q-10 and 8-hydroxy-2’-deoxyguanosine in the CSF of patients with Alzheimer’s disease demonstrate that mitochondrial oxidative damage and/or oxidative DNA damage contributes to the neurodegenerative process. J. Neurol. 2010, 257, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Isobe, C.; Abe, T.; Terayama, Y. Increase in the oxidized/total coenzyme Q-10 ratio in the cerebrospinal fluid of Alzheimer’s disease patients. Dement Geriatr. Cogn. Disord. 2009, 28, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T.; Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P.; Schapira, A.H.; Marsden, C.D. New insights into the cause of Parkinson’s disease. Neurology 1992, 42, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W. A radical hypothesis for neurodegeneration. Trends Neurosci. 1993, 16, 439–444. [Google Scholar] [CrossRef]
- Schapira, A.H.; Hartley, A.; Cleeter, M.W.; Cooper, J.M. Free radicals and mitochondrial dysfunction in Parkinson’s disease. Biochem. Soc. Trans. 1993, 21, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Isobe, C.; Murata, T.; Sato, C.; Terayama, Y. Increase of oxidized/total coenzyme Q-10 ratio in cerebrospinal fluid in patients with Parkinson’s disease. J. Clin. Neurosci. 2007, 14, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.C.; Tan, H.H.; Goh, S.K.; Subramaniam, T.; Sum, C.F.; Tan, I.K.; Lee, B.L.; Ong, C.N. Oxidative burden in prediabetic and diabetic individuals: Evidence from plasma coenzyme Q(10). Diabetes Med. 2006, 23, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Folkers, K.; Osterborg, A.; Nylander, M.; Morita, M.; Mellstedt, H. Activities of vitamin Q10 in animal models and a serious deficiency in patients with cancer. Biochem. Biophys. Res. Commun. 1997, 234, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Jolliet, P.; Simon, N.; Barré, J.; Pons, J.Y.; Boukef, M.; Paniel, B.J.; Tillement, J.P. Plasma coenzyme Q10 concentrations in breast cancer: Prognosis and therapeutic consequences. Int. J. Clin. Pharmacol. Ther. 1998, 36, 506–509. [Google Scholar] [PubMed]
- Rusciani, L.; Proietti, I.; Paradisi, A.; Rusciani, A.; Guerriero, G.; Mammone, A.; de Gaetano, A.; Lippa, S. Recombinant interferon alpha-2b and coenzyme Q10 as a postsurgical adjuvant therapy for melanoma: A 3-year trial with recombinant interferon-alpha and 5-year follow-up. Melanoma Res. 2007, 17, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Mano, T.; Iwase, K.; Hayashi, R.; Hayakawa, N.; Uchimura, K.; Makino, M.; Nagata, M.; Sawai, Y.; Oda, N.; Hamada, M.; et al. Vitamin E and coenzyme Q concentrations in the thyroid tissues of patients with various thyroid disorders. Am. J. Med. Sci. 1998, 315, 230–232. [Google Scholar] [PubMed]
- Portakal, O.; Ozkaya, O.; Erden Inal, M.; Bozan, B.; Koşan, M.; Sayek, I. Coenzyme Q10 concentrations and antioxidant status in tissues of breast cancer patients. Clin. Biochem. 2000, 33, 279–284. [Google Scholar] [CrossRef]
- Arroyo, A.; Santos-Ocaña, C.; Ruiz-Ferrer, M.; Padilla, S.; Gavilán, A.; Rodríguez-Aguilera, J.C.; Navas, P. Coenzyme Q is irreplaceable by demethoxy-coenzyme Q in plasma membrane of Caenorhabditis elegans. FEBS Lett. 2006, 580, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Farquharson, A.J.; Ramírez-Tortosa, M.C.; Grant, I.; Milne, L.; Huertas, J.R.; Battino, M.; Mataix, J.; Wahle, K.W.J. Coenzyme Q differentially modulates phospholipid hydroperoxide glutathione peroxidase gene expression and free radicals production in malignant and non-malignant prostate cells. Biofactors 2003, 18, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Jonassen, T.; Davis, D.E.; Larsen, P.L.; Clarke, C.F. Reproductive fitness and quinone content of Caenorhabditis elegans clk-1 mutants fed coenzyme Q isoforms of varying length. J. Biol. Chem. 2003, 278, 51735–51742. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Boutis, P.; Hekimi, S. Mutations in the clk-1 gene of Caenorhabditis elegans affect developmental and behavioral timing. Genetics 1995, 139, 1247–1259. [Google Scholar] [PubMed]
- Asencio, C.; Navas, P.; Cabello, J.; Schnabel, R.; Cypser, J.R.; Johnson, T.E.; Rodríguez-Aguilera, J.C. Coenzyme Q supports distinct developmental processes in Caenorhabditis elegans. Mech. Ageing Dev. 2009, 130, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Gavilán, A.; Asencio, C.; Cabello, J.; Rodríguez-Aguilera, J.C.; Schnabel, R.; Navas, P. C. elegans knockouts in ubiquinone biosynthesis genes result in different phenotypes during larval development. Biofactors 2005, 25, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Hihi, A.K.; Gao, Y.; Hekimi, S.J. Ubiquinone is necessary for Caenorhabditis elegans development at mitochondrial and non-mitochondrial sites. Biol. Chem. 2002, 277, 2202–2206. [Google Scholar] [CrossRef] [PubMed]
- Asencio, C.; Rodríguez-Aguilera, J.C.; Ruiz-Ferrer, M.; Vela, J.; Navas, P. C. elegans knockouts in ubiquinone biosynthesis genes result in different phenotypes during larval development. FASEB J. 2003, 17, 1135–1137. [Google Scholar] [PubMed]
- Vajo, Z.; King, L.M.; Jonassen, T.; Wilkin, D.J.; Ho, N.; Munnich, A.; Clarke, C.F.; Francomano, C.A. Conservation of the Caenorhabditis elegans timing gene clk-1 from yeast to human: A gene required for ubiquinone biosynthesis with potential implications for aging. Mamm. Genome 1999, 10, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Levavasseur, F.; Miyadera, H.; Sirois, J.; Tremblay, M.L.; Kita, K.; Shoubridge, E.; Hekimi, S. Ubiquinone is necessary for mouse embryonic development but is not essential for mitochondrial respiration. J. Biol. Chem. 2001, 276, 46160–46164. [Google Scholar] [CrossRef] [PubMed]
- Nakai, D.; Yuasa, S.; Takahashi, M.; Shimizu, T.; Asaumi, S.; Isono, K.; Takao, T.; Suzuki, Y.; Kuroyanagi, H.; Hirokawa, K.; et al. Mouse homologue of coq7/clk-1, longevity gene in Caenorhabditis elegans, is essential for coenzyme Q synthesis, maintenance of mitochondrial integrity, and neurogenesis. Biochem. Biophys. Res. Commun. 2001, 289, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Boitier, E.; Degoul, F.; Desguerre, I.; Charpentier, C.; François, D.; Ponsot, G.; Diry, M.; Rustin, P.; Marsac, C. A case of mitochondrial encephalomyopathy associated with a muscle coenzyme Q10 deficiency. J. Neurol. Sci. 1998, 156, 41–46. [Google Scholar] [CrossRef]
- Musumeci, O.; Naini, A.; Slonim, A.E.; Skavin, N.; Hadjigeorgiou, G.L.; Krawiecki, N.; Weissman, B.M.; Tsao, C.Y.; Mendell, J.R.; Shanske, S.; et al. Familial cerebellar ataxia with muscle coenzyme Q10 deficiency. Neurology 2001, 56, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, N.; Hughes, B.; Bigras, E.; Shoubridge, E.; Hekimi, S. Evolutionary conservation of the clk-1-dependent mechanism of longevity: Loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev. 2005, 19, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- López, L.C.; Schuelke, M.; Quinzii, C.M.; Kanki, T.; Rodenburg, R.J.; Naini, A.; Di Mauro, S.; Hirano, M. Leigh syndrome with nephropathy and CoQ 10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am. J. Hum. Genet. 2006, 79, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Quinzii, C.; Naini, A.; Salviati, L.; Trevisson, E.; Navas, P.; DiMauro, S.; Hirano, M. A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q 10 deficiency. Am. J. Hum. Genet. 2006, 78, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Mollet, J.; Giurgea, I.; Schlemmer, D.; Dallner, G.; Chretien, D.; Delahodde, A.; Bacq, D.; de Lonlay, P.; Munnich, A.; Rötig, A. Prenyldiphosphate synthase, subunit 1 (PDSS1) and OH-benzoate polyprenyltransferase (COQ2) mutations in ubiquinone deficiency and oxidative phosphorylation disorders. J. Clin. Investig. 2007, 117, 765. [Google Scholar] [CrossRef] [PubMed]
- Lagier-Tourenne, C.; Tazir, M.; López, L.C.; Quinzii, C.M.; Assoum, M.; Drouot, N.; Busso, C.; Makri, S.; Ali-Pacha, L.; Benhassine, T.; et al. ADCK3, an ancestral kinase, is mutated in a form of recessive ataxia associated with coenzyme Q 10 deficiency. Am. J. Hum. Genet. 2008, 82, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.J.; Bitner-Glindzicz, M.; Meunier, B.; Costello, H.; Hargreaves, I.P.; López, L.C.; Hirano, M.; Quinzii, C.M.; Sadowski, M.I.; Hardy, J.; Singleton, A.; Clayton, P.T.; Rahman, S. A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset primary coenzyme Q 10 deficiency: A potentially treatable form of mitochondrial disease. Am. J. Hum. Genet. 2009, 84, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, S.; Quinzii, C.M.; Hirano, M. Mutations in coenzyme Q10 biosynthetic genes. J. Clin. Investig. 2007, 117, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Ogasahara, S.; Engel, A.G.; Frens, D.; Mack, D. Muscle coenzyme Q deficiency in familial mitochondrial encephalomyopathy. Proc. Natl. Acad. Sci. USA 1989, 86, 2379–2382. [Google Scholar] [CrossRef] [PubMed]
- Rötig, A.; Appelkvist, E.L.; Geromel, V.; Chretien, D.; Kadhom, N.; Edery, P.; Lebideau, M.; Dallner, G.; Munnich, A.; Ernster, L.; et al. Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet 2000, 356, 391–395. [Google Scholar] [CrossRef]
- Artuch, R.; Brea-Calvo, G.; Briones, P.; Aracil, A.; Galván, M.; Espinós, C.; Corral, J.; Volpini, V.; Ribes, A.; Andreu, A.L.; et al. Cerebellar ataxia with coenzyme Q10 deficiency: Diagnosis and follow-up after coenzyme Q10 supplementation. J. Neurol. Sci. 2006, 246, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Quinzii, C.M.; López, L.C.; Naini, A.; di Mauro, S.; Hirano, M. Human CoQ10 deficiencies. Biofactors 2008, 32, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, S.L.; Florkowski, C.M.; Richards, A.M.; Lever, M.; Young, J.M.; George, P.M. Coenzyme Q10; an adjunctive therapy for congestive heart failure? N. Z. Med. J. 2009, 122, 74–79. [Google Scholar] [PubMed]
- Rosenfeldt, F.; Marasco, S.; Lyon, W.; Wowk, M.; Sheeran, F.; Bailey, M.; Esmore, D.; Davis, B.; Pick, A.; Rabinov, M.; et al. Coenzyme Q10 therapy before cardiac surgery improves mitochondrial function and in vitro contractility of myocardial tissue. J. Thorac. Cardiovasc. Surg. 2005, 129, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.F. Mitochondrial dysfunction and oxidative damage in Alzheimer’s and Parkinson’s diseases and coenzyme Q10 as a potential treatment. J. Bioenerg. Biomembr. 2004, 36, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Shults, C.W.; Oakes, D.; Kieburtz, K.; Beal, M.F.; Haas, R.; Plumb, S.; Juncos, J.L.; Nutt, J.; Shoulson, I.; Carter, J.; et al. Effects of coenzyme Q10 in early Parkinson disease: Evidence of slowing of the functional decline. Arch. Neuro 2002, 59, 154–1550. [Google Scholar] [CrossRef]
- Thomas, B.; Beal, M.F. Mitochondrial therapies for Parkinson’s disease. Mov. Disord. 2010, 25, S155–S160. [Google Scholar] [CrossRef] [PubMed]
- Dumont, M.; Lin, M.T.; Beal, M.F. Neuroprotective strategies involving ROS in Alzheimer disease. J. Alzheimers Dis. 2010, 20, S633–S643. [Google Scholar] [CrossRef] [PubMed]
- Dumont, M.; Beal, M.F. Neuroprotective strategies involving ROS in Alzheimer disease. Free Radic. Biol. Med. 2011, 51, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dai, G.; Li, G.; Yang, E.S. Coenzyme Q10 reduces beta-amyloid plaque in an APP/PS1 transgenic mouse model of Alzheimer’s disease. J. Mol. Neurosci. 2010, 41, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.E.; Lodi, R.; Rajagopalan, B.; Bradley, J.L.; Crilley, J.G.; Turner, C.; Blamire, A.M.; Manners, D.; Styles, P.; Schapira, A.H.V.; et al. Antioxidant treatment of patients with Friedreich ataxia: Four-year follow-up. Arch. Neurol. 2005, 62, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.L.; Clarke, C.F. Extension of life-span in Caenorhabditis elegans by a diet lacking coenzyme Q. Science 2002, 295, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Dillin, A.; Hsu, A.-L.; Arantes-Oliveira, N.; Lehrer-Graiwer, J.; Hsin, H.; Fraser, A.G.; Kamath, R.S.; Ahringer, J.; Kenyon, C. Rates of behavior and aging specified by mitochondrial function during development. Science 2002, 298, 2398–2401. [Google Scholar] [CrossRef] [PubMed]
- Saiki, R.; Lunceford, A.L.; Bixler, T.; Dang, P.; Lee, W.; Furukawa, S.; Larsen, P.L.; Clarke, C.F. Altered bacterial metabolism, not coenzyme Q content, is responsible for the lifespan extension in Caenorhabditis elegans fed an Escherichia coli diet lacking coenzyme Q. Aging Cell 2008, 7, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Lönnrot, K.; Holm, P.; Lagerstedt, A.; Huhtala, H.; Alho, H. The effects of lifelong ubiquinone Q10 supplementation on the Q9 and Q10 tissue concentrations and life span of male rats and mice. Biochem. Mol. Biol. Int. 1998, 44, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Fujii, K.; Yao, J.; Kishida, H.; Hosoe, K.; Sawashita, J.; Takeda, T.; Mori, M.; Higuchi, K. Reduced coenzyme Q10 supplementation decelerates senescence in SAMP1 mice. Exp. Gerontol. 2006, 41, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.A.; Forster, M.J.; Sumien, N. Coenzyme Q10 supplementation reverses age-related impairments in spatial learning and lowers protein oxidation. Age 2012, 35, 1821–1834. [Google Scholar] [CrossRef] [PubMed]
- Tarry-Adkins, J.L.; Blackmore, H.L.; Martin-Gronert, M.S.; Fernandez-Twinn, D.S.; McConnell, J.M.; Hargreaves, I.P.; Giussani, D.A.; Ozanne, S.E. Coenzyme Q10 prevents accelerated cardiac aging in a rat model of poor maternal nutrition and accelerated postnatal growth. Mol. Metabol. 2013, 2, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.J.; Quiles, J.L.; López-Frías, M.; Huertas, J.R.; Mataix, J. Effect of lifelong coenzyme Q10 supplementation on age-related oxidative stress and mitochondrial function in liver and skeletal muscle of rats fed on a polyunsaturated fatty acid (PUFA)-rich diet. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Pamplona, R.; Ramirez-Tortosa, M.C.; Naudí, A.; Portero-Otin, M.; Araujo-Nepomuceno, E.; López-Frías, M.; Battino, M.; Ochoa, J.J. Coenzyme Q addition to an n-6 PUFA-rich diet resembles benefits on age-related mitochondrial DNA deletion and oxidative stress of a MUFA-rich diet in rat heart. Mech. Ageing Dev. 2010, 131, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.J.; Pamplona, R.; Ramirez-Tortosa, M.C.; Granados-Principal, S.; Perez-Lopez, P.; Naudí, A.; Portero-Otin, M.; López-Frías, M.; Battino, M.; Quiles, J.L. Age-related changes in brain mitochondrial DNA deletion and oxidative stress are differentially modulated by dietary fat type and coenzyme Q 10. Free Radic. Biol. Med. 2011, 50, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- González-Alonso, A.; Ramírez-Tortosa, C.L.; Varela-López, A.; Roche, E.; Arribas, M.I.; Ramírez-Tortosa, M.C.; Giampieri, F.; Ochoa, J.J.; Quiles, J.L. Sunflower oil but not fish oil resembles positive effects of virgin olive oil on aged pancreas after life-long coenzyme Q addition. Int. J. Mol. Sci. 2015, 16, 23425–23445. [Google Scholar] [CrossRef] [PubMed]
- Varela-Lopez, A.; Bullon, P.; Battino, M.; Ramirez-Tortosa, M.C.; Ochoa, J.J.; Cordero, M.D.; Ramirez-Tortosa, C.L.; Rubini, C.; Zizzi, A.; Quiles, J.L. Coenzyme Q Protects Against Age-Related Alveolar Bone Loss Associated to n-6 Polyunsaturated Fatty Acid Rich-Diets by Modulating Mitochondrial Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2015, glv063. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Ciaffoni, F.; Safwat, G.M.; Aspichueta, P.; Ochoa, B.; Bravo, E.; Botham, K.M. Hepatic VLDL assembly is disturbed in a rat model of nonalcoholic fatty liver disease: Is there a role for dietary coenzyme Q? J. Appl. Physiol. 2009, 107, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, D.V.; Chandraiah, G.; Meena, A.K.; Ramarao, P.; Kumar, M.N.V.R. The co-encapsulated antioxidant nanoparticles of ellagic acid and coenzyme Q10 ameliorates hyperlipidemia in high fat diet fed rats. J. Nanosci. Nanotechnol. 2009, 9, 6741–6746. [Google Scholar] [CrossRef] [PubMed]
- Safwat, G.M.; Pisanò, S.; D’Amore, E.; Borioni, G.; Napolitano, M.; Kamal, A.A.; Ballanti, P.; Botham, K.M.; Bravo, E. Induction of non-alcoholic fatty liver disease and insulin resistance by feeding a high-fat diet in rats: Does coenzyme Q monomethyl ether have a modulatory effect? Nutrition 2009, 25, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Sohet, F.M.; Neyrinck, A.M.; Pachikian, B.D.; de Backer, F.C.; Bindels, L.B.; Niklowitz, P.; Menke, T.; Cani, P.D.; Delzenne, N.M. Coenzyme Q10 supplementation lowers hepatic oxidative stress and inflammation associated with diet-induced obesity in mice. Biochem. Pharmacol. 2009, 78, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Santos, M.A.; Juárez-Rojop, I.E.; Tovilla-Zárate, C.A.; Espinosa-García, M.T.; Juárez-Oropeza, M.A.; Ramón-Frías, T.; Bermúdez-Ocaña, D.Y.; Díaz-Zagoya, J.C. Coenzyme Q10 supplementation improves metabolic parameters, liver function and mitochondrial respiration in rats with high doses of atorvastatin and a cholesterol-rich diet. Lipids Health Dis. 2014, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Orlando, P.; Silvestri, S.; Brugè, F.; Tiano, L.; Kloting, I.; Falcioni, G.; Polidori, C. High-fat diet-induced met-hemoglobin formation in rats prone (WOKW) or resistant (DA) to the metabolic syndrome: Effect of CoQ10 supplementation. Biofactors 2014, 40, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Bello, R.I.; Gómez-Díaz, C.; Burón, M.I.; Alcaín, F.J.; Navas, P.; Villalba, J.M. Enhanced anti-oxidant protection of liver membranes in long-lived rats fed on a coenzyme Q10-supplemented diet. Exp. Gerontol. 2005, 40, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Díaz, C.; Burón, M.I.; Alcaín, F.J.; González-Ojeda, R.; González-Reyes, J.A.; Bello, R.I.; Herman, M.D.; Navas, P.; Villalba, J.M. Effect of dietary coenzyme Q and fatty acids on the antioxidant status of rat tissues. Protoplasma 2003, 221, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Santos-González, M.; Gómez Díaaz, C.; Navas, P.; Villalba, J.M. Modifications of plasma proteome in long-lived rats fed on a coenzyme Q10-supplemented diet. Exp. Gerontol. 2007, 42, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Delgado-Casado, N.; Delgado-Lista, J.; Perez-Martinez, P.; Tasset-Cuevas, I.; Santos-Gonzalez, M.; Caballero, J.; Garcia-Rios, A.; Marin, C.; Gutierrez-Mariscal, F.M.; et al. Postprandial antioxidant effect of the Mediterranean diet supplemented with coenzyme Q10 in elderly men and women. Age 2010, 33, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mariscal, F.M.; Perez-Martinez, P.; Delgado-Lista, J.; Yubero-Serrano, E.M.; Camargo, A.; Delgado-Casado, N.; Cruz-Teno, C.; Santos-Gonzalez, M.; Rodriguez-Cantalejo, F.; Castaño, J.P.; et al. Mediterranean diet supplemented with coenzyme Q10 induces postprandial changes in p53 in response to oxidative DNA damage in elderly subjects. Age 2012, 34, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Gonzalez-Guardia, L.; Rangel-Zuñiga, O.; Delgado-Lista, J.; Gutierrez-Mariscal, F.M.; Perez-Martinez, P.; Delgado-Casado, N.; Cruz-Teno, C.; Tinahones, F.J.; Villalba, J.M.; et al. Mediterranean diet supplemented with coenzyme Q10 modifies the expression of proinflammatory and endoplasmic reticulum stress-related genes in elderly men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67A, 3–10. [Google Scholar] [CrossRef] [PubMed]
- González-Guardia, L.; Yubero-Serrano, E.M.; Delgado-Lista, J.; Perez-Martinez, P.; Garcia-Rios, A.; Marin, C.; Camargo, A.; Delgado-Casado, N.; Roche, H.M.; Perez- Jimenez, F.; et al. Effects of the mediterranean diet supplemented with coenzyme Q10 on metabolomic profiles in elderly men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mariscal, F.M.; Yubero-Serrano, E.M.; Rangel-Zuñiga, O.A.; Marín, C.; García-Rios, A.; Perez-Martinez, P.; Delgado-Lista, J.; Malagón, M.M.; Tinahones, F.J.; Pérez-Jimenez, F.; et al. Postprandial activation of p53-dependent DNA repair is modified by mediterranean diet supplemented with coenzyme Q10 in elderly subjects. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 886–893. [Google Scholar] [CrossRef] [PubMed]
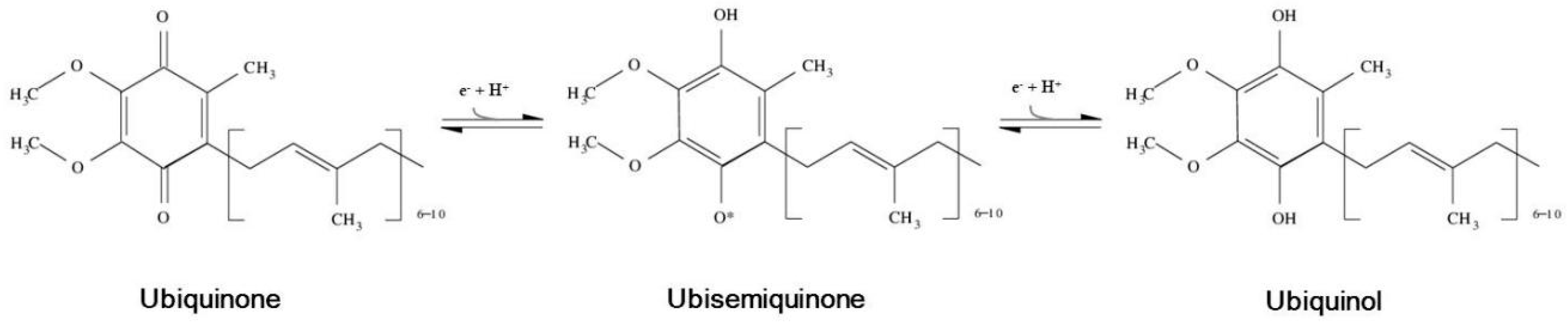
 ) or inhibitory (
) or inhibitory (  ) effects on gene expression. Abbreviations: α-tp: α-tocopherol; α-tp*−: α-tocopheryl anion radical; CoQ: oxidized coenzyme Q; CoQ·H: partially reduced coenzyme Q; CoQH2: fully reduced coenzyme Q; Cytb5R: NADH: cytochrome b5 reductase; e−: electron; FAD+: reduced flavine adenine dinucelotide; FADH2: reduced flavine adenine dinucleotide; H2O2: hydrogen peroxide; LOO*: lipid hydroperoxyl radical; LOOH: lipid hydroperoxyde; MAPKs: mitogen-activated protein kinases; mPTP: mitochondrial permeability transition pore; NAD+: oxidized nicotine adenine dinucleotide; NADH: reduced nicotine adenine dinucleotide; NQO1: NAD(P)H:Ubiquinone oxidase 1; O2·−: superoxide anion; ROS: reactive oxygen species; SIRT1: sirtuin1.
) effects on gene expression. Abbreviations: α-tp: α-tocopherol; α-tp*−: α-tocopheryl anion radical; CoQ: oxidized coenzyme Q; CoQ·H: partially reduced coenzyme Q; CoQH2: fully reduced coenzyme Q; Cytb5R: NADH: cytochrome b5 reductase; e−: electron; FAD+: reduced flavine adenine dinucelotide; FADH2: reduced flavine adenine dinucleotide; H2O2: hydrogen peroxide; LOO*: lipid hydroperoxyl radical; LOOH: lipid hydroperoxyde; MAPKs: mitogen-activated protein kinases; mPTP: mitochondrial permeability transition pore; NAD+: oxidized nicotine adenine dinucleotide; NADH: reduced nicotine adenine dinucleotide; NQO1: NAD(P)H:Ubiquinone oxidase 1; O2·−: superoxide anion; ROS: reactive oxygen species; SIRT1: sirtuin1.
 ) or inhibitory (
) or inhibitory ( 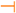 ) effects on gene expression. Abbreviations: α-tp: α-tocopherol; α-tp*−: α-tocopheryl anion radical; CoQ: oxidized coenzyme Q; CoQ·H: partially reduced coenzyme Q; CoQH2: fully reduced coenzyme Q; Cytb5R: NADH: cytochrome b5 reductase; e−: electron; FAD+: reduced flavine adenine dinucelotide; FADH2: reduced flavine adenine dinucleotide; H2O2: hydrogen peroxide; LOO*: lipid hydroperoxyl radical; LOOH: lipid hydroperoxyde; MAPKs: mitogen-activated protein kinases; mPTP: mitochondrial permeability transition pore; NAD+: oxidized nicotine adenine dinucleotide; NADH: reduced nicotine adenine dinucleotide; NQO1: NAD(P)H:Ubiquinone oxidase 1; O2·−: superoxide anion; ROS: reactive oxygen species; SIRT1: sirtuin1.
) effects on gene expression. Abbreviations: α-tp: α-tocopherol; α-tp*−: α-tocopheryl anion radical; CoQ: oxidized coenzyme Q; CoQ·H: partially reduced coenzyme Q; CoQH2: fully reduced coenzyme Q; Cytb5R: NADH: cytochrome b5 reductase; e−: electron; FAD+: reduced flavine adenine dinucelotide; FADH2: reduced flavine adenine dinucleotide; H2O2: hydrogen peroxide; LOO*: lipid hydroperoxyl radical; LOOH: lipid hydroperoxyde; MAPKs: mitogen-activated protein kinases; mPTP: mitochondrial permeability transition pore; NAD+: oxidized nicotine adenine dinucleotide; NADH: reduced nicotine adenine dinucleotide; NQO1: NAD(P)H:Ubiquinone oxidase 1; O2·−: superoxide anion; ROS: reactive oxygen species; SIRT1: sirtuin1.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varela-López, A.; Giampieri, F.; Battino, M.; Quiles, J.L. Coenzyme Q and Its Role in the Dietary Therapy against Aging. Molecules 2016, 21, 373. https://doi.org/10.3390/molecules21030373
Varela-López A, Giampieri F, Battino M, Quiles JL. Coenzyme Q and Its Role in the Dietary Therapy against Aging. Molecules. 2016; 21(3):373. https://doi.org/10.3390/molecules21030373
Chicago/Turabian StyleVarela-López, Alfonso, Francesca Giampieri, Maurizio Battino, and José L. Quiles. 2016. "Coenzyme Q and Its Role in the Dietary Therapy against Aging" Molecules 21, no. 3: 373. https://doi.org/10.3390/molecules21030373
APA StyleVarela-López, A., Giampieri, F., Battino, M., & Quiles, J. L. (2016). Coenzyme Q and Its Role in the Dietary Therapy against Aging. Molecules, 21(3), 373. https://doi.org/10.3390/molecules21030373








