Methanolic Extract of Pien Tze Huang Induces Apoptosis Signaling in Human Osteosarcoma MG63 Cells via Multiple Pathways
Abstract
:1. Introduction
2. Results
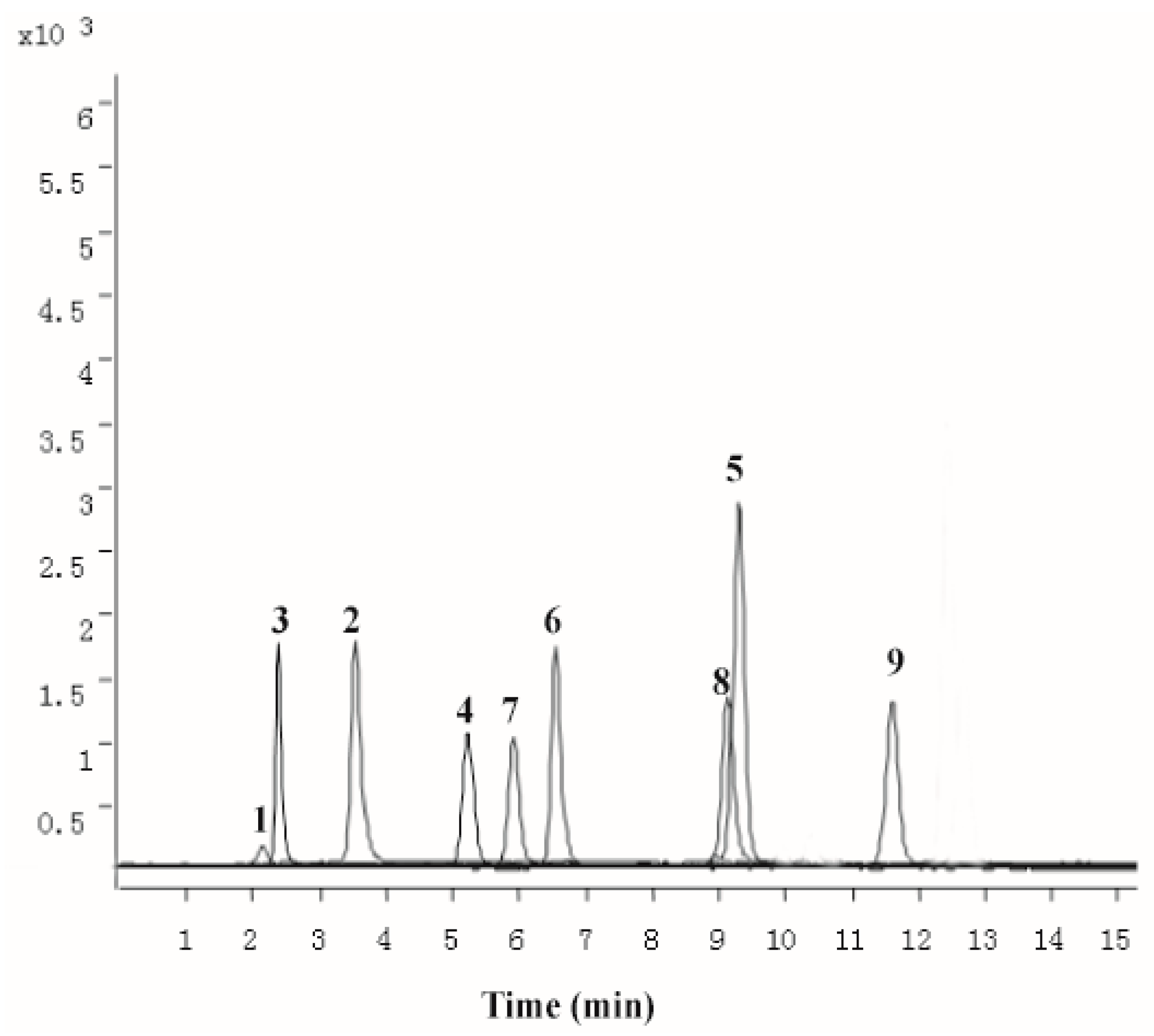
2.1. Chemical Characterization of PZH
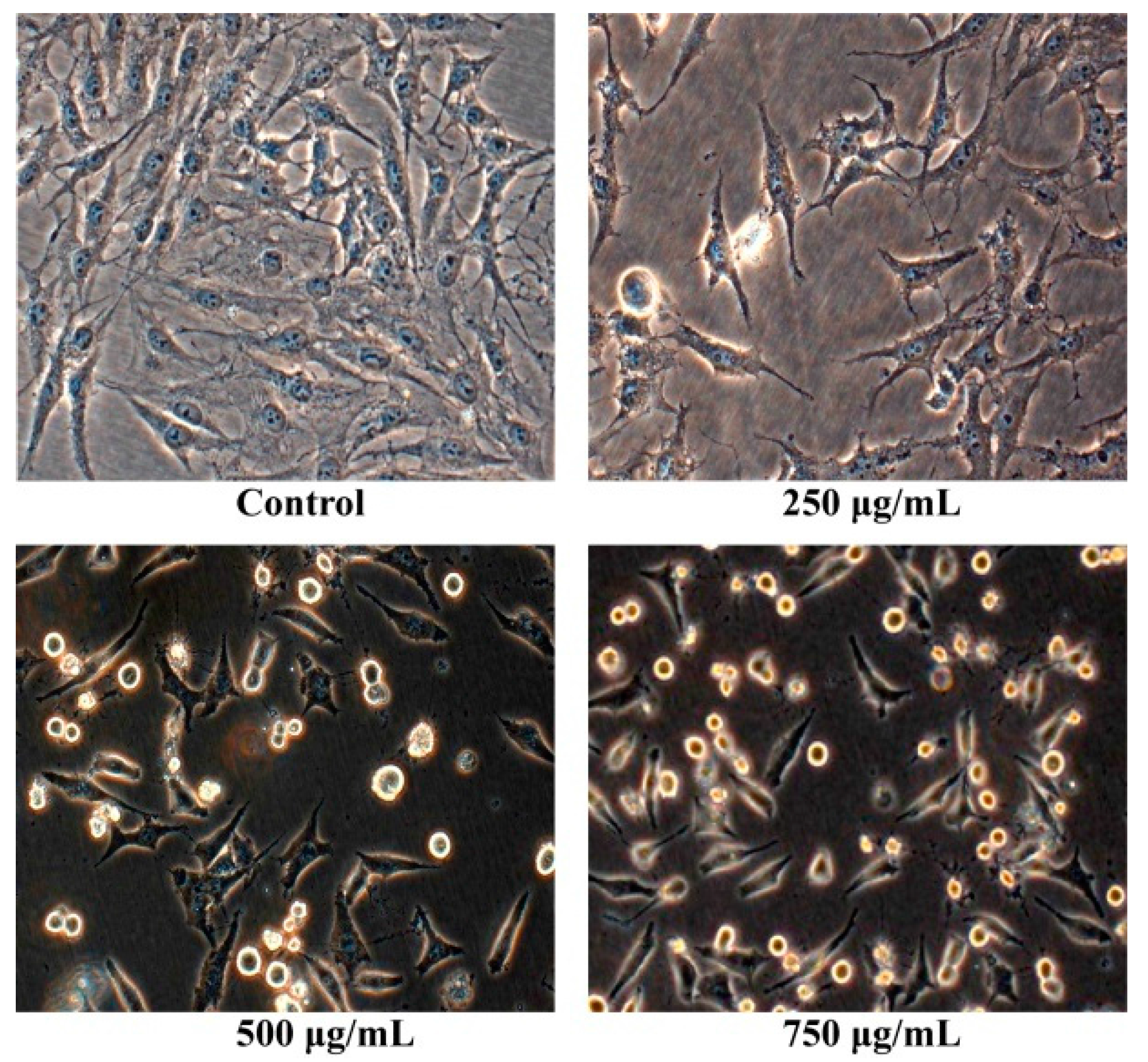
2.2. Morphology Observation
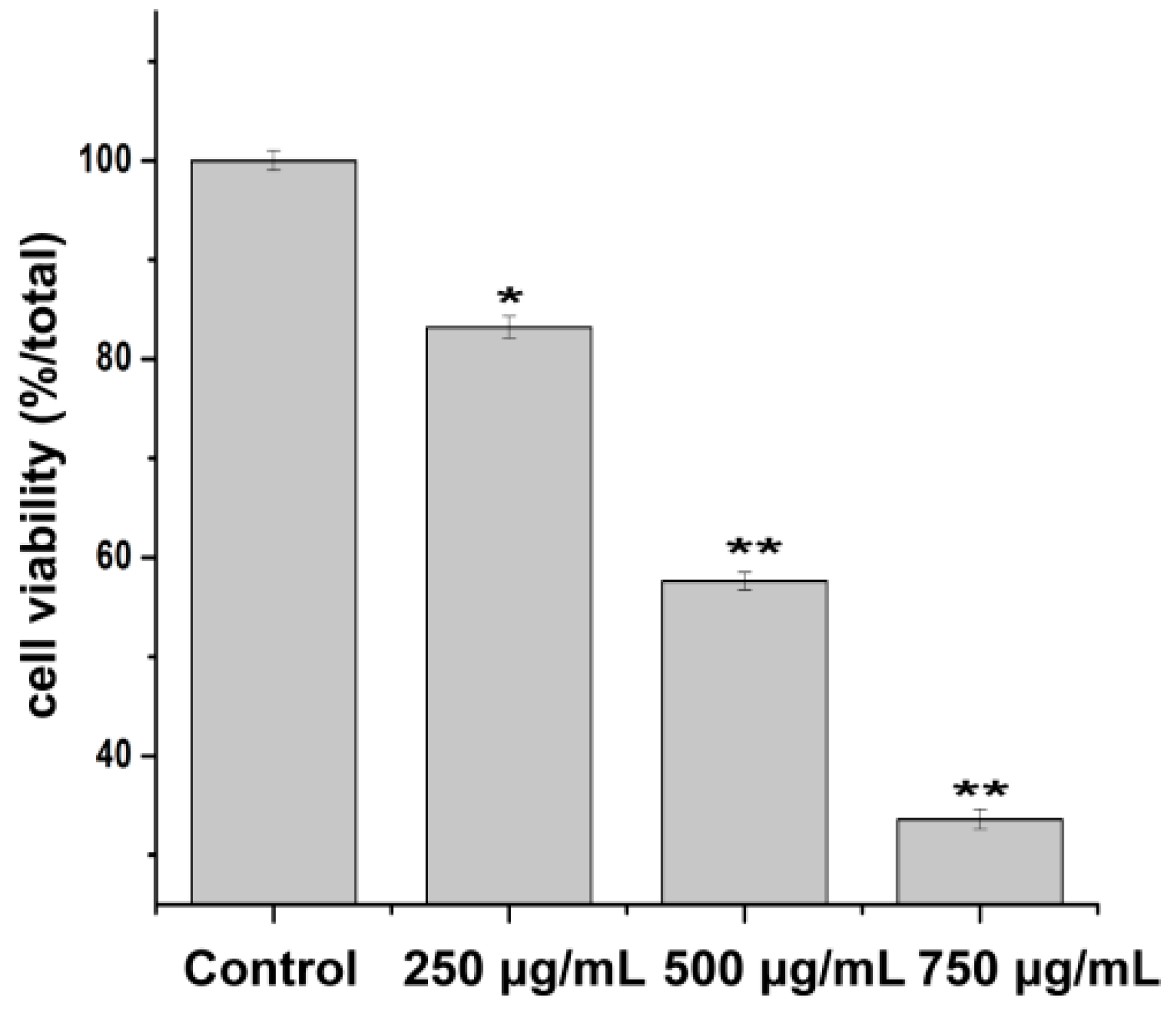
2.3. Effect of PZH on Cells Proliferation
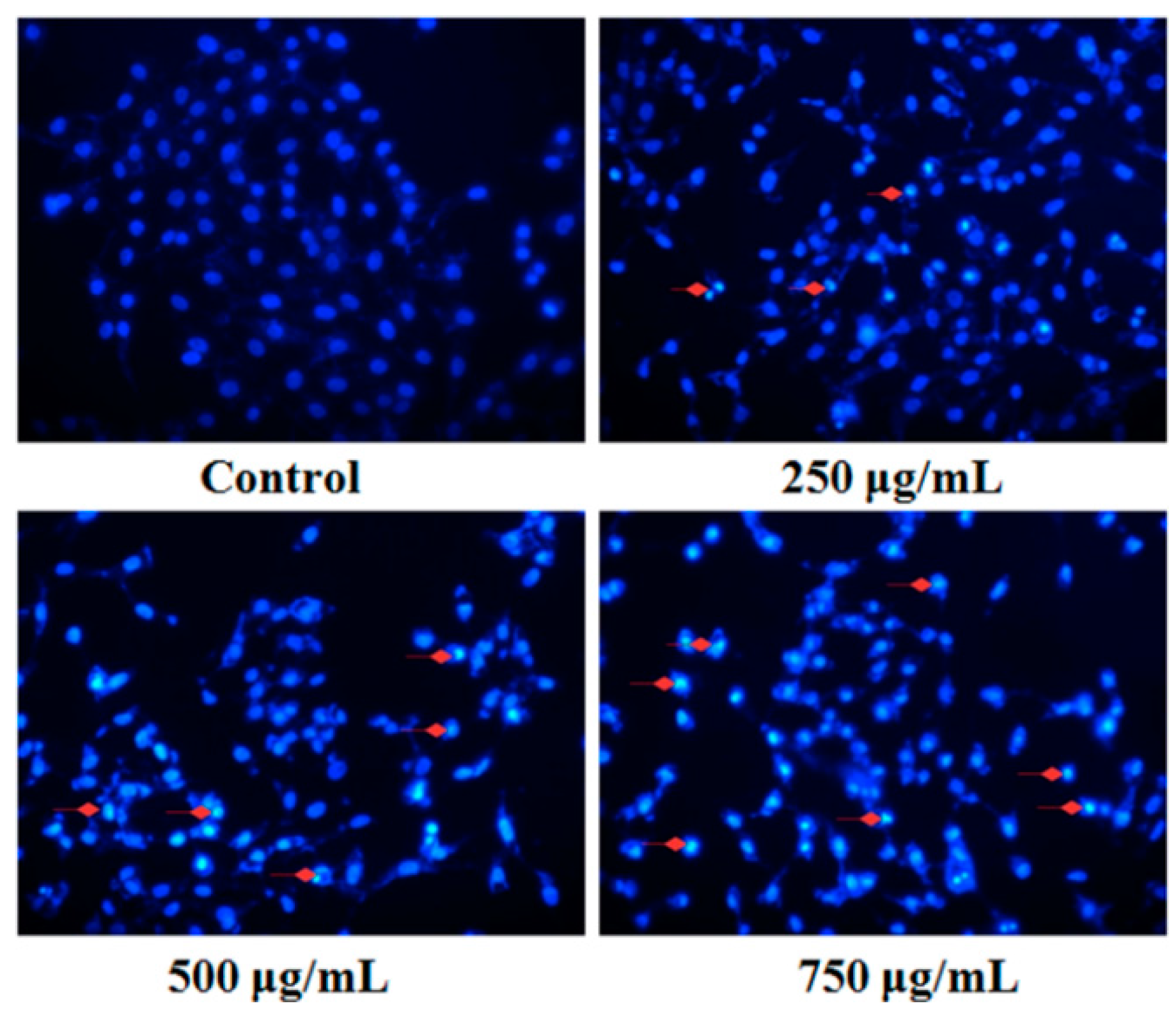
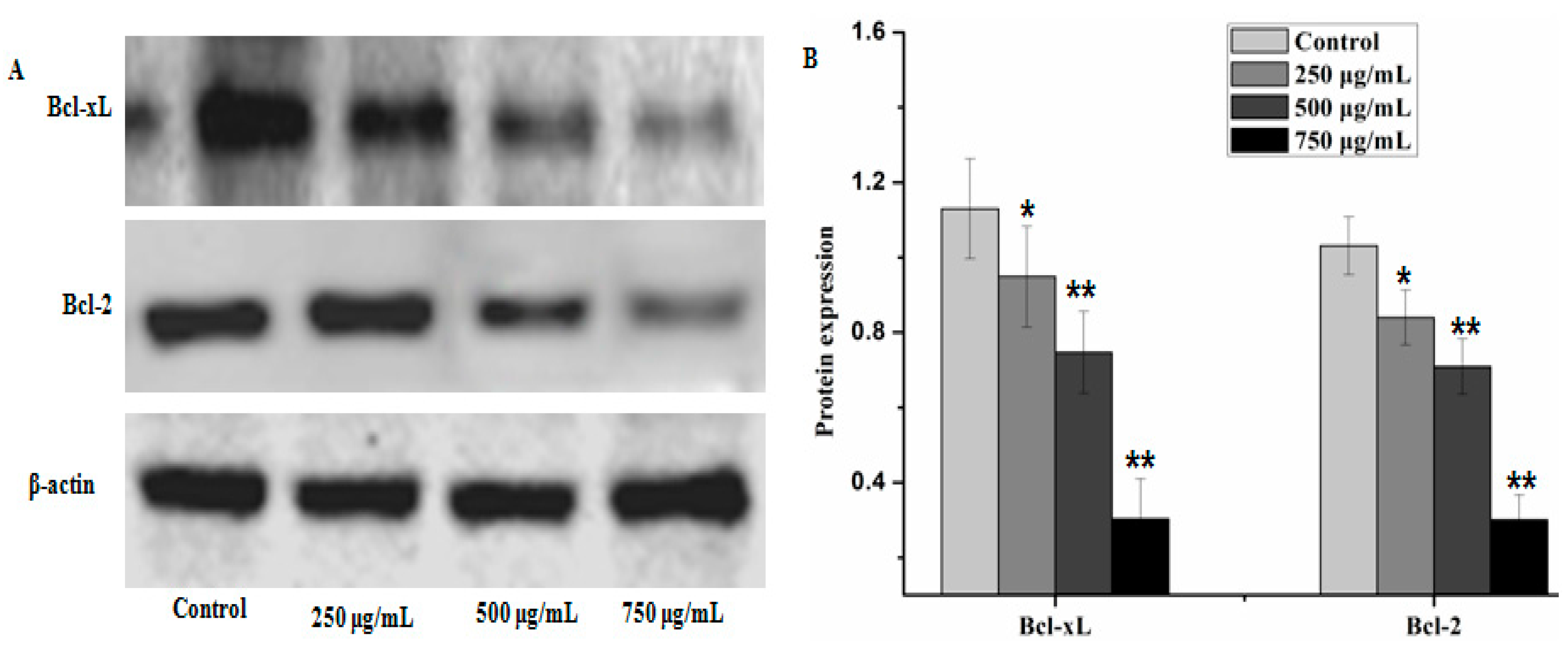
2.4. Effect of PZH on Cells Apoptosis
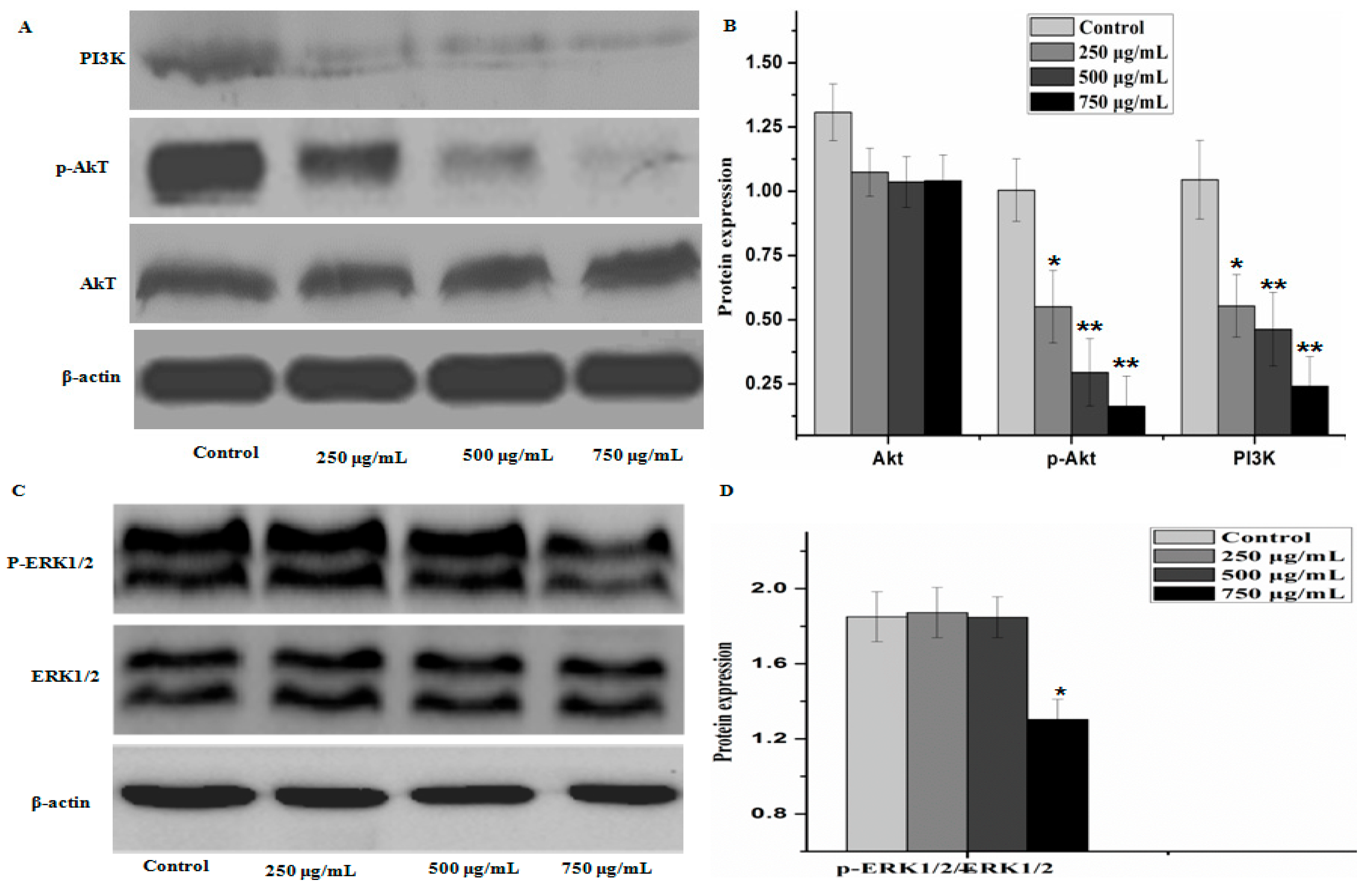
2.5. Effect of PZH on Protein Expression of PI3K/Akt Signal Pathway
3. Discussion
4. Materials and Methods
4.1. Chemical Characterization of PZH
4.2. Cell Culture and Treatment
4.3. Cell Viability Assay
4.4. Cells Apoptosis
4.4.1. Fluorescent Staining
4.4.2. DNA Fragmentation Analysis
4.5. Western-Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kappler, M.; Bache, M.; Bartel, F. Knockdown of survivin expressionby small interfering RNA reduces the clonogenic survival of human sarcoma cell lines independently of p53. Cancer Gene Ther. 2004, 11, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009, 152, 3–13. [Google Scholar] [PubMed]
- Marina, N.; Gebhardt, M.; Teot, L.; Gorlick, R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004, 9, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.L.; Liu, G.H. Progress of osteosarcoma therapy. Orthop. J. China 2009, 17, 1158–1169. [Google Scholar]
- Cagliero, E.; Ferracini, R.; Morello, E.; Scotlandi, K.; Manara, M.C.; Buracco, P.; Comandone, A.; Baroetto Parisi, R.; Baldini, N. Reversal of multidrug resistance using valspodar(PSC833) and doxorubicin in osteosarcoma. Oncol. Rep. 2004, 5, 1023–1031. [Google Scholar]
- Serra, M.; Scotlandi, K.; Reverter-Branchat, G.; Ferrari, S.; Manara, M.C.; Benini, S.; Incaprera, M.; Bertoni, F.; Mercuri, M.; Briccoli, A.; et al. Value of p-glycopretein and Clinicopathologic factor as the basis for new treatment strategies in high grand osteosarcoma of the extremities. J. Clin. Oncol. 2003, 3, 536–542. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the Peoples Republic of China, 2010 ed.; Chinese Medical Science and Technology Press: Beijing, China, 2010; pp. 573–575. [Google Scholar]
- Zhang, Y.; Wang, Q.H.; Zang, L. Effects of Pien Tze Huang on the Cell cycle of Human Osteocarcom U2OS Cells. J. Jiangxi Coll. Trad. Chin. Med. 2012, 24, 19–22. [Google Scholar]
- Zhang, L.; Zeng, Q.Q.; Lin, J.H. Inhibitory effect of human P27KIP1 gene AVV virus combining with Chinese herb Pien Tze Huang on human osteosarcoma transplant mice model. China J. Tradit. Chin. Med. Pharm. 2009, 24, 511–515. [Google Scholar]
- Fu, Y.; Yang, D.H.; Zhang, L. Effects of Pien Tze Huang on the migration and invasion of osteosarcoma MG63 cell. J. Chin. Med. 2013, 28, 1577–1580. [Google Scholar]
- Guan, J.Y.; Zhang, L. Effects of Pien Tze Huang on MMP-9 expression of osteosarcoma U2OS cell. Fujian J. TCM 2011, 42, 48–50. [Google Scholar]
- Zhang, L.; Yu, B.; Lin, J.H. Apoptosis induction of traditional Chinese herb Pien Tze Huang in human osteosarcoma U2OS cells. China J. Orthop. Trauma 2009, 22, 265–268. [Google Scholar]
- Kong, W.; Jin, C.; Xiao, X.; Zhao, Y.; Liu, W.; Li, Z.; Zhang, P. Determination of multicomponent contents in Calculus bovis by ultra-performance liquid chromatography-evaporative light scattering detection and its application for quality control. J. Sep. Sci. 2010, 33, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B. Pharmacological activity of sanchi ginseng (Panax notoginseng). J. Pharm. Pharmacol. 2006, 58, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.Q.; Zhang, M.; Zhou, Q.; Shi, Q.; Wang, Y.J. Muscone protects vertebral end-plate degeneration by antiinflammatory property. Clin. Orthop. Relat. Res. 2010, 468, 1600–1610. [Google Scholar]
- Wei, G.; Chen, D.F.; Lai, X.P.; Liu, D.H.; Deng, R.D.; Zhou, J.H.; Zhang, S.X.; Li, Y.W.; Li, H.; Zhang, Q.D. Muscone exerts neuroprotection in an experimental model of stroke via inhibition of the fas pathway. Nat. Prod. Commun. 2012, 7, 1069–1074. [Google Scholar] [PubMed]
- Ishizaki, K.; Kinbara, S.; Miyazawa, N.; Takeuchi, Y.; Hirabayashi, N.; Kasai, H.; Araki, T. Effect of sodium tauroursodeoxycholate (UR-906) on liver dysfunction in bile duct-ligated rats. Eur. J. Pharmacol. 1997, 333, 207–213. [Google Scholar] [CrossRef]
- Dufer, M.; Horth, K.; Wagner, R.; Schittenhelm, B.; Prowald, S.; Wagner, T.F.; Oberwinkler, J.; Lukowski, R.; Gonzalez, F.J.; Krippeit-Drews, P.; et al. Bile acids acutely stimulate insulin secretion of mouse β-cells via farnesoid X receptor activation and K(ATP) channel inhibition. Diabetes 2012, 61, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Q.; Shi, A.P.; Fan, Z.M. Apoptosis of T-47D Cells Induced by Cinobufacini via a Caspase-3-dependent Manner. Chem. Res. Chin. Univ. 2014, 30, 108–113. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. Apoptosomes: Engines for caspase activation. Curr. Opin. Cell Biol. 2002, 14, 715–720. [Google Scholar] [CrossRef]
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 2001, 276, 7320–7326. [Google Scholar] [CrossRef] [PubMed]
- Reddig, P.J.; Juliano, R.L. Clinging to life: Cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005, 24, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Goodyer, C.; LeBlanc, A. Selective and protracted apoptosis in human primary neurons microinjection with active caspase-3,-6,-7 and-8. J. Neuresci. 2000, 20, 8384–8389. [Google Scholar]
- Adams, J.M.; Cory, S. The Bcl-2 protein family: Arbiters of cell survival. Science 1998, 281, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Oltvai, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Clem, R.J.; Cheng, E.H.; Karp, C.L.; Kirsch, D.G.; Ueno, K.; Takahashi, A.; Kastan, M.B.; Griffin, D.E.; Earnshaw, W.C.; Veliuona, M.A.; et al. Modulation of cell death by Bcl-XL through caspase interaction. Proc. Natl. Acad. Sci. USA 1993, 95, 554–559. [Google Scholar] [CrossRef]
- Or, Y.Y.; Hui, A.B.; Tam, K.Y.; Huang, D.P.; Lo, K.W. Characterization of chromosome 3q and 12q amplicons in nasopharyngeal carcinoma cell lines. Int. J. Oncol. 2005, 26, 49–56. [Google Scholar] [CrossRef]
- Nyakem, M.; Tazzari, P.L.; Finelli, C.; Bosi, C.; Follo, M.Y.; Grafone, T.; Piccaluga, P.P.; Martinelli, G.; Cocco, L.; Martelli, A.M. Frequent elevation of Akt kinaae phosphorylation in blood marrow and peripheral blood mononuclear ceils from high-risk myelodysplastic syndrome patients. Leukemia 2006, 20, 230–238. [Google Scholar]
- Yuan, Z.Q.; Sun, M.; Feldman, R.I.; Wang, G.; Ma, X.; Jiang, C.; Coppola, D.; Nicosia, S.V.; Cheng, J.Q. Frequent activation of AKT2 and induction of apoptosis by inhibition of phosphoin0sitide-3-OH kinase/Akt pathway in human ovarian cancer. Oncogene 2000, 19, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
- Dicholson, K.M.; Anderson, N.G. The protein kinase B/Akt signaling pathy in human maliganacy. Cell Signal 2002, 14, 381–395. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, L.P.; Guo, Y.F.; Li, Z.; MO, S.L. Wogonin Downregulates P-Weel and P-Akt Expression in RPMI8226 Cells to Inhibit Cell Proliferation. J. Trop. Med. 2012, 12, 1301–1303. [Google Scholar]
- Datta, S.R.; Brunet, A.; Grcenberg, M.E. Cellular survival: A play in three Akts. Genes Dev. 1999, 13, 2905–2927. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Q.; Zhao, H.Y.; Xu, W.; Chu, K.D.; Hong, Z.F.; Peng, J.; Chen, L.D. Rapid simultaneous determination of twelve major components in Pien Tze Huang by ultra-performance liquid chromatography coupled with triple quadrupole mass spectrometry. J. Sep. Sci. 2013, 36, 3866–3873. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chu, K.D.; Li, H.; Huang, M.; Zheng, H.; Sha, M.; Zhang, X.; Chen, L. Bauhinia championii Extraction Treatment of Collagen-Induced Arthritis via Downregulation of the Expression of TLR4, MyD88 and NF-κB. Am. J. Chin. Med. 2013, 41, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Zhang, L.; Hong, Z.; Zheng, H.; Li, N.; Gao, H.; Chen, B.; Zhao, Y. Methanolic Extract of Pien Tze Huang Induces Apoptosis Signaling in Human Osteosarcoma MG63 Cells via Multiple Pathways. Molecules 2016, 21, 283. https://doi.org/10.3390/molecules21030283
Fu Y, Zhang L, Hong Z, Zheng H, Li N, Gao H, Chen B, Zhao Y. Methanolic Extract of Pien Tze Huang Induces Apoptosis Signaling in Human Osteosarcoma MG63 Cells via Multiple Pathways. Molecules. 2016; 21(3):283. https://doi.org/10.3390/molecules21030283
Chicago/Turabian StyleFu, Yong, Li Zhang, Zhenqiang Hong, Haiyin Zheng, Nan Li, Hongjian Gao, Boyi Chen, and Yi Zhao. 2016. "Methanolic Extract of Pien Tze Huang Induces Apoptosis Signaling in Human Osteosarcoma MG63 Cells via Multiple Pathways" Molecules 21, no. 3: 283. https://doi.org/10.3390/molecules21030283
APA StyleFu, Y., Zhang, L., Hong, Z., Zheng, H., Li, N., Gao, H., Chen, B., & Zhao, Y. (2016). Methanolic Extract of Pien Tze Huang Induces Apoptosis Signaling in Human Osteosarcoma MG63 Cells via Multiple Pathways. Molecules, 21(3), 283. https://doi.org/10.3390/molecules21030283




