Abstract
Five new bis(3-hydroxy-4-pyridinone) tetradentate chelators were synthesized in this study. The structures of these tetradentate chelators were characterized by 1H-NMR, 13C-NMR, FT-IR, UV-vis, and mass spectral analyses. The binding abilities of these tetradentate chelators for uranyl ion at pH 7.4 were also determined by UV spectrophotometry in aqueous media. Results showed that the efficiencies of these chelating agents are dependent on the linker length. Ligand 4b is the best chelator and suitable for further studies.
1. Introduction
Uranium is introduced into the body by ingestion, inhalation, or through wounds. The risk of uranium contamination has considerably magnified because of the extensive use of uranium as nuclear fuel in fission reactors and as weapon-grade nuclear material. The hexavalent uranyl ion [UO22+, U(VI)] is the most stable form in vivo [1] and is complexed in the blood by chelating agents, such as proteins or carbonates. Meanwhile, tissues, especially the kidney and bones, accumulate uranium for months to years, which will induce cancer and chemical intoxication [2,3,4]. Thus, uranium should be eliminated from the body by administration of nontoxic chelating agents that can form stable complexes with the uranyl ion.
Among the different chelators, 3-Hydroxy-4-pyridinones (3,4-HOPO) have emerged as one of the hotspots in studies that focus on heavy metal chelators because of their special bidentate structure, highly selective chelating capacity, and significant physiological activities [5,6,7,8,9,10,11,12]. To date, three kinds of 3,4-HOPO derivatives are available, namely bidentate hydroxypyridinones [5,6], tetradentate hydroxypyridinones [7,8,9], and hexadentate hydroxypyridinones [10,11]. The ideal design for chelators is to synthesize the hydroxypyridinones, which have excellent chelating efficacy and high selectivity of interaction, with special biological receptors in one molecular unity. The chelating capacity of bidentate hydroxypyridinones is usually inferior to that of hexadentate desferrioxamine [13,14]. Hexadentate hydroxypyridinones have a higher chelating ability than hexadentate desferrioxamine, but the poor absorption caused by their high molecular weight limits their application [15]. Thus, tetradentate hydroxypyridinones have been one of most extensively investigated compounds among heavy metal chelators [16]. Recent studies have reported that the tetradentate hydroxypyridinones exhibit better assays in vivo such as high chelating efficacy for Fe and Ga as well as excellent hydrophilic character [17,18,19,20]. However, few studies have examined the hydroxypyridinone chelating uranyl ions. In this paper, a series of new tetradentate hydroxypyridinone chelators is reported, and the binding affinities towards a uranyl cation (UO22+) were examined with UV spectrophotometry.
2. Results and Discussion
2.1. Synthesis
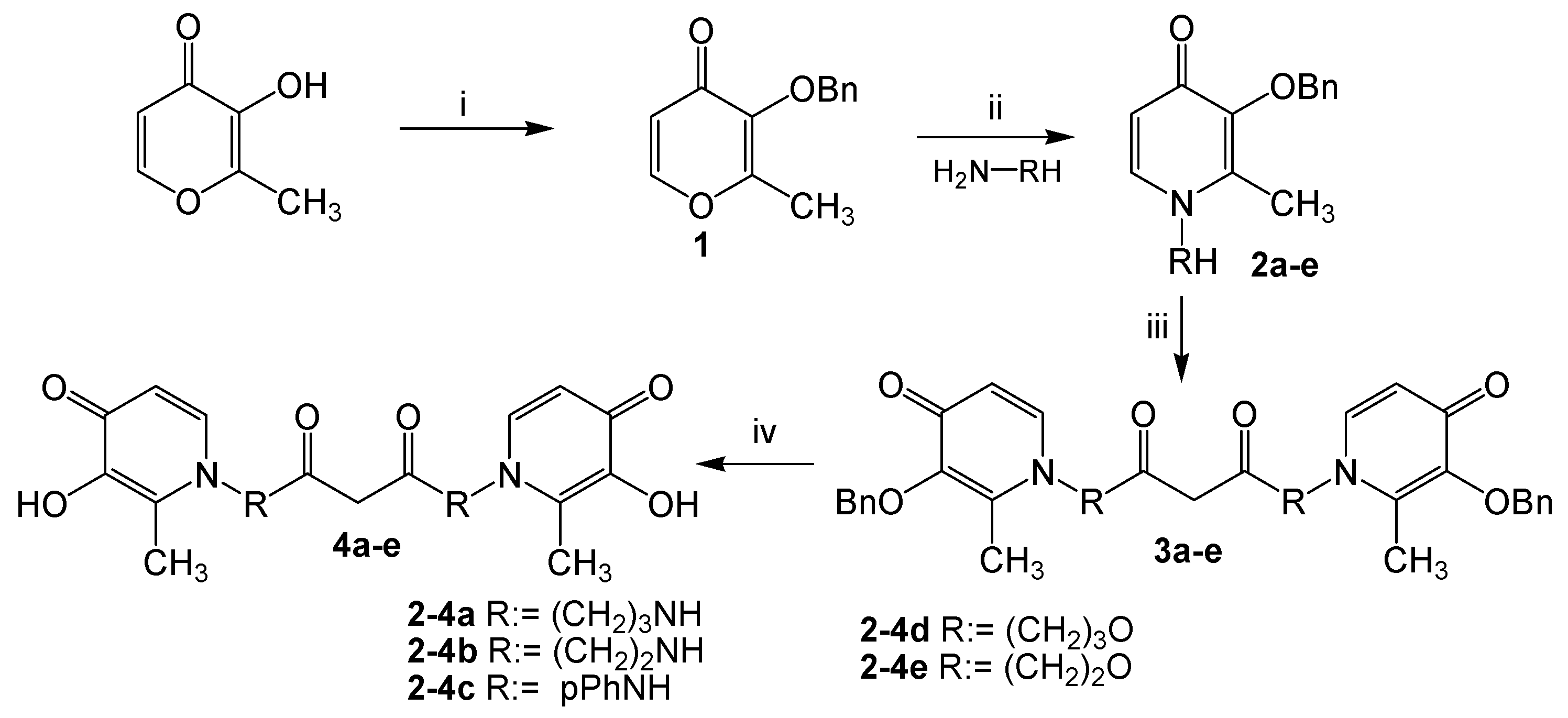
The synthesis of tetradentate hydroxypyridinones 4a–e is shown in Scheme 1. Starting from the commercially available 3-hydroxy-2-methyl-4-pyrone (maltol), the hydroxyl was protected with benzyl bromide and proceeded in excellent yield (93%) to produce 3-benzyloxy-2-methyl-4-pyrone 1. Then 3-Benzyloxy-2-methyl-4-pyrone 1 was directly condensed with diamines or ammonia alcohols to provide benzyloxy-pyridone derivatives 2a–e in 75%–89% yields. This method was similar to that described by Santos [18] but with minor modifications. In this study, for benzyloxy-pyridone 2a, compared with the method of Santos, the molar ratio of amines with pyrone 1 was increased from 1:1 to 3:1, and the corresponding yield of 2a evidently increased from 40% to 87%. The structures of benzyloxy-pyridones 2a–e contained a reactive hydroxyl or amino group, which could easily react with malonyl dichloride to yield the corresponding condensed products 3a–e. The malonyl dichloride is extremely active, so the reaction should be conducted in an ice bath and anhydrous conditions. Removal of benzyl from the oxygen of 3a–e catalytic hydrogenation conditions (14.5 psi H2, 10% Pd/C as catalyst) proceeded smoothly with good yield (71%–90%) to produce tetradentate hydroxypyridinones 4a–e.
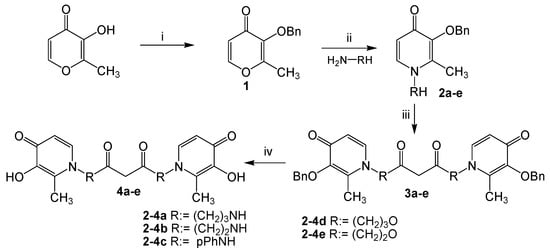
Scheme 1.
Synthesis for the ligands 4a–e. Reagents and conditions: (i) BnBr, CH3OH, 75 °C, reflux 6 h; (ii) EtOH/H2O, NaOH, reflux 3 h, 10 M HCl, until pH = 1, 10 M NaOH, pH = 11; (iii) CH2(COCl)2, dried CH2Cl2, 0 °C, 8 h; (iv) H2, Pd/C, CH3OH, 4 h.
2.2. Characterization
All products were purified and characterized by FT-IR, NMR, UV-vis and MS, and all characterizations were in accordance with the structures of the products.
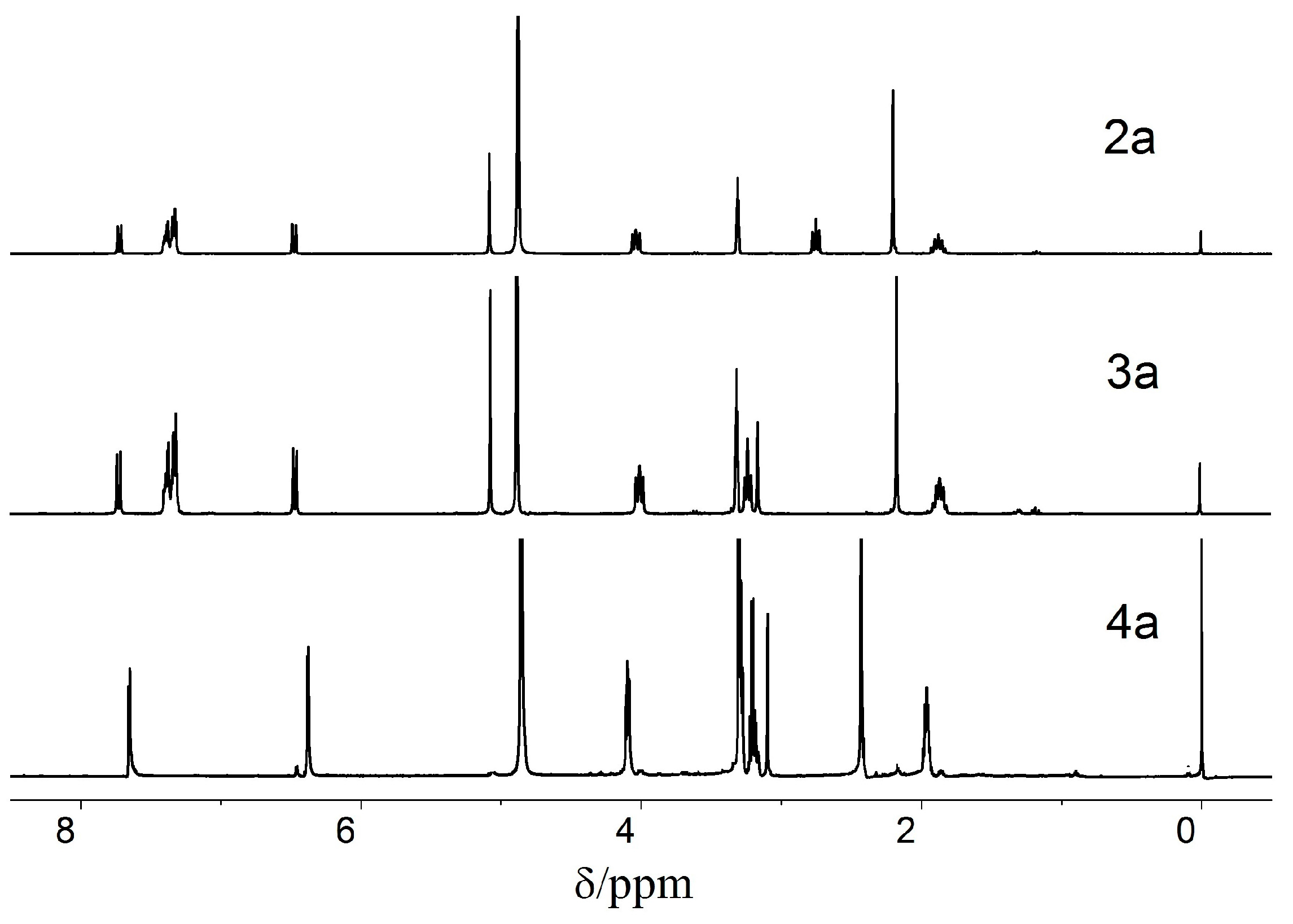
The 1H-NMR spectra of compounds 2–4a are shown in Figure 1. Compared with 2a, the signal of NHCH2CH2- in 3a shifted to the low chemical field (from 2.75 to 3.31) because of the electrophilic effect of the –CONH, and the singlet of the –COCH2CO– in 3a appeared at δ = 3.20 ppm. Compound 4a was obtained after the hydroxyls of 3a were deprotected. The peaks at 7.34 ppm of the benzene ring and 5.07 ppm of the methylene disappeared when the benzyl group was removed, and the signal of the CH3-Pys shifted from 2.16 ppm to 2.42 ppm.
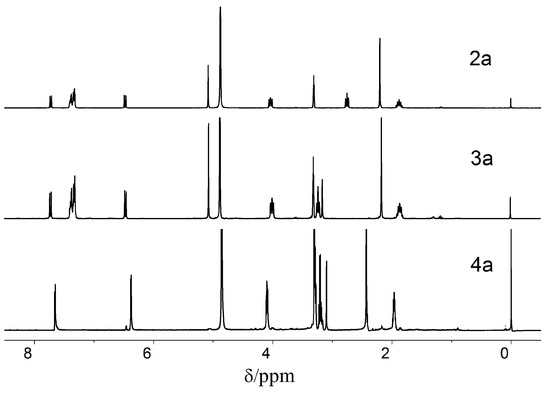
Figure 1.
The 1H-NMR spectra of 2a (CD3OD), 3a (CD3OD) and 4a (DMSO-d6).
The 1H-NMR spectra of 2b–e, 3b–e, and 4b–e have similar results. The 1H-NMR spectra of 4b and 4c exhibit two doublets at 7.6 ppm–7.7 ppm and 6.45 ppm–6.55 ppm with JAB = 7.5 Hz for the two nonequivalent protons in the pyridine ring. Compared with 4d and 4e, the signals of the pyridine ring protons appeared in the higher field because the oxygen atom is more electronegative than the nitrogen atom. The signals of the two nonequivalent protons in the pyridine rings of 4d and 4e appeared at 8.30 ppm–8.33 ppm (d, J = 7.2 Hz, 1H) and 7.23 ppm–7.25 ppm (d, J = 7.2 Hz, 1H), respectively. Additionally, the signals of –COCH2CO– in 4d and 4e appeared in a lower field than in 4b and 4c under the same condition. The singlet of –COCH2CO– in 4b and 4c appeared at 3.0 ppm–3.1 ppm, whereas that of 4d and 4e appeared at 3.6 ppm–3.8 ppm, respectively.
2.3. Complexation
Although present metal-complexation studies are focused on a set of three-charged hard metal ions [21,22] (e.g., Fe, Al, and Ga), the current study on decorporation [23] for UO22+ is based on the extreme damage to the environment caused by uranyl cations (UO22+) in biological systems. In this paper, the complexation behavior of tetradentate hydroxypyridinones 4a–e and the uranyl cation was evaluated by the spectrophotometric method [24,25].
We defined:
M = UO22+; L = ligands 4a–e
C = [M] + [L]; x = [M]/C
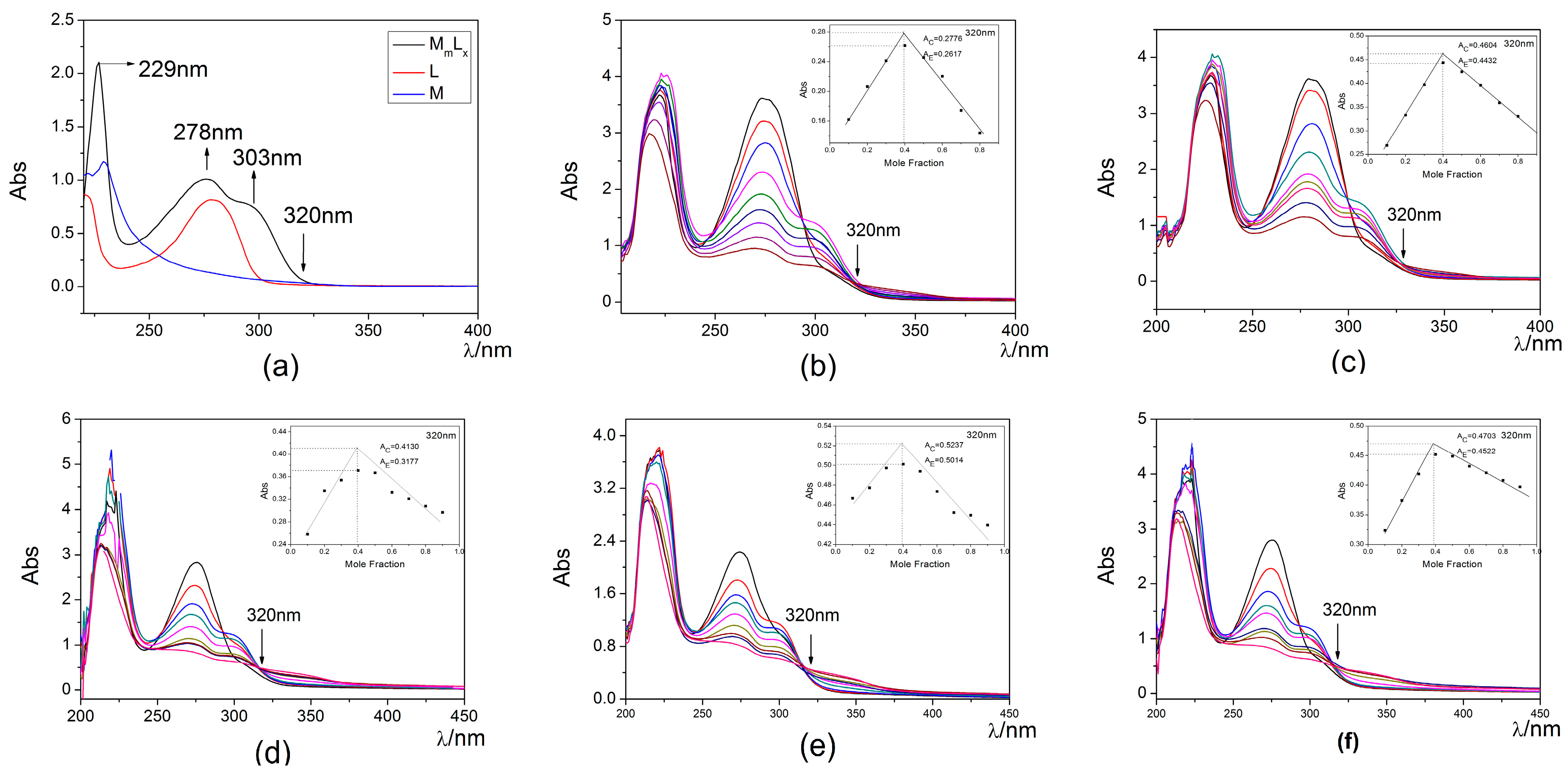
The coordination numbers (M:L) and the corresponding complex stability constants of the five tetradentate hydroxypyridinones 4a–e toward UO22+ at pH 7.4 were measured with the method of equivalent molarity. The UV-vis spectra of the various metal-to-ligand molar ratios for the UO22+-ligand 4a–e system at pH 7.4 are shown in Figure 2. UO22+ has an absorption peak at 229 nm, the peak at 278 nm was a characteristic absorption of ligand 4a, and a new peak at 303 nm appeared, which indicated that a new peak was the characteristic of the UO22+-ligand 4a complexation (Figure 2a). To construct the Job plot, the absorbance values were normalized according to the complex absorbance, and the experimental absorbance values of the UO22+-ligand 4a complexation at pH 7.4 are plotted against the mole fraction (Figure 2b). The result shows that the complex stoichiometry for the complexation of UO22+-ligand 4a at pH 7.4 is M/L = 2:3 (x = 0.4). This value indicates that the three ligand molecules could bridge two bis-chelated UO22+ centers under the effect of polar solvent molecules to complete their coordination sphere at pH 7.4.

Figure 2.
UV-vis absorption spectra of the various metal-to-ligand molar ratios for the UO22+-Ligand 4a–e system at pH 7.4: C = 4.00 × 10−4 M, T = 25 °C, I = 0.1 M KNO3: (a) The spectrophotometric absorption curves of the UO22+, ligand 4a and UO22+-ligand 4a; (b) UO22+-Ligand 4a system; (c) UO22+-Ligand 4b system; (d) UO22+-Ligand 4c system; (e) UO22+-Ligand 4d system; (f) UO22+-Ligand 4e system. All illustrations were obtained from the wavelength of 320 nm.
Then ligands 4a–e complex with the uranyl, and the formation constants (log K) for the ligands could be calculated as follows:
| 2 M | + | 3 L | = | M2L3 | |
| Initial concentration: | xC | (1 − x)C | 0 | ||
| Equilibrium concentration: | [M]E | [L]E | [M2L3]E | ||
| Complete reaction: | 0 | 0 | [M2L3]C |
Based on the Lambert-Beer law:
The complex stability constant of 4a and UO22+ at pH 7.4 was calculated by Equation (1), and the corresponding logKcondU-L 4a at 7.4 pH levels was 21.7. The corresponding complex stability constants of other ligands were also calculated by Equation (1), and the results are shown in Table 1.

Table 1.
Summary of the logKcond of ligand-UO22+ (pH = 7.4 ± 0.1).
Although the structures of Ligands 4a–e are similar, some consistent differences are apparent. The change of the linker length has a great influence on the their U(VI) chelation efficiency [26,27,28]. As the length of the linker increases, the angle formed between the uranium and two phenolic oxygen donors also becomes greater, and it leads to an increase of the strain in this complex. [29] As a result of the strain imposed by the linker, that two carbon atoms may be considered the optimal length is consistent with the high efficacy of ligands 4b and 4e for in vivo uranyl chelation. Ligands 4b and 4e exhibit the highest Kcond at pH 7.4, and the corresponding logKcond are 22.7 and 22.2. The modest reduction of body uranium in animals by the injection of bidentate Tiron (4,5-dihydroxy-1,3-benzenedisulfonic acid, disodium salt) and its U(VI)-catechol complex (log KML ) is 15.9 [29,30,31], which suggests the U(VI)-Ligands 4b complex and U(VI)-Ligands 4e complex Ligands 4b and 4e exhibit higher stability than the U(VI)-Tiron complex. Thus, Ligands 4b and 4e are most suitable for further studies.
3. Materials and Methods
3.1. General
The organic reagents used were pure commercial products from Aladdin. The solvents were purchased from Chengdu Kelong Chemical Reagents Co. (Sichuan, China). Anhydrous CH2Cl2 was distilled prior to use. The 300–400 mesh silica gel was purchased from Qingdao Hailang. The 1H-NMR, 13C-NMR spectra were recorded on Bruker Avance 300, Avance 400, or Avance 600 spectrometer (Carlsruhe, Germany). The FTIR spectra were obtained from Nicolet 380 FTIR spectrophotometer (Thermo Fisher Nicolet, Madison, WI, USA) with a resolution of 4 cm−1 from 400 cm−1 to 4000 cm−1. UV-vis spectrophotometer (Thermo Scientific Evolution 201, Waltham, MA, USA) used had a double-beam light source from 190 nm to 1100 nm. Mass spectral analysis was conducted using Varian 1200 LC/MS (Palo Alto, CA, USA).
3.2. Synthesis
Synthesis of 3-benzyloxy-2-methyl-4-pyrone (1). To a solution of 10.00 g 3-hydroxy-2-methyl-4-pyrone (79 mmol) containing the equivalent amount of NaOH (3.16 g, 79 mmol) in 100 mL methanol, 13 mL benzyl bromide (90 mmol) was dropwise added and then stirred for 6 h under reflux temperature. After cooling, the reaction mixture was evaporated under vacuum, and the residual oil was resolved in 50 mL dichloromethane and washed with 5% NaOH aqueous solution (5 × 30 mL) and water (3 × 50 mL). The organic solution was evaporated to dryness to obtain the pure product as pale oil (15.7 g, 93%). UV-vis (CH3OH): λmax = 219, 260 nm. FT-IR (KBr): ν = 3065, 3031, 2958, 2877, 1644, 1575, 1496, 1455, 1428, 1253, 1186, 1079, 973, 915, 832, 751, 703 cm−1. 1H NMR (300 MHz, CDCl3): δ =7.57 (d, J = 6.0 Hz, 1H), 7.25–7.40 (m, 5H), 6.33 (d, J = 6.0 Hz, 1H), 5.13 (s, 2H, CH2Ph), 2.06 (s, 3H, CH3) ppm. MS (APCI, CH3OH) m/z (%) = 217 (100) [M + H]+. C13H12O3 (216.3): calcd. C 72.22, H 5.56; found: C 72.43, H 5.47.
Synthesis of (3′-aminopropyl)-3-benzyloxy-2-methyl-4-pyridinone (2a). First 3 g 3-Benzyoxy-2-methyl-4-pyrone (13.9 mmol), 3.5 mL 1,3-diaminopropane (42.1 mmol) and 0.5 g NaOH were added in a EtOH–water mixture (20/15) mL, and then stirred at 75 °C for 3 h. After cooling, 2 M HCl was added until the pH = 1, and the reaction mixture was evaporated under vacuum. The residue was washed with acetone and dissolved in 20 mL water. Then the aqueous was basified with 10 M NaOH until pH = 12, and extracted with dichloromethane (5 × 30 mL). The organic solution was evaporated to dryness to obtain the faint yellow oil (3.5 g, 87%). UV-vis (CH3OH): λmax = 213, 277 nm. FT-IR (KBr ): ν = 3432 (N-H), 3063, 3030 (C-H, Ar), 2492, 2872 (C-H), 1622(C=O), 1550, 1250, 838, 752, 703 cm−1. 1H-NMR (300 MHz, CD3OD): δ =7.73 (d, J = 6.0 Hz, 1H), 7.30–7.42 (m, 5H), 6.47 (d, J = 7.4 Hz, 1H), 5.08 (s, 2H, CH2Ph), 4.03 (t, J = 7.5 Hz, 2H), 2.75 (t, J = 7.2 Hz, 2H, NH2CH2CH2), 2.19 (s, 3H, CH3), 1.87 (m, 2H, CH2CH2CH2) ppm. MS (APCI) m/z (%) = 273.0 (100) [M + H]+. C16H20O2N2 (272.1): calcd. C 70.59, H 7.35, N 10.29; found: C 70.61, H 7.37, N 10.24.
Synthesis of N,N′-bis(4-(3-benzyloxy-2-methyl-4-pyridinone)propyl)malonamide (3a). First 0.4 g (3′-aminopropyl)-3-benzyloxy-2-methyl-4-pyridinone (1.5 mmol) and 2 mL triethylamine were added in 50 mL dichloromethane, then mixture was stirred at 0 °C under N2. Then 75 μL malonyl dichloride was diluted with 20 mL dichloromethane and then dropwise added slowly and consecutively keeping the temperature at 0 °C for 8 h. The mixture was purified by chromatography on a silica-gel column with the methanol: chloroform = 1:6 as eluent, the pure product was white powder (0.29 g, 64%). UV-vis (CH3OH): λmax = 213, 279 nm. FT-IR (KBr): ν = 3226 (N-H), 3063 (C-H, Ar), 2935, 2879, 1654, 1624, 1559, 1518, 1249, 1249, 1218, 1160, 1036, 975, 833, 750, 703 cm−1. 1H-NMR (300 MHz, CD3OD): δ = 7.72 (d, J = 7.2 Hz, 2H), 7.29–7.41 (m, 10H), 6.48 (d, J = 7.2 Hz, 2H), 5.07 (s, 4H, CH2Ph), 4.00 (t, J = 7.5 Hz, 4H, NHCH2CH2CH2), 3.31 (t, J = 6.0 Hz, 4H, NHCH2CH2CH2), 3.20 (s, 2H, COCH2CO), 2.16 (s, 6H, CH3), 1.87 (m, 4H, CH2CH2CH2) ppm. MS (APCI) m/z (%) = 613 (100) [M + H]+. C35H40O6N4 (612): calcd. C 68.63, H 6.54, N 9.15; found: C 68.42, H 6.83, N 9.51.
Synthesis of N,N′-bis(4-(3-hydroxy-2-methyl-4-pyridinone)propyl)malonamide (4a). First 40 mg 10% Pd/C was added in a solution of N,N′-bis(4-(3-benzyloxy-2-methyl-4-pyridinone)propyl)malonamide (0.2 g, 3.7 mmol) in methanol (50 mL), then the mixture was stirred under H2 (1 atm) for 4 h at room temperature. After filtration, the solvent was evaporated under reduced pressure and the product was obtained as the white powder (0.13 g, 89%). UV-vis (CH3OH): λmax = 279 nm. FT-IR (KBr): ν = 3069, 2930, 1656 (C=OCH2), 1627 (PyC=O), 1561, 1509, 1251 (C=ONH2), 1035, 832 cm−1. 1H-NMR (600 MHz, DMSO-d6): δ =7.64 (d, J = 7.2 Hz, 2H), 6.48 (d, J = 7.2 Hz, 2H), 4.10 (t, J = 7.5 Hz, 4H, NHCH2CH2CH2), 3.31 (t, J = 6.0 Hz, 4H, NHCH2CH2CH2), 3.21 (s, 2H, COCH2CO), 2.42 (s, 6H, CH3), 1.96 (m, 4H, CH2CH2CH2) ppm. 13C-NMR (150 MHz, DMSO-d6): δ = 169.19, 168.48, 145.95, 137.55, 131.33, 128.01, 51.43, 46.52, 35.98, 30.06, 10.46 ppm. MS (APCI) m/z (%)= 217 (100) [M + 2H]+. C21H28O6N4 (432): calcd. C 58.33, H 6.48, N 12.96; found: C 57.97, H 6.51, N 12.85.
Synthesis of (3′-aminoethyl)-3-benzyloxy-2-methyl-4-pyridinone (2b). First 3 g 3-benzyoxy-2-methyl-4-pyrone (13.9 mmol) and 3.3 mL ethanediamine (49.3 mmol) were added in a EtOH–water mixture (20/15) mL, then 0.5 g NaOH was added and stirred at 70 °C for 4 h. After cooling, 2 M HCl was added until the pH = 1 and the solvent was evaporated. The residue was washed with acetone and dissolved in 20 mL water. The obtained solution was basified with 10 M NaOH until pH ≈ 12 and extracted with dichloromethane (5 × 30 mL). The organic phase was evaporated and obtained the flavescent oil (2.84 g, 74%). UV-vis (CH3OH): λmax = 213, 277 nm. FT-IR (KBr): ν = 3433, 1591, 1490, 1384, 1332, 1103, 824, 592 cm−1. 1H-NMR (300 MHz, CD3OD): δ = 7.67 (d, J = 7.5 Hz, 1H), 7.30–7.42 (m, 5H), 6.46 (d, J = 7.5 Hz, 1H), 5.07 (s, 2H, CH2Ph), 3.96 (t, J = 7.5 Hz, 2H, NH2CH2CH2), 2.84 (t, J = 7.2 Hz, 2H, NH2CH2CH2), 2.17 (s, 3H, CH3) ppm. MS(APCI) m/z (%) = 259 (100) [M + H]+. C15H18O2N2 (258.1): calcd. C 69.77, H 6.98, N 10.85; found: C 70.23, H 7.17, N 10.49.
Synthesis of N,N′-bis(4-(3-benzyloxy-2-methyl-4-pyridinone)ethyl)malonamide (3b). First 0.4 g (3′-aminoethyl)-3-benzyloxy-2-methyl-4-pyridinone (1.5 mmol) and 2 mL triethylamine were added in 50 mL dichloromethane, then mixture was stirred at 0 °C under N2. Then 75 μL malonyl dichloride was diluted with 20 mL dichloromethane and then dropwise added slowly and consecutively keeping the temperature at 0 °C for 8 h. The mixture was purified by chromatography on a silica-gel column with the methanol: chloroform = 1:6 as eluent, and the pure product was white powder (0.26 g, 58%). UV-vis (CH3OH) λmax = 213, 279 nm. FT-IR (KBr): ν = 2987, 1961, 1676, 1623, 1548, 1507, 1395, 1252, 1164, 1034, 880, 835, 752, 703, 613 cm−1. 1H-NMR (300 MHz, CD3OD): δ = 7.62 (d, J = 7.5 Hz, 2H), 7.28–7.44 (m, 10H), 6.44 (d, J = 7.5 Hz, 2H), 5.04 (s, 4H, CH2Ph), 4.07 (t, J = 6.0 Hz, 4H, NHCH2CH2), 3.45 (t, J = 6.0 Hz, 4H, NHCH2CH2), 3.08 (s, 2H, COCH2CO), 2.16 (s, 6H, CH3). MS (APCI) m/z (%) = 585 (100) [M + H]+. C33H36N6O4 (584): calcd. C 67.81, H 6.16, N 9.56; found: C 67.55, H 5.87, N 9.38.
Synthesis of N,N′-bis(4-(3-hydroxy-2-methyl-4-pyridinone)ethyl)malonamide (4b). First 40 mg 10% Pd/C was added in a solution of N,N′-bis(4-(3-benzyloxy-2-methyl-4-pyridinone)ethyl)malonamide 3b (0.2 g, 3.7 mmol) in methanol (50 mL), then the mixture was stirred under H2 (1 atm) for 4 h at room temperature. After filtration, the solvent was evaporated under reduced pressure and the product was obtained as the white powder (0.12 g, 83%). UV-vis (CH3OH): λmax = 279 nm. FT-IR (KBr): ν = 3429, 3069, 2093, 1654, 1627, 1561, 1509, 1353, 1251, 1035, 831 cm−1. 1H-NMR (600 MHz, CD3OD): δ = 7.62 (d, J = 7.5 Hz, 2H), 6.46 (d, J = 7.5 Hz, 2H), 4.07 (t, J = 6.0 Hz, 4H, NHCH2CH2), 3.45 (t, J = 6.0 Hz, 4H, NHCH2CH2), 3.08 (2H, s, COCH2CO), 2.16(6H, s, CH3) ppm. MS (APCI) m/z (%) = 405 (100) [M + H]+. C19H24O6N4 (404): calcd. C 56.44, H 5.94, N 13.86; found: C 56.38, H 6.25, N 13.74.
Synthesis of N-(4-aminophenyl)-3-benzyloxy-2-methyl-4-pyridinone (2c). First 4.6 g 3-benzyoxy-2-methyl-4-pyrone (21.3 mmol) and 6.9 g p-phenylenediamine (63.9 mmol) were added in a mixture solvent of 40 mL EtOH and 20 mL water, then 0.5 g NaOH was added and stirred at 90 °C for 8 h. After cooling, 2 M HCl was added until the pH = 1 and the solvent was evaporated. The residue was washed with acetone and dissolved in 20 mL water. The obtained solution was basified with 10 M NaOH until pH = 12 and extracted with dichloromethane (5 × 30 mL). The organic phase was evaporated under reduced pressure and the residue was dissolved in 10 mL ethanol. Then 200 mL diethyl ether was added to afford the light yellow rude product, which was purified by chromatography on a silica gel column with a mixture of methanol: chloroform = 1:20 as eluent to afford a white powder (6.25 g, 69%). UV-vis (CH3OH): λmax = 207, 250, 279 nm. FT-IR(KBr): ν = 3434, 3347 (N-H), 3232 (C-H), 2961 (C-H), 1619 (C=O), 1567, 1511, 1479, 1358, 1281, 1162, 953, 843, 764, 703 cm−1. 1H-NMR (300 MHz, CDCl3): δ = 7.44 (d, J = 6.8 Hz, 2H, 7.28–7.36 (m, 3H), 7.23 (d, J = 6.8 Hz, 1H), 6.94 (d, J = 8.5 Hz, 2H), 6.66 (d, J = 8.5 Hz, 2H), 6.41 (d, J = 6.8 Hz, 1H), 5.24 (s, 2H, CH2Ph), 1.82 (t, J = 7.5 Hz, 3H, CH3). MS (ESI) m/z (%) = 307 (100) [M + H]+. C19H18O2N2 (306): calcd. C 74.51, H 5.88, N 9.15; found: C 74.32, H 5.92, N 9.13.
Synthesis of N,N′-bis(4-(3-benzyloxy-2-methyl-4-pyridinone)phenyl)malonamide (3c). First 1.6 g N-(4-aminophenyl)-3-benzyloxy-2-methyl-4-pyridinone 2c was stirred at 0 °C under N2. Then 300 μL malonyl dichloride was diluted with 20 mL dichloromethane and then dropwise added slowly and consecutively keeping the temperature at 0 °C for 8 h. The mixture was purified by chromatography on a silica-gel column with the methanol:chloroform = 1:8 as eluent, and the pure product was faint yellow powder (1.5 g, 86%). UV-vis (CH3OH): λmax = 208, 251, 281 nm. FT-IR (KBr): ν = 3247, 3189, 3056, 2926, 1697, 1625, 1546, 1508, 1409, 1341, 1287, 1172, 1004, 988, 834, 751, 701 cm−1. 1H-NMR (300 MHz, CD3OD): δ = 7.64 (d, J = 8.5 Hz, 4H), 7.22–7.40 (m, 2H), 7.03 (d, J = 8.5 Hz, 4H), 6.66 (d, J = 7.5 Hz, 4H), 6.42 (d, J = 7.5 Hz, 2H), 5.08 (s, 4H, CH2Ph), 3.45 (s, 2H, COCH2CO), 1.71 (t, 6H, CH3) ppm. MS (ESI) m/z (%) = 681 (100) [M + H]+. C41H36O6N4 (680): calcd. C 72.35, H 5.29, N 8.24; found: C 72.24, H 5.35, N 8.20.
Synthesis of N,N′-bis(4-(3-hydroxy-2-methyl-4-pyridinone)phenyl)malonamide (4c). First 50 mg 10% Pd/C was added in a solution of N,N′-bis(4-(3-benzyloxy-2-methyl-4-pyridinone)phenyl)malonamide 3c (0.23 g, 3.38 mmol) in methanol (50 mL), then the mixture was stirred under H2 (1 atm) for 4 h at room temperature. After filtration, the solvent was evaporated under reduced pressure and the crude product was obtained as the white powder, which was recrystallized from methanol/diethyl ether (0.15 g, 90%). UV-vis (CH3OH): λmax = 209, 251, 292 nm. FT-IR (KBr): ν = 3245 (N-H), 3121, 3063, 2928 (C-H), 1693 (HNC=O), 1626(PyC=O), 1605, 1538, 1505, 1411, 1297, 1243, 1116, 1041, 834 cm−1. 1H-NMR (600 MHz, CD3OD): δ = 7.87 (d, J = 7.8 Hz, 4H), 7.64 (d, J = 7.2 Hz, 2H), 7.38 (d, J = 8.5 Hz, 4H), 6.53 (d, J = 7.2 Hz, 2H), 3.63 (s, 2H, COCH2CO), 2.15 (s, 6H, CH3) ppm. 13C-NMR (150 MHz, CD3OD): δ = 165.70, 161.32, 143.03, 139.64, 137.69, 138.63, 136.65, 125.81, 119.98, 109.70, 29.39, 12.41 ppm. MS (ESI) m/z (%) = 523 (100) [M + Na]+. C27H24O6N4 (500): calcd. C 64.80; H 4.80, N 11.20; found: C 64.47, H 4.96, N 10.96.
Synthesis of (3′-hydroxypropyl)-3-benzyloxy-2-methyl-4-pyridinone (2d). First 3 g 3-benzyoxy-2-methyl-4-pyrone (13.9 mmol), 3.5 mL 1,3-propanol amine (45.8 mmol) and 0.5 g NaOH were added in a EtOH–water mixture (20/15) mL, and then stirred at 70 °C for 6 h. After cooling, 2 M HCl was added until the pH = 1 and the reaction solution was evaporated under vacuum. The residue was washed with acetone and dissolved in 20 mL water. The obtained solution was basified with 10 M NaOH until pH = 10 and extracted with dichloromethane (5 × 30 mL). The organic phase was evaporated and obtained the snow white powder (2.9 g, 75%). UV-vis (CH3OH): λmax = 216, 274 nm. FT-IR (KBr): ν = 3336, 1632, 1522, 1494, 1419, 1352, 1263, 1159, 1157, 1084, 1035, 960, 842, 754, 706 cm−1. 1H-NMR (300 MHz, CD3OD): δ = 7.62 (d, J = 7.4 Hz, 1H), 7.32–7.46 (5H, m), 6.46 (d, J = 7.4 Hz, 1H), 5.06 (s, 2H, CH2Ph), 4.13 (t, J = 7.1 Hz, 2H, HOCH2CH2), 4.03 (t, J = 7.1 Hz, 2H, HOCH2CH2CH2), 2.13 (s, 3H, CH3), 2.00 (t, J = 6.2 Hz, 2H, HOCH2CH2CH2) ppm. MS (APCI) m/z (%) = 274 (100) [M + H]+. C16H19O3N (273): calcd. C 70.33,H 6.96, N 5.13; found: C 70.08, H 6.78, N 5.26.
Synthesis of N,N′-bis(4-(3-benzyloxy-2-methyl-4-pyridinone)hydroxypropyl)malonicester (3d). First 0.5 g (3′-hydroxypropyl)-3-benzyloxy-2-methyl-4-pyridinone (1.83 mmol) and 2 mL triethylamine were added in 50 mL dichloromethane, then mixture was stirred at 0 °C under N2. Then 100 μL malonyl dichloride was diluted with 20 mL dichloromethane and then dropwise added slowly and consecutively keeping the temperature at 0 °C for 6 h. The mixture was purified by chromatography on a silica-gel column with the methanol:chloroform =1:8 as eluent, and the pure product was faint yellow oil (0.2 g, 37%). UV-vis (CH3OH): λmax = 218, 274 nm. FT-IR (KBr): ν = 2925, 1742, 1647, 1494, 1384, 1263, 1159, 1083, 1035, 842, 753, 706 cm−1. 1H-NMR (300 MHz, CD3OD): δ = 7.18–7.36 (m, 12 H), 6.35 (d, J = 7.4 Hz, 2H), 5.08 (s, 4 H, CH2Ph), 4.06 (d, J = 4.5 Hz, 4H, OCH2CH2CH2), 3.83 (t, J = 6.6 Hz, 2H, OCH2CH2CH2), 3.36 (s, 2H, COCH2CO), 2.02 (s, 6H, CH3), 1.85–1.95 (m, 4H, OCH2CH2CH2) ppm. MS (APCI) m/z (%) = 308 (100) [M + 2 H]+. C35H38O8N2 (614): calcd. C 68.40, H 6.19, N 4.55; found: C 68.28, H 6.42, N 4.37.
Synthesis of N,N′-Bis(4-(3-hydroxy-2-methyl-4-pyridinone)-hydroxyethyl)-malonic ester (4d). First 40 mg 10% Pd/C was added in a solution of N,N′-bis(4-(3-hydroxy-2-methyl-4-pyridinone)hydroxyethyl)-malonic ester (0.22 g, 0.34 mmol) in methanol (50 mL), then the mixture was stirred under H2 (1 atm) for 6 h at room temperature. After filtration, the solvent was evaporated under reduced pressure and the crude product was obtained as the white powder (0.11 g, 71%). UV-vis (CH3OH): λmax = 284 nm. FT-IR (KBr) ν/cm−1: 3399, 2965, 2426, 1731, 1632, 1508, 1383, 1255, 1162, 1032, 827. 1H-NMR (600 MHz, CD3OD): δ = 7.99 (d, J = 7.2 Hz, 2H), 6.88 (d, J = 7.2 Hz, 2H), 4.39 (t, J = 4.5 Hz, 4H, OCH2CH2CH2), 4.22 (t, J = 6.6 Hz, 4H, OCH2CH2CH2), 3.67 (s, 2H, COCH2CO), 2.17 (s, 6H, CH3), 2.00–2.20 (m, 4H, OCH2CH2CH2); MS (APCI) m/z (%) = 435 (100) [M + H]+. C21H26O8N2 (434): calcd. C 58.06, H 5.99, N 6.45; found: C 58.13, H 6.07, N 6.21.
Synthesis of (3′-Hydroxyethyl)-3-benzyloxy-2-methyl-4-pyridinone (2e). First 4.3 g 3-benzyoxy-2-methyl-4-pyrone (19.9 mmol), 3.3 mL 2-aminoethanol (49.3 mmol) and 0.5 g NaOH were added in a mixture solvent of 20 mL EtOH and 15 mL water mixture, and then stirred at 70 °C for 6 h. After cooling, 2 M HCl was added until the pH = 1 and the reaction solution was evaporated under vacuum. The residue was washed with acetone and dissolved in 20 mL water. The obtained solution was basified with 10 M NaOH until pH = 10 and extracted with dichloromethane (5 × 30 mL). The organic phase was evaporated and obtained the snow white powder (4.6 g, 89%). UV-vis (CH3OH): λmax = 215, 270 nm. FT-IR (KBr): ν = 3335, 1636 (C=O), 1523, 1492, 1342, 1295, 1271, 1157, 1079, 1075, 1032, 969, 831, 768, 708 cm−1. 1H-NMR (300 MHz, CD3OD): δ = 8.32 (1 H, d, J = 7.2 Hz), 7.36–7.44 (m, 5H), 7.23 (d, J = 7.2 Hz, 1H), 5.17 (s, 2H, CH2Ph), 4.47 (t, J = 7.5 Hz, 2H, HOCH2CH2), 3.89 (t, J = 7.5 Hz, 2H, HOCH2CH2), 2.51 (s, 3H, CH3) ppm. MS (APCI) m/z (%)= 260 (100) [M + H]+. C15H17O3N (259): calcd. C 69.50, H 6.56, N 5.41; found: C 69.84, H 6.72, N 5.38.
Synthesis of N,N′-bis(4-(3-benzyloxy-2-methyl-4-pyridinone)-Hydroxyethyl)-malonic ester (3e). First 0.5 g (3′-hydroxyethyl)-3-benzyloxy-2-methyl-4-pyridinone (1.9 mmol) and 2 mL triethylamine were added in 50 mL dichloromethane, then mixture was stirred at 0 °C under N2. Then 100 μL malonyl dichloride was diluted with 20 mL dichloromethane and then dropwise added slowly and consecutively keeping the temperature at 0 °C for 6 h. The mixture was purified by chromatography on a silica-gel column with the methanol: chloroform=1:8 as eluent, and the pure product was faint yellow oil (0.12 g, 35%). UV/vis (CH3OH): λmax = 220, 273 nm. FT-IR (KBr): ν = 2921, 2851, 1748, 1625, 1561, 1512, 1251, 1230, 1108, 1064, 825, 737, 701 cm−1. 1H-NMR (400 MHz, CD3OD): δ = 8.32 (d, J = 7.2 Hz, 2H), 7.36–7.44 (m, 10H), 7.26 (d, J = 7.2 Hz, 2H), 5.17 (s, 4H, CH2Ph), 4.47 (t, J = 7.8 Hz, 2H, OCH2CH2), 3.89 (t, J = 7.8 Hz, 2H, OCH2CH2), 3.69 (s, 2H, COCH2CO), 2.51(s, 3H, CH3). MS (APCI) m/z (%) = 587 (100) [M + H]+. C33H34O8N2 (586): calcd. C 67.58,; H 5.80, N 4.78; found: C 67.66, H 6.01, N 4.64.
Synthesis of N,N′-Bis(4-(3-hydroxy-2-methyl-4-pyridinone)-hydroxyethyl)-malonic ester (4e). First 40 mg 10% Pd/C was added in a solution of N,N′-bis(4-(3-hydroxy-2-methyl-4-pyridinone)-hydroxyethyl)-malonic ester (0.19 g, 0.34 mmol) in methanol (50 mL), then the mixture was stirred under H2 (1 atm) for 6 h at room temperature. After filtration, the solvent was evaporated under reduced pressure and the crude product was obtained as the white powder, which was recrystallized from methanol/diethyl ether (0.10 g, 76%). UV-vis (CH3OH) λmax = 281 nm. FT-IR (KBr): ν = 3367, 2963, 1736, 1628, 1567, 1508, 1460, 1384, 1352, 1251, 1150, 1110, 1026, 831, 599 cm−1. 1H-NMR (600 MHz, CD3OD): δ = 8.31 (d, J = 7.2 Hz, 2H), 7.25 (d, J = 7.2 Hz, 2H), 4.38 (t, J = 7.8 Hz, 2H, OCH2CH2), 3.91 (t, J = 7.8 Hz, 2H, OCH2CH2), 3.67 (s, 2H, COCH2CO), 2.49 (s, 3H, CH3). MS (APCI) m/z (%) = 407 (100) [M + H]+. C19H22O8N2 (406): calcd. C 56.16, H 5.42, N 6.90; found: C 56.32, H 5.63, N 7.02.
3.3. Metal Complexation Solutions
In all the complexation studies in aqueous solution, the water was distilled three times and the atmospheric CO2 was excluded from the system with a purging steam of N2 under 80 °C. The UO2(NO3)2·6H2O is analytical grade, the buffered solution were NaAc/HAc (pH = 5.5) and Tris-HCl (pH = 7.4, 9.0). All the ligands were synthesized and dissolved into the water, except the solvent of the ligand 4c was DMSO:H2O = 1:9 to ensure all the ligands was dissolved. The ligands concentration (CL) was 2.0 × 10−3 mol/L and the UO22+ (CM) concentration was 2.0 × 10−3 mol/L, then added the solutions into the 10 mL comparison tubes by different volume, the total concentration (CL + CM) was 2.0 × 10−3 mol/L for all complexation samples.
4. Conclusions
In summary, five new ligands of bis(3-hydroxy-4-pyridinone) tetradentate ligands were synthesized and characterized. The stability constants determined in this study provide evidence for the extremely high affinities of the ligands for UO22+ complexes. The evident difference in logKcond, compared with the similar structural ligands, emphasized the superior affinity for UO22+ of the 4b ligand. At pH 7.4, the 4b ligand shows a high constant of 22.7, confirming this compound as an effective chelator for UO22+.
Acknowledgments
The authors are thankful for financial support from the National Natural Science Foundation of China (Project No. 21301142), Applied Basic Research Program of Sichuan Province (2014JY0170), Open Project of State Key Laboratory Cultivation Base for Nonmetal Composites and Functional (project no. 14zdfk05), Major Project of the Education Department of Sichuan Province (project no. 13ZA0172), Southwest University of Science and Technology Outstanding Youth Foundation (project no. 13zx9107).
Author Contributions
Bo Jin and Rufang Peng conceived and designed the experiments; Rongzong Zheng performed the experiments; Shijin Chu analyzed the data; Rongzong Zheng and Bo Jin wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hamilton, J.G. The metabolic properties of the fission products and actinide elements. Rev. Mod. Phys. 1948, 20, 718–728. [Google Scholar] [CrossRef]
- Brugge, D.J.; Lemos, L.D.; Oldmixon, B. Exposure pathways and health effects associated with chemical and radiological toxicity of natural uranium: A review. Rev. Environ. Health 2005, 20, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Morss, L.R.; Edelstein, N.M.; Fuger, J.; Katz, J.J. The Chemistry of the Actinide and Transactinide Elements; Springer: Dordrecht, The Netherlands, 2006; pp. 3339–3440. [Google Scholar]
- Guzmán, L.; Durán-Lara, E.F.; Donoso, W.; Nachtigall, F.M.; Santos, L.S. In Vivo Nanodetoxication for acute uranium exposure. Molecules 2015, 20, 11017–11033. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Bruenger, F.W.; Miller, S.C.; Afri, A.M. Molecular structure and biological and pharmacological properties of 3-hydroxy-2-methyl-1-(β-d-ribofuranosyl or pyranosyl)-4-pyridinone: Potential iron overload drugs for oral administration. Bio. Med. Chem. Lett. 1998, 8, 3007–3080. [Google Scholar] [CrossRef]
- Santos, M.A.; Gil, M.; Marques, S.; Gano, L.; Cantinho, G.J. N-Carboxyalkyl derivatives of 3-hydroxy-4-pyridinones: Synthesis, complexation with Fe(III), Al(III) and Ga(III) and in vivo evaluation. J. Inorg. Biochem. 2002, 92, 43–54. [Google Scholar] [CrossRef]
- Santos, M.A.; Gama, S.; Gano, L.; Farkas, E. Bis(3-hydroxy-4-pyridinone)-EDTA derivative as a potential therapeutic Al-chelating agent. Synthesis, solution studies and biological assays. J. Inorg. Biochem. 2005, 99, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Grazina, R.; Buglyo, P.; Gama, S.; Farkas, E. A new bipodal carboxy-bis (hydroxypyridinonate) ligand. Synthesis and complexation with copper(II), nicker(II) and zinc(II) in aqueous solution. Polyhedron 2002, 21, 2609–2616. [Google Scholar]
- Leite, A.; Silva, A.M.G.; Nunes, A.; Andrade, M.; Sousa, C.; Silva, L.C.; Gameiro, P.; Castro, B.D.; Rangel, M. Novel tetradentate chelators derived from 3-hydroxy-4-pyridinone units: synthesis, characterization and aqueous solution properties. Tetrahedron 2011, 67, 4009–4016. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, Z.D.; Neubert, H.; Kong, X.L.; Ma, Y.M.; Hider, R.C. High affinity iron(III) scavenging by a novel hexadentate 3-hydroxypyridin-4-one-based dendrimer: Synthesis and characterization. Bioorg. Med. Chem. Lett. 2005, 15, 5007–5011. [Google Scholar] [CrossRef] [PubMed]
- Grazina, R.; Gano, L.; Šebestík, J.; Santos, M.A. New tripodal hydroxypyridinone based chelating agents for Fe(III), Al(III) and Ga(III): Synthesis, physico-chemical properties and bioevaluation. J. Inorg. Biochem. 2009, 103, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Salaheldin, A.M.; Al-Sheikh, M.A. β-Enamino esters in heterocyclic synthesis: Synthesis of pyrazolone and pyridinone derivatives. Molecules 2010, 15, 4359–4368. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Kong, X.L.; Zhou, T.; Qiu, D.H.; Chen, Y.L.; Li, M.S. Synthesis, iron(III)-binding affinity and in vitro evaluation of 3-hydroxypyridin-4-one hexadentate ligands as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2011, 21, 6376–6380. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Marques, S.M.; Chaves, S. Hydroxypyridinones as privileged chelating structures for the design of medicinal drugs. Coord. Chem. Rev. 2012, 256, 240–259. [Google Scholar] [CrossRef]
- Crisponi, G.; Remeli, M. Iron chleating agent for the treatment of iron overload. Coord. Chem. Rev. 2008, 252, 1225–1240. [Google Scholar] [CrossRef]
- Santos, M.A. Recent development on 3-hydroxy-4-pyridinones with respect to their clinical applications Mono and combined ligand approaches. Coord. Chem. Rev. 2008, 252, 1213–1224. [Google Scholar] [CrossRef]
- Gama, S.; Gil, M.; Gano, L.; Farkas, E.; Santos, M.A. Combined chelation of bi-functional bis-hydroxypiridinone and mono-hydroxypiridinone: Synthesis, solution and in vivo evaluation. J. Inorg. Biochem. 2009, 103, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Gama, S.; Gano, L.; Cantinho, G.; Frakas, E. A new bis(3-hydroxy-4-pyridinone)-IDA derrivatives as a potential therapeutic chelating agent. Synthesis,metal-complexation and biological assays. Dalton Trans. 2004, 21, 3772–3781. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Neubert, H.; Liu, D.Y.; Liu, Z.D.; Ma, Y.M.; Kong, X.L.; Luo, W. Iron dendrimers: A novel approach for the treatment of Haemochromatosis. J. Med. Chem. 2006, 49, 4171–4182. [Google Scholar] [CrossRef] [PubMed]
- Leydier, A.; Lecerclé, D.; Rostaing, S.P.; Reguillon, A.F.; Taran, F.; Lemaire, M. Sequestering agent for uranyl chelation: New binaphtyl ligands. Tetrahedron Lett. 2011, 52, 3973–3977. [Google Scholar] [CrossRef]
- Hider, R.C.; Roy, S.; Ma, Y.M.; Kong, X.L.; Preston, J. The potential application of iron chelators for the treatment of neurodegenerative disease. Metallomics 2011, 3, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Grazina, R.; Neto, A.Q.; Cantinho, G.; Gano, L.; Patrício, L. Synthesis, chelating poperties towards gallium and biological evaluation of two N-substituted 3-hydroxy-4-pyridinones. J. Inorg. Biochem. 2000, 78, 303–311. [Google Scholar] [CrossRef]
- Leydier, A.; Lecerclé, D.; Rostaing, S.P.; Réguillona, A.F.; Taran, F.; Lemairea, M. Sequestering agents for uranyl chelation: New calixarene ligands. Tetrahedron 2008, 64, 11319–11324. [Google Scholar] [CrossRef]
- Hoehne, M.S.; Deblonde, G.J.P.; Abergel, R.J. Solution thermodynamic evaluation of hydroxypyridinonate chelators 3,4,3-LI(1,2-HOPO) and 5-LIO(Me-3,2-HOPO) for UO2(VI) and Th(IV) decorporation. Radiochim. Acta 2013, 101, 359–366. [Google Scholar] [CrossRef]
- Szigethy, G.; Raymond, K.N. Hexadentate Terephthalamide (bis-hydroxypyridinone) ligands for uranyl chelation: Structural and thermodynamic consequences of ligand variation. J. Am. Chem. Soc. 2011, 133, 7942–7956. [Google Scholar] [CrossRef] [PubMed]
- Chaves, S.; Capelo, A.; Areias, L.; Marpues, S.M.; Gano, L.; Esteves, M.A.; Santos, M.A. A novel tripodal tris-hydroxypyrimidinone sequestering agent for trivalent hard metal ions: Synthesis, complexation and in vivo studies. Dalton Trans. 2013, 42, 6033–6045. [Google Scholar] [CrossRef] [PubMed]
- Bartholomä, M.D. Recent developments in the design of bifunctional chelators for metal-based radiopharmaceuticals used in positron emission tomography. Inorg. Chim. Acta 2012, 389, 36–51. [Google Scholar] [CrossRef]
- Chaves, S.; Marques, S.M.; André, M.F. New tris(hydroxypyridinones) as iron and aluminium sequestering agents: Synthesis, complexation and in vivo studies. Chem. Eur. J. 2010, 16, 10535–10545. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.D.; Raymond, K.N. Uranyl sequestering agents: Correlation of properties and efficacy with structure for UO22+ complexes of linear tetradentate 1-methyl-3-hydroxy-2(1H)-pyridinone ligands. Inorg. Chem. 1999, 38, 308–315. [Google Scholar] [CrossRef]
- Domingo, J.L.; Ortega, A.; Llobet, J.M.; Paternain, J.L.; Corbella, J. The effects of uranium on reproduction, gestation, and postnatal survival in mice. Res. Commun. Pathol. Pharmacol. 1989, 64, 161–164. [Google Scholar]
- Stradling, G.N.; Gray, S.A.; Moody, J.C.; Ellender, M. Efficacy of Tiron for Enhancing the Excretion of Uranium from the Rat. Hum. Exp. Toxicol. 1991, 10, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: not availiable.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).