Three New Sesquiterpenoids and One New Sesquiterpenoid Derivative from Chinese Eaglewood
Abstract
:1. Introduction
2. Results and Discussion
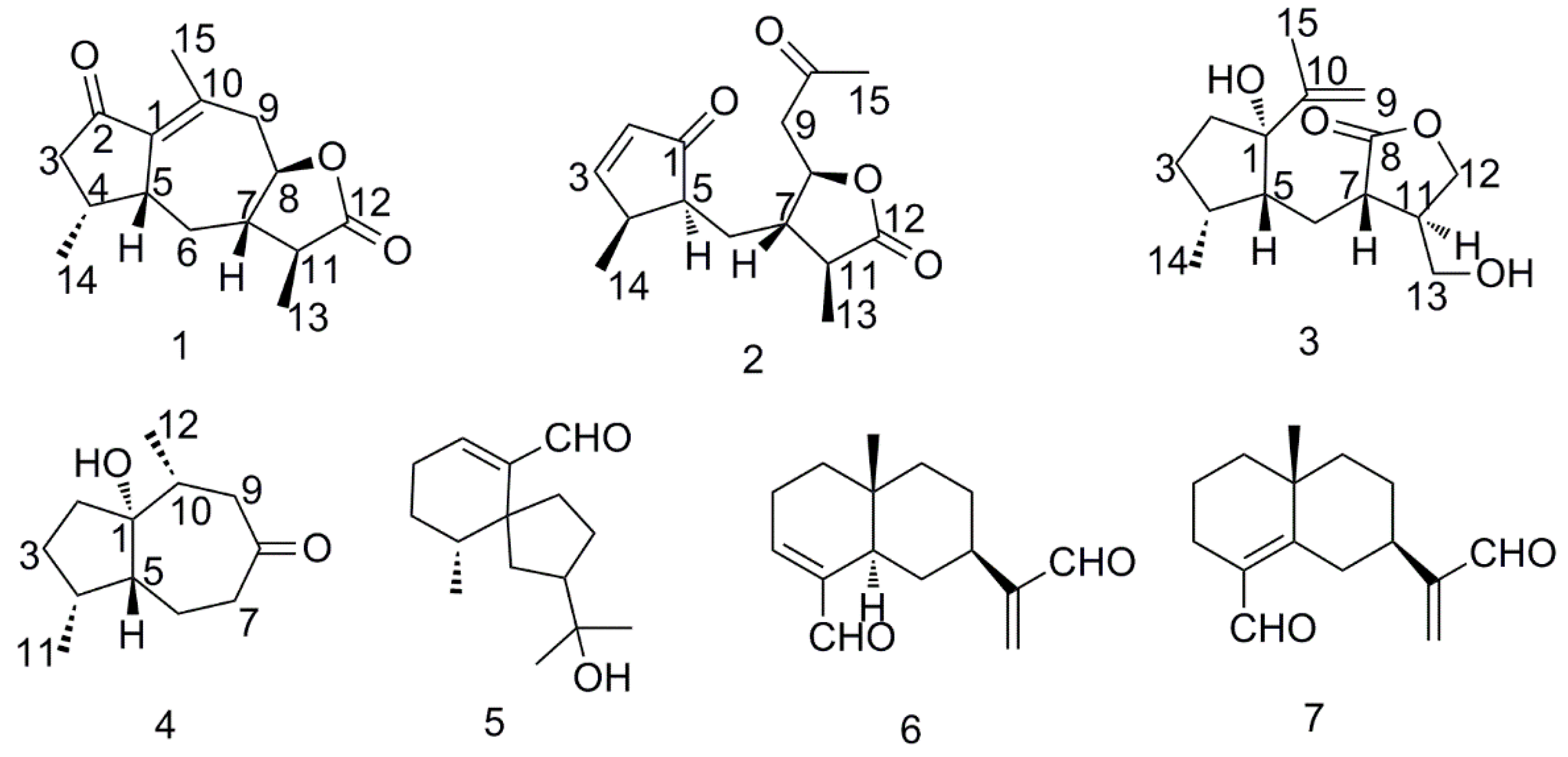
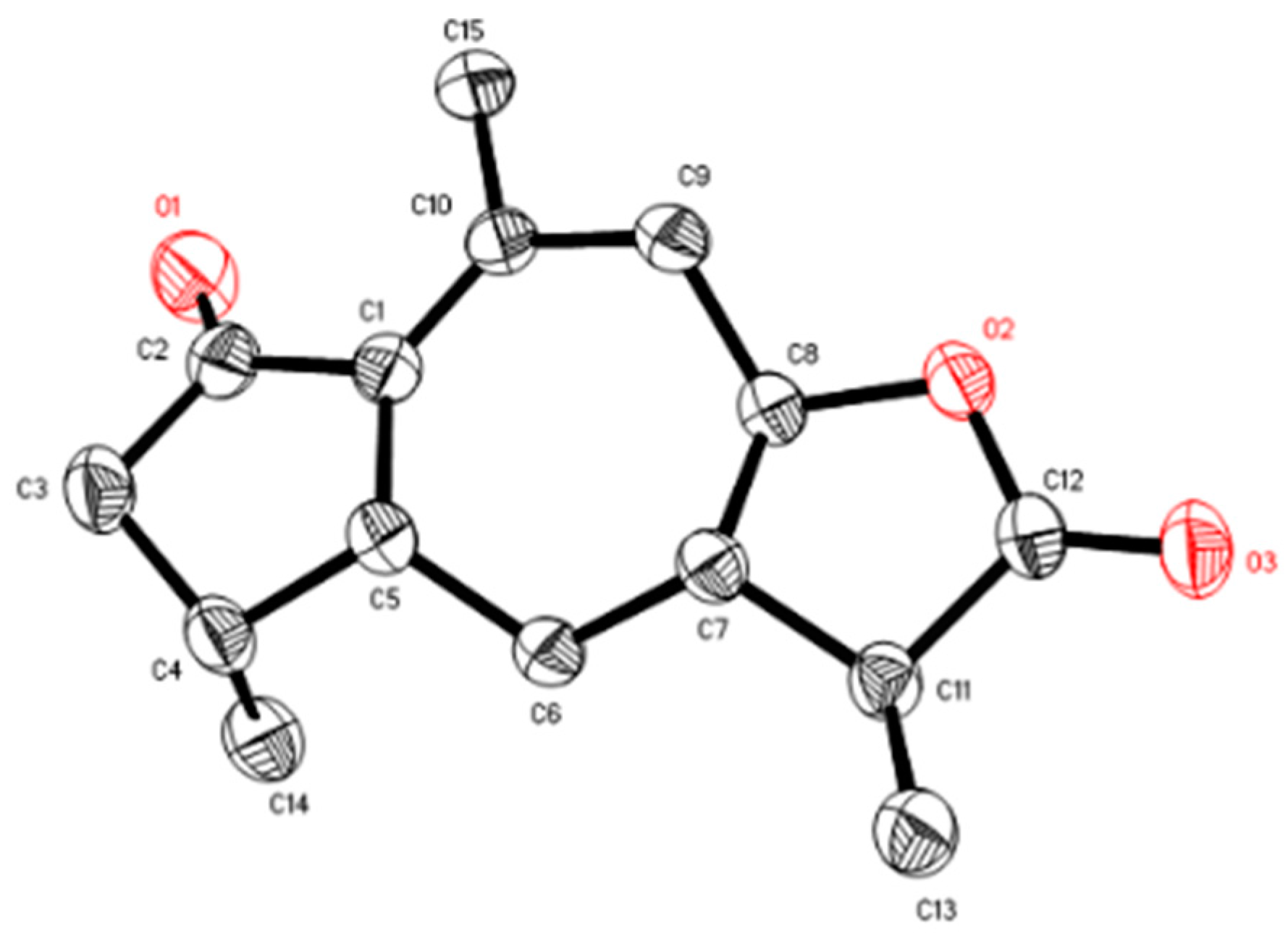
2.1. Structure Elucidation of Compounds
2.2. Evalution of Anti-Inflammatory Activity
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Data for 1–4
3.5. Anti-Inflammatory Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IR | Infrared |
| NMR | Nuclear magnetic resonance |
| HRESIMS | High resolution electrospray ionization mass spectroscopy |
| LPS | Lipopolysaccharide |
| UPLC | Ultra-performance liquid chromatography |
| EtOAc | Ethyl acetate |
| MeOH | Methanol |
| CC | Column chromatography |
References
- Liu, J.; Wu, J.; Zhao, Y.X.; Deng, Y.Y.; Mei, W.L.; Dai, H.F. A new cytotoxic 2-(2-phenylethyl) chromone from Chinese eaglewood. Chin. Chem. Lett. 2008, 19, 934–936. [Google Scholar] [CrossRef]
- Junshan, Y. Review of the chemical constituents isolated from chen-xiang. Nat. Prod. Res. Dev. 1998, 10, 99–103. [Google Scholar]
- Takemoto, H.; Ito, M.; Shiraki, T.; Yagura, T.; Honda, G. Sedative effects of vapor inhalation of agarwood oil and spikenard extract and identification of their active components. J. Nat. Med. 2008, 62, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Wei, J.H.; Yang, J.S.; Zhang, Z.; Yang, Y.; Gao, Z.H.; Sui, C.; Gong, B. Chemical constituents of agarwood originating from the endemic genus aquilaria plants. Chem. Biodivers. 2012, 9, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.-F.; Liu, J.; Zeng, Y.-B.; Han, Z.; Wang, H.; Mei, W.-L. A new 2-(2-phenylethyl) chromone from Chinese eaglewood. Molecules 2009, 14, 5165–5168. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.H.; Liu, J.M.; Lu, H.X.; Wei, Z.X. Two new 2-(2-phenylethyl) chromen-4-ones from aquilariasinensis (lour.) gilg. Helv. Chim. Acta 2012, 95, 951–954. [Google Scholar] [CrossRef]
- Li, W.; Cai, C.-H.; Dong, W.-H.; Guo, Z.-K.; Wang, H.; Mei, W.-L.; Dai, H.-F. 2-(2-phenylethyl) chromone derivatives from Chinese agarwood induced by artificial holing. Fitoterapia 2014, 98, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Yagura, T.; Ito, M.; Kiuchi, F.; Honda, G.; Shimada, Y. Four new 2-(2-phenylethyl) chromone derivatives from withered wood of aquilariasinensis. Chem. Pharm. Bull. 2003, 51, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qiao, L.; Ji, C.; Xie, D.; Gong, N.-B.; Lu, Y.; Zhang, J.; Dai, J.; Guo, S. Antidepressant abietanediterpenoids from Chinese eaglewood. J. Nat. Prod. 2013, 76, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.-L.; Yang, D.-L.; Wang, H.; Yang, J.-L.; Zeng, Y.-B.; Guo, Z.-K.; Dong, W.-H.; Li, W.; Dai, H.-F. Characterization and determination of 2-(2-phenylethyl) chromones in agarwood by GC-MS. Molecules 2013, 18, 12324–12345. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zeng, Q.; Ren, J.; Qin, J.; Zhang, S.; Shen, Y.; Zhu, J.; Zhang, F.; Chang, R.; Zhu, Y. Sesquiterpene lactones from inulafalconeri, a plant endemic to the himalayas, as potential anti-inflammatory agents. Eur. J. Med. Chem. 2011, 46, 5408–5415. [Google Scholar] [CrossRef] [PubMed]
- Rustaiyan, A.; Zare, K.; Biniyaz, T.; Fazlalizadeh, G. A seco-guaianolide and other sesquiterpene lactones from postiabombycina. Phytochemistry 1989, 28, 3127–3129. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y. [Studies on the constituents of aquilariasinensis (lour.) gilg. I. Isolation and structure elucidation of two new sesquiterpenes, baimuxinic acid and baimuxinal]. Yao Xue Xue Bao 1983, 18, 191–198. [Google Scholar] [PubMed]
- Bohlmann, F.; Zdero, C.; Cuatrecasas, J.; King, R.M.; Robinson, H. Neuesesquiterpene und norditerpeneausvertretern der gattunglibanothamnus. Phytochemistry 1980, 19, 1145–1148. [Google Scholar] [CrossRef]
- Wu, B.; Lee, J.G.; Lim, C.J.; Jia, S.D.; Kwon, S.W.; Hwang, G.S.; Park, J.H. Sesquiterpenoids and 2-(2-phenylethyl)-4H-chromen-4-one (2-(2-phenylethyl)-4H-1-benzopyran-4-one) derivatives from aquilariamalaccensisagar wood. Helv. Chim. Acta 2012, 95, 636–642. [Google Scholar] [CrossRef]
- Sample availability: Samples of the compounds 7βH-guaia-1(10)-en-12,8β-olide (1), 1,10-dioxo-4αH-5αH-7βH-11αH-1,10-secoguaia-2(3)-en-12,8β-olide (2), 1β-Hydroxy-4βH-5βH-7βH-11αH-8,9-secoguaia-9(10)-en-8,12-olide (3), 1α-Hydroxy-4α,10α-dimethyl-5βH-octahydro-azulen-8-one (4), baimuxinal (5), Selina-3,11-diene-12,15-dial (6), and Selina-4,11-diene-12,15-dial (7) are available from the authors.


| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | ||||
| 2a | 6.09 dd (6.0,2.4) | 1.67 m | 1.53 m | |
| 2b | 2.01 m | 1.97 m | ||
| 3a | 2.44 m | 7.55 dd (6.0,2.4) | 1.56 m | 1.29 m |
| 3b | 2.04 dd (16.0,5.6) | 2.00 m | 1.96 m | |
| 4 | 2.36 m | 2.6 m | 2.38 m | 2.77 m |
| 5 | 2.94 m | 1.91 m | 2.62 m | 1.66 m |
| 6a | 1.95 m | 1.90 m | 1.65 m | 1.27 m |
| 6b | 1.31 m | 1.66 m | 1.72 m | |
| 7a | 1.90 m | 2.25 m | 2.46 m | 2.41 m |
| 7b | 2.54 m | |||
| 8 | 3.86 td (9.6,3.6) | 4.53 m | ||
| 9a | 2.73 m | 2.89 m | 4.90 s | 2.19 dd (15.2,1.2) |
| 9b | 5.07 s | 2.71 m | ||
| 10 | 2.09 m | |||
| 11 | 2.45 m | 2.37 m | 2.41 m | 0.98 d (7.2) |
| 12a | 4.12 t (8.4) | 1.08 d (6.8) | ||
| 12b | 4.39 t (8.4) | |||
| 13a | 1.21 d (6.8) | 1.30 d (7.2) | 3.74 dd (10.8,4.2) | |
| 13b | 3.81 dd (10.8,4.2) | |||
| 14 | 0.96 d (6.8) | 1.25 d (7.2) | 1.06 d (7.2) | |
| 15 | 2.31 d (2.4) | 2.22 brs | 1.81 s | |
| 1-OH 4.13 d (0.4) b |
| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 138.5 s | 210.8 s | 85.8 s | 86.9 s |
| 2 | 209.4 s | 132.2 d | 39.0 t | 29.7 t |
| 3 | 49.0 t | 168.2 d | 32.1 t | 28.9 t |
| 4 | 32.5 d | 43.8 d | 33.4 d | 35.6 d |
| 5 | 47.2 d | 50.4 d | 44.0 d | 57.0 d |
| 6 | 30.2 t | 33.5 t | 25.0 t | 19.7 t |
| 7 | 56.0 d | 45.6 d | 38.8 d | 41.5 t |
| 8 | 81.4 d | 78.8 d | 179.6 s | 213.9 s |
| 9 | 44.0 t | 47.8 t | 110.1 t | 46.2 t |
| 10 | 150.3 s | 205.2 s | 149.6 s | 38.5 d |
| 11 | 43.5 d | 41.9 d | 44.8 d | 15.8 q |
| 12 | 180.7 s | 178.1 s | 68.6 t | 18.5 q |
| 13 | 12.7 q | 15.2 q | 61.7 t | |
| 14 | 16.8 q | 19.3 q | 17.8 q | |
| 15 | 22.9 q | 30.9 q | 19.5 q |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Peng, Q.; Han, Z.; Yang, L.; Wang, Z. Three New Sesquiterpenoids and One New Sesquiterpenoid Derivative from Chinese Eaglewood. Molecules 2016, 21, 281. https://doi.org/10.3390/molecules21030281
Zhao H, Peng Q, Han Z, Yang L, Wang Z. Three New Sesquiterpenoids and One New Sesquiterpenoid Derivative from Chinese Eaglewood. Molecules. 2016; 21(3):281. https://doi.org/10.3390/molecules21030281
Chicago/Turabian StyleZhao, Huan, Qinghua Peng, Zhuzhen Han, Li Yang, and Zhengtao Wang. 2016. "Three New Sesquiterpenoids and One New Sesquiterpenoid Derivative from Chinese Eaglewood" Molecules 21, no. 3: 281. https://doi.org/10.3390/molecules21030281
APA StyleZhao, H., Peng, Q., Han, Z., Yang, L., & Wang, Z. (2016). Three New Sesquiterpenoids and One New Sesquiterpenoid Derivative from Chinese Eaglewood. Molecules, 21(3), 281. https://doi.org/10.3390/molecules21030281






