Synthesis and Selective Cytotoxic Activities on Rhabdomyosarcoma and Noncancerous Cells of Some Heterocyclic Chalcones
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Evaluation of Cytotoxic Activities
3. Experimental Section
3.1. General Information
3.2. General Procedure for the Synthesis of Chalcones 1–20
3.3. Product Characterization
3.4. MTT Assay of Cytotoxic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Ward, E. Cancer statistics. CA Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef] [PubMed]
- Twombly, R. Cancer surpasses heart disease as leading cause of death for all but the very elderly. JNCI J. Natl. Cancer Inst. 2005, 97, 330–331. [Google Scholar] [CrossRef] [PubMed]
- Willam, H.M.; Sheri, L.S. Soft tissue sarcomas of childhood. Cancer Treat. Rev. 2004, 30, 269–280. [Google Scholar]
- Keller, C.; Guttridge, D.C. Mechanisms of impaired differentiation in rhabdomyosarcoma. FEBS J. 2013, 280, 4323–4334. [Google Scholar] [CrossRef] [PubMed]
- Pappo, A.S. Rhabdomyosarcoma and other soft tissue sarcomas of childhood. Curr. Opin. Oncol. 1995, 7, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Dagher, R.; Helman, L. Rhabdomyosarcoma: An Overview. Oncologist 1999, 4, 34–44. [Google Scholar] [PubMed]
- Firoozpour, L.; Edraki, N.; Nakhjiri, M.; Emami, S.; Safavi, M.; Ardestani, S.K.; Khoshneviszadeh, M.; Shfiee, A.; Foroumadi, A. Cytotoxic Activity Evaluation and QSAR study of Chromene—Based Chalcone. Arch. Pharm. Res. 2012, 35, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Baviskar, B.A.; Baviskar, B.; Shiradkar, M.R.; Deokate, U.A.; Khadabadi, S.S. Synthesis and Antimicrobial activity of some novel. Benzimidazolyl chalcones. Eur. J. Chem. 2009, 6, 196–200. [Google Scholar]
- Munawar, M.A.; Azad, M.; Siddiqui, H.L. Synthesis and antimicrobial studies of some quinolinylpyrimidine derivatives. J. Chin. Soc. 2008, 55, 394–400. [Google Scholar] [CrossRef]
- Azad, M.; Munawar, M.A.; Siddiqui, H.L. Antimicrobial activity and synthesis of quinoline-base chalcones. J. Appl. Sci. 2007, 7, 2485–2489. [Google Scholar]
- Kalirajan, R.; Sivakumar, S.U.; Jubie, S.; Gowramma, B.; Suresh, B. Synthesis and biological evaluation of some heterocyclic derivatives of chalcones. Int. J. ChemTech Res. 2009, 1, 27–34. [Google Scholar]
- Talia, J.M.; Debattista, N.B.; Pappano, N.B. New antimicrobial combinations: Substituted chalcones-oxacillin against methicillin resistant Staphylococcus aureus. Braz. J. Microbiol. 2011, 42, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Eumkeb, G.; Siriwong, S.; Phitaktim, S.; Rojtinnakorn, N.; Sakdarat, S. Synergistic activity and mode of action of flavonoids isolated from smaller galangal and amoxicillin combinations against amoxicillin-resistant Escherichia coli. J. Appl. Microbiol. 2012, 112, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Shibata, H.; Arakaki, N.; Higuti, T. 6,7-dihydroxyflavone dramatically intensifies the susceptibility of methicillin-resistant or -sensitive Staphylococcus aureus to beta-lactams. Antimicrob. Agents Chemother. 2004, 48, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Babasaheb, P.B.; Sachin, A.P.; Rajesh, N.G. Synthesis and biological evaluation of nitrogencontaining chalcones as possible anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. Lett. 2010, 20, 730–733. [Google Scholar]
- Vogel, S.; Barbic, M.; Jürgenliemk, G.; Heilmann, J. Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur. J. Med. Chem. 2010, 45, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.-D.; Park, H.; Kim, H.P.; Ecker, G.F.; Thai, K.-M. Inhibitory activity of prostaglandin E2 production by the synthetic 2′-hydroxychalcone analogues: Synthesis and SAR study. Bioorg. Med. Chem. Lett. 2009, 19, 1650–1653. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-T.; O, K.-J.; Chun, J.-C.; Hwang, K.-J. Synthesis of dihydroxylated chalcone derivatives with diverse substitution patterns and their radical scavenging ability toward DPPH free radicals. Bull. Korean Chem. Soc. 2008, 29, 1125–1130. [Google Scholar]
- Doan, T.N.; Tran, T.-D. Synthesis, antioxidant and antimicrobial activities of a novel series of chalcones, pyrazolic chalcones, and allylic chalcones. Pharmacol. Pharm. 2011, 2, 282–288. [Google Scholar] [CrossRef]
- Sivakumar, P.M.; Prabhakar, P.K.; Doble, M. Synthesis, antioxidant evaluation, and quantitative structure–activity relationship studies of chalcones. Med. Chem. Res. 2011, 20, 482–492. [Google Scholar] [CrossRef]
- Vogel, S.; Ohmayer, S.; Brunner, G.; Heilmann, J. Natural and non-natural prenylated chalcones: Synthesis, cytotoxicity and anti-oxidative activity. Bioorg. Med. Chem. 2008, 16, 4286–4293. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, C.; Santibañez, J.F.; Donoso-Tauda, O.; Escobar, C.A.; Ramirez-Tagle, R. Structural antitumoral activity relationships of synthetic chalcones. Int. J. Mol. Sci. 2009, 10, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Modzelewska, A.; Pettit, C.; Achanta, G.; Davidson, N.E.; Huang, P.; Khan, S.R. Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorg. Med. Chem. 2006, 14, 3491–3495. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Heilmann, J. Synthesis, cytotoxicity, and antioxidative activity of minor prenylated chalcones from Humulus lupulus. J. Nat. Prod. 2008, 71, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Kashi Reddy, M.; Viswanath, A. The design and development of imidazothiazole-chalcone derivatives as potential anticancer drugs. Expert Opin Drug Discov. 2013, 8, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.P.; Lima, R.T.; Choosang, K.; Pakkong, P.; de São José Nascimento, M.; Vasconcelos, M.H.; Pinto, M.; Silva, A.M.; Cidade, H. Synthesis of a natural chalcone and its prenyl analogs—Evaluation of tumor cell growth-inhibitory activities, and effects on cell cycle and apoptosis. Chem. Biodivers. 2012, 9, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Forejtníková, H.; Lunerová, K.; Kubínová, R.; Jankovská, D.; Marek, R.; Suchŷ, V.; Vondrácek, J. Chemoprotective and toxic potentials of synthetic and natural chalcones and dihydrochalcones in vitro. Toxicology 2005, 208, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Orlikova, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Yoon, H.; Ahn, S.; Kim, D.W.; Kim, S.H.; Koh, D.; Lee, Y.H.; Lim, Y. Chromenylchalcones showing cytotoxicity on human colon cancer cell lines and in silico docking with aurora kinases. Bioorg. Med. Chem. 2013, 21, 4250–4258. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, C.; Moorthy, N.S.; Ramasamy, S.; Vanam, U.; Manivannan, E.; Karunagaran, E.; Trivedi, P. Advances in Chalcones with Anticancer Activities. Recent Pat. Anticancer Drug Discov. 2015, 10, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Priya, M.K.; Muhammad, S.A.; Ravi, S. Novel phenothiazine compounds as third generation BCR-ABL tyrosine kinase inhibitors. Int. J. Pharm. Biol. Sci. 2015, 6, 707–716. [Google Scholar]
- Saranya, A.V.; Ravi, S. In vitro antifungal activity of Chalcones with phenothiazine ring. Res. J. Recent Sci. 2012, 1, 40–43. [Google Scholar]
- Tran, T.-D.; Nguyen, T.-T.-N.; Do, T.-H.; Huynh, T.-N.-P.; Tran, C.-D.; Thai, K.-M. Synthesis and Antibacterial Activity of some heterocyclic chalcone analogues alone and in combination with antibiotics. Molecules 2012, 17, 6684–6696. [Google Scholar] [CrossRef] [PubMed]
- Butkiewicz, K. Polarographic Behaviour of Pyridyl Analogues of Chalcone: Part 1. Polarographic reduction of isomeric 1-(2-hydroxyphenyl)-3-[2(3 or 4)-pyridyl]-2-propen-1-ones and 1-(2-hydroxy-4-methoxyphenyl)-3-[2(3 or 4)-pyridyl]-2-propen-1-ones. Electroanal. Chem. Interfacial Electrochem. 1972, 39, 407–417. [Google Scholar] [CrossRef]
- Kim, K.T.; Cho, S.G.; Han, Y.S.; Nah, S.Y.; Park, H.D.; Yeo, E.J.; Moon, S.H. Inhibitory effects of naringenin, kaempherol, and apigenin on cholesterol biosynthesis in HepG2 and MCF-7 cells. Food Sci. Biotechnol. 2008, 17, 1361–1364. [Google Scholar]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Sample Availability: No Available.

| No. | Structure of Phenothiazinyl Chalcones | Ring B | Ref. | |||
|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | |||
| 1 | 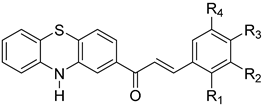 | H | H | N(CH3)2 | H | <N> 1 |
| 2 | H | H | OCH2C6H5 | H | <N> 1 | |
| 3 | H | Br | H | H | <N> 1 | |
| 4 | H | H | Cl | H | [31] | |
| 5 | H | H | H | H | [32] | |
| 6 | H | H | OCH3 | H | [31] | |
| 7 | Cl | H | H | H | <N> 1 | |
| 8 | OCH3 | H | OCH3 | H | <N> 1 | |
| 9 | 2-thiophenyl | <N> 1 | ||||
| No. | Chalcone Structure | Ring A | Ring B | Ref. | |||
|---|---|---|---|---|---|---|---|
| X | R1 | R2 | R3 | R4 | |||
| 10 | 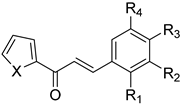 | S | H | H | Cl | H | [32] |
| 11 | S | OH | H | H | H | [33] | |
| 12 | S | H | H | N(CH3)2 | H | [33] | |
| 13 | S | H | OCH3 | OCH3 | OCH3 | [33] | |
| 14 | O | H | OCH3 | OCH3 | OCH3 | [33] | |
| 15 | O | H | H | N(CH3)2 | H | [33] | |
| 16 | 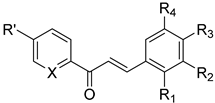 | R′: H; X: N | H | OCH3 | OCH3 | OCH3 | [33] |
| 17 * | R′: H; X: C-OH | 3-pyridyl | [34] | ||||
| 18 * | R′: H; X: C-OH | 2-pyridyl | [34] | ||||
| 19 * | R′:OCH3; X: C-OH | 3-pyridyl | [34] | ||||
| 20 * | R′:OCH3;X: C-OH | 2-pyridyl | [34] | ||||
| Compounds | IC50 (μM) | Compounds | IC50 (μM) |
|---|---|---|---|
| 1 | Nd | 11 | 48.77 ± 2.42 |
| 2 | Nd | 12 | Nd |
| 3 | Nd | 13 | 26.64 ± 1.42 |
| 4 | Nd | 14 | 29.06 ± 1.82 |
| 5 | Nd | 15 | 77.34 ± 4.69 |
| 6 | Nd | 16 | 19.57 ± 1.04 |
| 7 | 18.04 ± 1.63 | 17 | 92.85 ± 6.14 |
| 8 | 19.65 ± 1.63 | 18 | 91.12 ± 2.45 |
| 9 | 12.51 ± 1.74 | 19 | 26.62 ± 1.72 |
| 10 | 49.55 ± 3.20 | 20 | 20.77 ± 1.33 |
| Paclitaxel was used as the reference | 10.84 ± 0.47 | ||
| Compounds | % Cell Viability on Noncancer LLC-PK1 cells | ||
|---|---|---|---|
| 100 μM | 50 μM | 10 μM | |
| 7 | 94.8 ± 7.6 | 109.4 ± 10.9 | 100.2 ± 6.5 |
| 8 | 105.2 ± 5.4 | 103.2 ± 6.4 | 97.3 ± 10.2 |
| 9 | 97.1 ± 5.0 | 98.2 ± 7.3 | 98.8 ± 10.6 |
| 16 | 104.3 ± 6.5 | 101.9 ± 12.1 | 99.1 ± 3.1 |
| Paclitaxel (reference) | 20 μM | 10 μM | 2 μM |
| 75.0 ± 3.6 | 108.1 ± 8.2 | 103.4 ± 6.0 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, T.-H.; Nguyen, D.-M.; Truong, V.-D.; Do, T.-H.-T.; Le, M.-T.; Pham, T.-Q.; Thai, K.-M.; Tran, T.-D. Synthesis and Selective Cytotoxic Activities on Rhabdomyosarcoma and Noncancerous Cells of Some Heterocyclic Chalcones. Molecules 2016, 21, 329. https://doi.org/10.3390/molecules21030329
Do T-H, Nguyen D-M, Truong V-D, Do T-H-T, Le M-T, Pham T-Q, Thai K-M, Tran T-D. Synthesis and Selective Cytotoxic Activities on Rhabdomyosarcoma and Noncancerous Cells of Some Heterocyclic Chalcones. Molecules. 2016; 21(3):329. https://doi.org/10.3390/molecules21030329
Chicago/Turabian StyleDo, Tuong-Ha, Dai-Minh Nguyen, Van-Dat Truong, Thi-Hong-Tuoi Do, Minh-Tri Le, Thanh-Quan Pham, Khac-Minh Thai, and Thanh-Dao Tran. 2016. "Synthesis and Selective Cytotoxic Activities on Rhabdomyosarcoma and Noncancerous Cells of Some Heterocyclic Chalcones" Molecules 21, no. 3: 329. https://doi.org/10.3390/molecules21030329
APA StyleDo, T.-H., Nguyen, D.-M., Truong, V.-D., Do, T.-H.-T., Le, M.-T., Pham, T.-Q., Thai, K.-M., & Tran, T.-D. (2016). Synthesis and Selective Cytotoxic Activities on Rhabdomyosarcoma and Noncancerous Cells of Some Heterocyclic Chalcones. Molecules, 21(3), 329. https://doi.org/10.3390/molecules21030329








