Abstract
Nine oxylipin mimics were designed and synthesized starting from d-mannose. Their antifungal activity against three citrus postharvest pathogens was evaluated by spore germination assay. The results indicated that all the compounds significantly inhibited the growth of Penicillium digitatum, Penicillium italicum and Aspergillus niger. The compound (3Z,6Z,8S,9R,10R)-octadeca-3,6-diene-8,9,10-triol (3) exhibited excellent inhibitory effect on both Penicillium digitatum (IC50 = 34 ppm) and Penicillium italicum (IC50 = 94 ppm). Their in vivo antifungal activities against citrus postharvest blue mold were tested with fruit inoculated with the pathogen Penicillium italicum. The compound (3R,4S)-methyl 3,4-dihydroxy-5-octyltetrahydrofuran-2-carboxylate (9) demonstrated significant efficacy by reducing the disease severity to 60%. The antifungal mechanism of these oxylipin mimics was postulated in which both inhibition of pathogenic mycelium and stimuli of the host oxylipin-mediated defense response played important roles.
1. Introduction
The biologically active compounds oxidized from polyunsaturated fatty acids in aerobic organisms are collectively termed oxylipins, which play an important role in a variety of functions including growth, aging, development, and defense responses to environmental stimuli [1,2,3,4]. Generally, oxylipins are enzymatically or spontaneously synthesized de novo in response to mechanical injury, pathogen attack and other environmental inputs [5,6,7]. The chemical structures of oxylipins are diverse including hydroxyl, aldehyde, epoxy, ketol and divinyl-ether derivatives. It has been reported that quite a few oxylipins and their metabolisms play important roles in defense against microbial pathogens [8,9]. For example, linolenic acid and linoleic acid exhibited antifungal activity against Rhizoctonia solani and Pythium ultimum [10]. The compound 12-oxo-10,15(Z)-phytodienoic acid strongly inhibited mycelial growth and spore germination of eukaryotic microbes [8]. Jasmonic acid could suppress the reproductive development and secondary metabolism of Aspergillus specie [11,12]. Free fatty acid methyl ester fractions isolated from Linum usitatissimum L. seeds exhibited antifungal activity against toxigenic Apsergillus [13,14].
Citrus fruit is one of the most abundant agricultural economic crops in the world due to its taste and nutritive properties. However, decay during postharvest storage always brings significant loss in the economy [15]. The most common culprits are green and blue mold [16,17,18,19]. At present, the application of germicides is a principal method to control postharvest decay. The safety and endurance of these compounds are mainly considered, besides the preservative effect. Oxylipin, which is a natural plant metabolite and generally low in toxicity, was reported to show notable antifungal activity. In this paper, a series of oxylipin mimics were designed and synthesized (Scheme 1). Their antifungal effects on P. digitatum, P. italicum and Aspergillus niger in Petri dishes were also investigated.
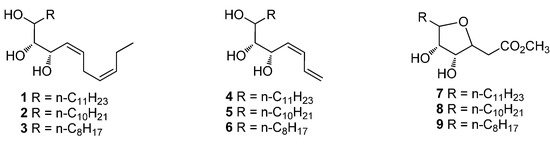
Scheme 1.
The structures of designed oxylipin mimics.
Scheme 1.
The structures of designed oxylipin mimics.
2. Results and Discussion
2.1. Synthetic Chemistry
In this work, we designed a few oxylipin mimics which were mainly unsaturated alkane with a multi-hydroxyl group forming an aliphatic carbon chain ranging from 15 to 21. Furthermore, a few mimics with a tetrahydrofuran ring were also synthesized to investigate the influence of different structures on antifungal activities [2,20].
The compounds 1–9 were synthesized from d-mannose, which provided inherent multi-hydroxyl groups. The synthetic route of compounds 1–9 was shown in Scheme 2. The compound 2,3:5,6-di-O-isopropylidene-d-mannose (10), prepared according to the literature [21], was reacted with the corresponding Grignard reagent to give compound 11. Oxidation of compound 11 with periodic acid [22] afforded compound 12. The Wittig/Horner-Wadsworth-Emmons reaction of compound 12 and subsequent deprotection resulted in the desired oxylipin mimics 1–9 [23,24]. All the synthesized products were characterized by 1H-NMR and HRMS.
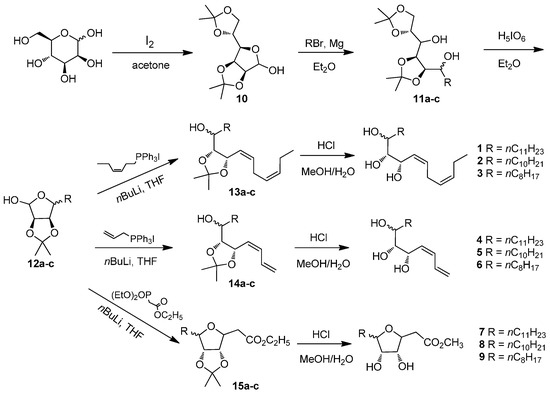
Scheme 2.
The synthetic route to compounds 1–9. 11a, 12a, 13a, 14a, 15a: R = nC11H23; 11b, 12b, 13b, 14b, 15b: R = nC10H21; 11c, 12c, 13c, 14c, 15c: R = nC8H17.
Scheme 2.
The synthetic route to compounds 1–9. 11a, 12a, 13a, 14a, 15a: R = nC11H23; 11b, 12b, 13b, 14b, 15b: R = nC10H21; 11c, 12c, 13c, 14c, 15c: R = nC8H17.
2.2. Evaluation of in Vitro Antifungal Activity
All the synthesized compounds were screened to investigate their antifungal activities against P. digitatum, P. italicum and A. niger on Petri dishes using the agar dilution method. Linolenic acid was reported to inhibit a few fungi [10,25] and was thus used as the reference compound. IC50, the concentration inhibiting 50% of fungal activity, was calculated and shown in Table 1. All the tested compounds showed significant antifungal activities against P. digitatumi and diverse activities against P. italicum and A. niger. Compound 2 strongly reduced the conidia germination of P. digitatumi with the IC50 value of 23 ppm. Compound 3 demonstrated substantial antifungal activity against P. italicum (IC50 = 94 ppm). Compound 9 inhibited the growth of A. niger with the IC50 value of 202 ppm.

Table 1.
IC50 values of the tested compounds against three citrus postharvest pathogens.
| Compound | IC50 for Penicillium digitatumi (ppm) | IC50 for Penicillium italicum (ppm) | IC50 for Aspergillus niger (ppm) |
|---|---|---|---|
| 1 | 35.54 ± 1.75 | 133.12 ± 2.27 | 256.22 ± 18.93 |
| 2 | 22.73 ± 3.43 | 114.01 ± 7.63 | 385.56 ± 12.74 |
| 3 | 33.64 ± 1.85 | 94.41 ± 7.62 | 311.04 ± 5.71 |
| 4 | 47.59 ± 0.13 | 156.97 ± 10.13 | 243.64 ± 11.31 |
| 5 | 31.45 ± 3.26 | 191.37 ± 8.14 | 301.01 ± 3.54 |
| 6 | 28.43 ± 0.82 | 182.33 ± 13.50 | 263.81 ± 8.84 |
| 7 | 201.65 ± 6.48 | 272.83 ± 18.73 | 257.99 ± 9.32 |
| 8 | 59.73 ± 4.31 | 208.85 ± 12.28 | 333.15 ± 13.62 |
| 9 | 123.69 ± 8.79 | 212.60 ± 11.08 | 202.40 ± 2.99 |
| Linolenic acid | 57.96 ± 2.16 | 234.21 ± 10.43 | 272.14 ± 5.47 |
Compounds 1–9 showed antifungal activities against three pathogens. It is reasonable to speculate that compounds possessing many hydroxylic groups are able to interact with fungal cytoderm composed mainly from chitin. This results in increased cytoderm permeability [26]. If considering structural features, triols 1–6 containing long aliphatic chains showed better antifungal activities than compounds 7–9, which were characterized by cyclic structure and a lower number of hydroxyl groups. It seems that the length of the chain for similar structures did not show an apparent difference. Overall, oxylipin mimic treatment could result in the inhibition of germination or spore death.
2.3. Evaluation of in Vivo Antifungal Activity
To further explore the antifungal activities of these oxylipin mimics, the test to control citrus postharvest blue mold on fruit inoculated with the pathogens was conducted. Results summarized in Table 2 showed that all the compounds reduced the severity of blue mold to some extent as compared to the control after 10 days. The most effective, compound 3, in spore germination assay simply reduced the disease severity to 82.5%. However, compound 9 exhibited the best antifungal activity in vivo. Compound 9 reduced the disease severity to 60%, which is more effective than that of linolenic acid (65%).

Table 2.
The disease index after 10 days on citrus treated with tested compounds.
| Compound | Disease Index (%) |
|---|---|
| Control | 97.5 |
| 1 | 80.0 |
| 2 | 75.0 |
| 3 | 82.5 |
| 4 | 90.0 |
| 5 | 77.5 |
| 6 | 65.0 |
| 7 | 62.5 |
| 8 | 65.0 |
| 9 | 60.0 |
| Linolenic acid | 65.0 |
It is noteworthy that the antifungal activities of the tested compounds against P. italicum on citrus and in vitro Petri dish are inconsistent. Compounds 7–9, which displayed moderate inhibitory effect on P. italicum in a Petri dish, showed better a inhibitory effect in vivo than those compounds without an ester group. It is possible that the ester group could be a contributing factor which could be converted to acid by hydrolase. It is well known that the antifungal activity of oxylipin is not simply due to the inhibition of the growth of mycelia or killing fungi spores directly. Oxylipin may act as a signal-transmitting substance to activate the immunomodulation properties of the host to induce a systemic defense response [6]. The reactive oxygen species [27] and related enzymes [1,28] such as lipoxygenase, α-dioxygenase and allene oxide synthase could also be influenced by oxylipin. Whether the compounds in this work demonstrate a significant effect through similar mechanisms is worth investigating in the future.
3. Experimental Section
3.1. General
All reagents were commercially available and used without further purification. Solvents were dried prior to use. All the reactions were performed under argon atmosphere. The 1H-NMR spectra were collected at room temperature on 400 MHz Bruker AM spectrometers (Bruker Corporation, Madison, WI, USA). The residual solvent signals were taken as the reference (7.26 ppm for 1H-NMR and 77.0 ppm for 13C-NMR in CDCl3). Chemical shift (δ) is reported in ppm, coupling constants (J) are given in Hz. The following abbreviations classify the multiplicity: s = singlet, brs = broad singlet, d = doublet, t = triplet, m = multiplet or unresolved. HR-MS (ESI) spectra were recorded on Waters Q-Tof premier™ (Waters Corporation, Milford, MA, USA) and Bruker MicroTOF II mass spectrometer (Bruker Corporation). The 1H-NMR and HRMS spectra were attached in the Supplementary Materials.
3.2. Synthesis of Compounds 1–9
(2R,4S,5R)-1,2:4,5-Diisopropylideneundecyl-1,2,3,4,5,6-hexaol (11a). To a suspension of magnesium turnings (3.65 g, 0.15 mol) in 100 mL of anhydrous ether, 1-bromoundecane (4.7 g, 0.02 mol) was added. The reaction was initiated by addition of 0.1 mL dibromoethane. Then 1-bromoundecane (18.8 g, 0.08 mol) was added dropwise to keep gently refluxing. After 3 h, the mixture was cooled to room temperature and then −10 °C, which was followed by addition of compound 10 (2.6 g, 0.01 mol). After completion of addition, the mixture was warmed to room temperature and stirred for 2 h. Ice water was added to quench the reaction and the resulting suspension was filtered through Celite. The filtrate was concentrated and purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:5) to give compound 11a as a colorless oil (3.54 g, 85%). 1H-NMR (400 MHz, CDCl3) δ: 0.87 (t, J = 6.8 Hz, 3H, CH3), 1.23–1.53 (m containing 4 s, 32H, 4CH3, 10CH2), 3.59–3.62 (m, 1H, CH), 3.77–3.88 (m, 1H, CH), 3.97–4.14 (m, 4H, 2CH, CH2), 4.34–4.41 (m, 1H, CH); HRMS (ESI) m/z 439.3036 (calcd. for C24H44O6Na [M + Na]+, 439.3036).
(3R,4S)-3,4-Isopropylidene-5-undecyltetrahydrofuran-2,3,4-triol (12a). A mixture of periodic acid (3.84 g, 16.95 mmol) and compound 11a (2.35 g, 5.65 mmol) in 200 mL of anhydrous ether was stirred for 4 h at room temperature. The suspension was filtered to remove the extra periodic acid. The filtrate was washed with saturated NaHCO3 solution to neutral and then washed with brine. The organic phase was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:10) to give compound 12a as a colorless oil (1.55 g, 87%). 1H-NMR (400 MHz, CDCl3) δ: 0.86 (t, J = 6.8 Hz, 3H, CH3), 1.24–1.46 (m containing 2 s, 24H, 2CH3, 9CH2), 1.68 (dd, J = 14.7, 7.2 Hz, 2H, CH2), 3.31(s, 1H, OH), 4.10 (dt, J = 6.8, 3.6 Hz, 1H, CH), 4.56–4.57 (m, 1H, CH), 4.60–4.64 (m, 1H, CH), 5.32 (d, J = 1.6 Hz, 1H, CH); HRMS (ESI) m/z 337.2355 (calcd. for C18H34O6Na [M + Na]+, 337.2354).
(3Z,6Z,8S,9R)-8,9-Isopropylidene-heneicosa-3, 6-diene-8, 9, 10-triol (13a). n-Butyl lithium (9.88 mL, 1.6 M) was added dropwise to a solution of (Z)-hex-3-ene triphenylphosphonium iodine (5.81 g, 12.3 mmol) in 70 mL of THF at −30 °C. After 0.5 h, a solution of compound 12a (1.10 g, 3.5 mmol) in 5 mL of THF was added to the mixture, which was then allowed to warm to 0 °C. The reaction was continued for another 6 h. The resulting mixture was filtered. The filtrate was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:20) to give compound 13a as a colorless oil (0.65 g, 49%). 1H-NMR (400 MHz, CDCl3) δ: 0.88 (t, J = 6.8 Hz, 3H, CH3), 0.97 (t, J = 7.4 Hz, 3H, CH3), 1.26–1.51 (m containing 2 s, 26H, 2CH3, 10CH2), 2.02–2.10 (m, 2H, CH2), 2.80–2.86 (m, 2H, CH2), 3.56–3.59 (m 1H, CH), 3.97–4.00 (m, 1H, CH), 4.98 (dd, J = 8.6, 6.8 Hz, 1H, CH), 5.24–5.74 (m, 4H, 2CH=CH); HRMS (ESI) m/z 403.3191 (calcd. for C24H44O3Na [M + Na]+, 403.3188).
(3Z,6Z,8S,9R,10R)-Henicosa-3,6-diene-8,9,10-triol (1). Compound 13a (0.46 g, 1.2 mmol) was added to a solution of concentrated HCl (11 mL) in water/methanol (75 mL, v/v = 1:9). The mixture was stirred for 3 h at room temperature and then neutralized with NaOH solution. The resulting mixture was concentrated to remove methanol. The residue was diluted with EtOAc and washed with water. The organic phase was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:2) to give compound 1 as a colorless oil (0.32 g, 78%). 1H-NMR (400 MHz, CDCl3) δ: 0.87 (t, J = 6.8 Hz, 3H, CH3), 0.97 (t, J = 7.5 Hz, 3H, CH3), 1.25–1.51 (m, 20H, 10CH2), 2.02–2.08 (m, 2H, CH2), 2.15 (brs, 3H, 3OH), 2.79–2.89 (m, 2H, CH2), 3.28–3.48 (m, 1H, CH), 3.64–3.85 (m, 1H, CH), 4.22–4.67 (m, 1H, CH), 5.27–5.83 (m, 4H, CH=CH); HRMS (ESI) m/z 363.2875 (calcd. for C21H40O3Na [M + Na]+, 363.2875).
(3Z,6Z,8S,9R,10R)-Icosa-3,6-diene-8,9,10-triol (2). To a suspension of magnesium turnings (4.86 g, 0.2 mol) in 200 mL of anhydrous ether, 1-bromodecane (8.85 g, 0.04 mol) was added. The reaction was initiated by addition of 0.1 mL dibromoethane. Then 1-bromodecane (35.39 g, 0.16 mol) was added dropwise to keep gently refluxing. After 3 h, the mixture was cooled to room temperature and then −10 °C, which was followed by addition of compound 10 (5.21 g, 0.02 mol). After completion of addition, the mixture was warmed to room temperature and stirred for 2 h. Ice water was added to quench the reaction and the resulting suspension was filtered through Celite. The filtrate was concentrated and purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:5) to give compound 11b as a colorless oil (7.08 g, 88%). A mixture of periodic acid (11.97 g, 52.5 mmol) and compound 11b (7.04 g, 17.5 mmol) in 250 mL of anhydrous ether was stirred for 4 h at room temperature. The suspension was filtered to remove the extra periodic acid. The filtrate was washed with saturated NaHCO3 solution to neutral and then washed with brine. The organic phase was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:10) to give compound 12b as a colorless oil (4.16 g, 79%). n-Butyl lithium (11.8 mL, 1.6 M) was added dropwise to a solution of (Z)-hex-3-ene triphenylphosphonium iodine (7.44 g, 15.75 mmol) in 80 mL of THF at −30 °C. After 0.5 h, a solution of compound 12b (1.35 g, 4.5 mmol) in 5 mL of THF was added to the mixture, which was then allowed to warm to 0 °C. The reaction was continued for another 6 h. The resulting mixture was filtered. The filtrate was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:20) to give compound 13b as a colorless oil (0.76 g, 46%). Compound 13b (0.75 g, 2.05 mmol) was added to a solution of concentrated HCl (8.5 mL) in water/methanol (100 mL, v/v = 1:9). The mixture was stirred for 3 h at room temperature and then neutralized with NaOH solution. The resulting mixture was concentrated to remove methanol. The residue was diluted with EtOAc and washed with water. The organic phase was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:2) to give compound 2 as a colorless oil (0.48 g, 72%). 1H-NMR (400 MHz, CDCl3) δ: 0.88 (t, J = 6.4 Hz, 3H, CH3), 0.97 (t, J = 7.5 Hz, 3H, CH3), 1.24–1.73 (m, 18H, 9CH2), 2.00–2.10 (m containing brs, 3H, CH2, OH), 2.44 (brs, 1H, OH), 2.63 (brs, 1H, OH), 2.77–2.91 (m, 2H, CH2), 3.39–3.46 (m, 1H, CH), 3.66–3.83 (m, 1H, CH), 4.22–4.30 (m, 0.5H, CH), 4.64–4.66 (m, 0.5H, CH), 5.28–5.83 (m, 4H, 2CH=CH); HRMS (ESI) m/z 349.2713 (calcd. for C20H38O3Na [M + Na]+, 349.2706).
(3Z,6Z,8S,9R,10R)-Octadeca-3,6-diene-8,9,10-triol (3). To a suspension of magnesium turnings (4.86 g, 0.2 mol) in 200 mL of anhydrous ether, 1-bromoctane (7.72 g, 0.04 mol) was added. The reaction was initiated by addition of 0.1 mL dibromoethane. Then 1-bromodecane (30.9 g, 0.16 mol) was added dropwise to keep gently refluxing. After 3 h, the mixture was cooled to room temperature and then −10 °C, which was followed by addition of compound 10 (5.2 g, 0.02 mol). After completion of addition, the mixture was warmed to room temperature and stirred for 2 h. Ice water was added to quench the reaction and the resulting suspension was filtered through Celite. The filtrate was concentrated and purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:5) to give compound 11c as a colorless oil (5.91 g, 79%). A mixture of periodic acid (10.77 g, 47.25 mmol) and compound 11c (5.90 g, 15.75 mmol) in 250 mL of anhydrous ether was stirred for 4 h at room temperature. The suspension was filtered to remove the extra periodic acid. The filtrate was washed with saturated NaHCO3 solution to neutral and then washed with brine. The organic phase was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:10) to give compound 12c as a colorless oil (3.36 g, 78%). n-Butyl lithium (10.5 mL, 1.6 M) was added dropwise to a solution of (Z)-hex-3-ene triphenylphosphonium iodine (6.61 g, 14 mmol) in 80 mL of THF at −30 °C. After 0.5 h, a solution of compound 12c (1.1 g, 4 mmol) in 5 mL of THF was added to the mixture, which was then allowed to warm to 0 °C. The reaction was continued for another 6 h. The resulting mixture was filtered. The filtrate was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:20) to give compound 13c as a colorless oil (0.48 g, 36%). Compound 13c (0.47 g, 1.4 mmol) was added to a solution of concentrated HCl (6 mL) in water/methanol (100 mL, v/v = 1:9). The mixture was stirred for 3 h at room temperature and then neutralized with NaOH solution. The resulting mixture was concentrated to remove methanol. The residue was diluted with EtOAc and washed with water. The organic phase was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:2) to give compound 3 as a colorless oil (0.39 g, 75%). 1H-NMR (400 MHz, CDCl3) δ: 0.88 (t, J = 6.8 Hz, 3H, CH3), 0.96(t, J = 7.5 Hz, 3H, CH3), 1.26–1.72(m, 14H, CH2), 2.02–2.06(m, 2H, CH2), 2.20 (brs, 2H, 2OH), 2.60(brs, 1H, OH), 2.77–2.86 (m, 2H, CH2), 3.44–3.48 (m, 1H, CH), 3.65(s, 1H, CH), 4.23 (s, 1H, CH), 5.30–5.37 (m, 1H, =CH), 5.43–5.49(m, 1H, =CH), 5.58–5.64 (m, 1H, =CH), 5.77–5.84(m, 1H, =CH); HRMS (ESI) m/z 321.2405 (calcd. for C18H34O3Na [M + Na]+, 321.2400).
(5S,6R,7R,Z)-Octadeca-1,3-diene-5,6,7-triol (4). n-Butyl lithium (8.5 mL, 1.6 M) was added dropwise to a solution of allyl triphenylphosphonium bromide (4.02 g, 10.5 mmol) in 60 mL of THF at −10 °C. After 0.5 h, a solution of compound 12a (0.94 g, 3.0 mmol) in 5 mL of THF was added to the mixture, which was then allowed to warm to 20 °C. The reaction was continued for another 6 h. The resulting mixture was filtered. The filtrate was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:20) to give compound 14a as a colorless oil (0.39 g, 40%). Compound 14a (0.37 g, 1.1 mmol) was added to a solution of concentrated HCl (10 mL) in water/methanol (70 mL, v/v = 1:9). The mixture was stirred for 3 h at room temperature and then neutralized with NaOH solution. The resulting mixture was concentrated to remove methanol. The residue was diluted with EtOAc and washed with water. The organic phase was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:3) to give compound 4 as a colorless oil (0.24 g, 74%). 1H-NMR (400 MHz, CDCl3) δ: 0.88 (t, J = 6.8 Hz, 3H, CH3), 1.01–1.73 (m, 20H, 10CH2), 3.34–3.37 (m, 1H, CH), 3.54–3.97 (m, 2H, 2CH), 5.14–5.34 (m, 2H, =CH2), 5.57–5.82 (m, 1H, =CH), 6.22–6.72 (m, 2H, 2CH=); HRMS (ESI) m/z 321.2404 (calcd. for C18H34O3Na [M + Na]+, 321.2400).
(5S,6R,7R,Z)-Heptadeca-1,3-diene-5,6,7-triol (5). n-Butyl lithium (11.8 mL, 1.6 M) was added dropwise to a solution of allyl triphenylphosphonium bromide (6.77 g, 15.75 mmol) in 80 mL of THF at −10 °C. After 0.5 h, a solution of compound 12b (1.35 g, 4.5 mmol) in 5 mL of THF was added to the mixture, which was then allowed to warm to 20 °C. The reaction was continued for another 6 h. The resulting mixture was filtered. The filtrate was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:20) to give compound 14b as a colorless oil (0.65 g, 45%). Compound 14b (0.65 g, 2 mmol) was added to a solution of concentrated HCl (8 mL) in water/methanol (50 mL, v/v = 1:9). The mixture was stirred for 3 h at room temperature and then neutralized with NaOH solution. The resulting mixture was concentrated to remove methanol. The residue was diluted with EtOAc and washed with water. The organic phase was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:3) to give compound 5 as a colorless oil (0.38 g, 68%). 1H-NMR (400 MHz, CDCl3) δ: 0.87 (t, J = 6.8 Hz, 3H), 1.25–1.62 (m, 18H), 2.27–2.85 (brs, 1H, OH), 3.04–3.18 (brs, 1H, OH), 3.83–3.89 (m, 2H, 2CH), 3.99–4.00 (m, 1H, CH), 4.04 (s, 1H, OH), 4.74–4.89 (m, 2H, =CH2), 5.22–5.55 (m, 2H, 2=CH), 6.15–6.66 (m, 1H, =CH); HRMS (ESI) m/z 307.2250 (calcd. for C17H32O3Na [M + Na]+, 307.2244).
(5S,6R,7R,Z)-Pentadeca-1,3-diene-5,6,7-triol (6). n-Butyl lithium (10.5 mL, 1.6 M) was added dropwise to a solution of allyl triphenylphosphonium bromide (6.02 g, 14 mmol) in 80 mL of THF at −10 °C. After 0.5 h, a solution of compound 12c (1.1 g, 4 mmol) in 5 mL of THF was added to the mixture, which was then allowed to warm to 20 °C. The reaction was continued for another 6 h. The resulting mixture was filtered. The filtrate was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:20) to give compound 14c as a colorless oil (0.49 g, 41%). Compound 14c (0.48 g, 1.6 mmol) was added to a solution of concentrated HCl (6.7 mL) in water/methanol (40 mL, v/v = 1:9). The mixture was stirred for 3 h at room temperature and then neutralized with NaOH solution. The resulting mixture was concentrated to remove methanol. The residue was diluted with EtOAc and washed with water. The organic phase was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:3) to give compound 6 as a colorless oil (0.31 g, 76%). 1H NMR (400 MHz, CDCl3) δ: 0.89 (t, J = 6.4 Hz, 3H, CH3), 1.25–1.59 (m, 14H, 7CH2), 2.98 (brs, 3H, 3OH), 3.33–3.36 (m, 0.5H, CH), 3.41–3.42 (m, 0.5H, CH), 3.50–3.53 (m, 0.5H, CH), 3.62–3.65 (m, 0.5H, CH), 3.77–3.86 (m, 0.5H, CH), 3.94–4.03 (m, 0.5H, CH), 4.31–4.78 (m, 1H, =CH2), 5.12–5.33 (m, 1.5H, =CH2, =CH), 5.49–5.58 (m, 0.5H, =CH), 5.71–5.85 (m, 0.5H, =CH), 6.13–6.24 (m, 0.5H, =CH), 6.29–6.37 (m, 0.5H, =CH), 6.56–6.70 (m, 0.5H, =CH); HRMS (ESI) m/z 279.1937 (calcd. for C15H28O3Na [M + Na]+, 279.1931).
(3R,4S)-Methyl 3,4-dihydroxy-5-undecyl-tetrahydrofuran-2-acetate (7). n-Butyl lithium (5.5 mL, 1.6 M) was added dropwise to a solution of triethyl phosphonoacetate (1.93 g, 8.6 mmol) in 70 mL of THF at −30 °C. After 0.5 h, a solution of compound 12a (1.89 g, 6.0 mmol) in 5 mL of THF was added to the mixture, which was then allowed to warm to room temperature. The reaction mixture was stirred overnight and then filtered. The filtrate was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:20) to give compound 15a as a colorless oil (1.02 g, 44%). Compound 15a (1.0 g, 2.6 mmol) was added to a solution of concentrated HCl (20 mL) in water/methanol (150 mL, v/v = 1:9). The mixture was stirred for 3 h at room temperature and then neutralized with NaOH solution. The resulting mixture was concentrated to remove methanol. The residue was diluted with EtOAc and washed with water. The organic phase was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:3) to give compound 7 as a colorless oil (0.51 g, 69%). 1H-NMR (400 MHz, CDCl3) δ: 0.86 (t, J = 6.8 Hz, 3H, CH3), 1.24–1.72 (m, 20H, 10CH2), 2.58–2.86 (m, 2H, CH2), 3.60–3.90 (m containing s, 5H, CH, OCH3), 3.96–4.02 (m, 1H, CH), 4.79–4.98 (m, 1H, CH); HRMS (ESI) m/z 353.2287 (calcd. for C18H34O5Na [M + Na]+, 353.2298).
(3R,4S)-Methyl 5-decyl-3,4-dihydroxytetrahydrofuran-2-acetate (8). n-Butyl lithium (4.1 mL, 1.6 M) was added dropwise to a solution of triethyl phosphonoacetate (1.45 g, 6.45 mmol) in 70 mL of THF at −30 °C. After 0.5 h, a solution of compound 12b (1.3 g, 4.3 mmol) in 5 mL of THF was added to the mixture, which was then allowed to warm to room temperature. The reaction mixture was stirred overnight and then filtered. The filtrate was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:20) to give compound 15b as a colorless oil (0.76 g, 48%). Compound 15b (0.74 g, 2 mmol) was added to a solution of concentrated HCl (8 mL) in water/methanol (50 mL, v/v = 1:9). The mixture was stirred for 3 h at room temperature and then neutralized with NaOH solution. The resulting mixture was concentrated to remove methanol. The residue was diluted with EtOAc and washed with water. The organic phase was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:3) to give compound 8 as a colorless oil (0.47 g, 75%). 1H-NMR (400 MHz, CDCl3) δ: 0.86 (t, J = 0.90 Hz, 3H, CH3), 1.23–1.55 (m, 18H, 9CH2), 2.61 (dd, J = 16.2, 7.4 Hz, 1H, CH2), 2.75 (dd, J = 16.2, 5.9 Hz, 1H, CH2), 3.78 (s, 3H, OCH3), 3.71–3.75 (m, 1H, CH), 3.78–3.85 (m, 2H, 2CH), 3.95–4.00 (m, 1H, CH); 13C NMR (100 MHz, CDCl3) δ: 14.1 (CH3), 22.6 (CH2), 25.5 (CH2), 29.3 (CH2), 29.5 (2CH2), 29.6 (3CH2), 31.9 (CH2), 38.3 (CH2), 52.0 (OCH3), 74.8 (CH), 74.9 (CH), 78.4 (CH), 84.3 (CH),172.5 (C=O); HRMS (ESI) m/z 339.2144 (calcd. for C17H32O5Na [M + Na]+, 339.2142).
(3R,4S)-Methyl 3,4-dihydroxy-5-octyltetrahydrofuran-2-acetate (9). n-Butyl lithium (4.3 mL, 1.6 M) was added dropwise to a solution of triethyl phosphonoacetate (1.28 g, 5.7 mmol) in 80 mL of THF at −10 °C. After 0.5 h, a solution of compound 12c (1.04 g, 3.8 mmol) in 5 mL of THF was added to the mixture, which was then allowed to warm to 20 °C. The reaction was continued for another 6 h. The resulting mixture was filtered. The filtrate was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:20) to give compound 15c as a colorless oil (0.53 g, 41%). Compound 15c (0.52 g, 1.5 mmol) was added to a solution of concentrated HCl (6 mL) in water/methanol (40 mL, v/v = 1:9). The mixture was stirred for 3 h at room temperature and then neutralized with NaOH solution. The resulting mixture was concentrated to remove methanol. The residue was diluted with EtOAc and washed with water. The organic phase was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography on silica gel (ethyl acetate/petroleum ether = 1:3) to give compound 9 as a colorless oil (0.32 g, 74%). 1H-NMR (400 MHz, CDCl3) δ: 0.85 (t, J = 7.2 Hz, 3H, CH3), 1.23–1.73 (m, 14H, 7 CH2), 2.58–2.85 (m, 2H, CH2), 3.57–4.01 (m containing s, 6H, CH3, 3CH), 4.55–4.99 (m, 1H, CH); HRMS (ESI) m/z 289.2007 (calcd. for C15H29O5 [M + H]+, 289.2010).
3.3. Antifungal Biology Assay
3.3.1. Strain
The P. digitatum, P. italicum and A. niger strain was obtained from the College of Horticulture and Forestry Sciences, Huazhong Agricultural University. The strain was incubated with potato dextrose agar (PDA). The P. italicum conidial suspension was prepared by diluting with 0.1% Tween-80 sterile water. After three days incubation, the concentration was adjusted to 1 × 106 conidia mL−1 by diluting with sterile distilled water using a hemocytometer.
3.3.2. In Vitro Antifungal Activity [29]
The mycelium was inoculated by a hole punch uniformly in 70 mm sterile plastic Petri dishes. Afte a pre-study, the tested compounds were diluted with PDA to 15, 25, 50, 75, 150 ppm for P. digitatum and 50, 100, 200, 300, 400 ppm for P. italicum and A. niger detection. Control plates contained culture medium without tested compounds. All the Petri dishes were incubated in biochemical incubator at 28 °C. Colony diameters (D) were measured after fourdays for P. digitatum, seven days for P. italicum and A. niger. Three measurements were taken on each plates and in triplicate for each concentration. The mean value was used for statistical analysis. Inhibitory rate% = [(Dcontrol − Dtest)/Dcontrol] × 100%.
Plot was set with inhibitory rate as Y axial and concentration as X axial. IC50, the half maximal concentration to inhibit 50% of fungal activity, was calculated from the plot.
3.3.3. In Vivo Antifungal Activity
Citrus reticulate used for experiments were collected from orchards in Wuhan, China. After being stored for one week at room temperature, tested fruits were selected for uniformity of size and maturity without any physical injury or infection. All fruits were washed with tap water and dried with tissue carefully before use. A uniform wound (3 mm deep and 4 mm wide) was made at the equator of each orange with a sterile needle. Then, 20 min later, 20 μL P. italicum conidial suspension was injected into each wound. After 2 h, 20 μL 300 ppm oxylipin mimics were inoculated and 20 μL sterile water for control. The tested fruits were stored in artificial climatic box at 28 °C with the relative humidity of 90%. The disease incidence and lesion diameters were recorded for 10 days. Analysis of 10 fruits, in triplicate for each compound was done.
The disease index for fruit was observed by assessing the extent of total disease symptoms on each fruit surface using the following scale:
- 0: lesion diameter = 0 mm (no visible disease symptoms);
- 1: 1 mm ≤ lesion diameter ≤ 10 mm;
- 2: 10 mm < lesion diameter ≤ 20 mm;
- 3: 20 mm < lesion diameter ≤ 30 mm;
- 4: lesion diameter > 30 mm.
The disease index was calculated using the formula [30]: ∑(disease scale × number of fruit in each class)/(number of total fruit × highest disease scale) × 100. Lesion diameter was measured by a vernier caliper using the cross method, and the unit was milimeter.
3.4. Statistical Analysis
The analysis was carried out using Statistical Package of Social Sciences (SPSS 17.0, SPSS Inc. Chicago, IL, USA).
4. Conclusions
In conclusion, a series of oxylipin mimics were designed and synthesized. The in vitro bioassay indicated that all these oxylipin mimics showed significant inhibitory effect to the growth of P. digitatum, P. italicum and A. niger. The in vivo experiment on citrus-inoculated P. italicum. demonstrated that the most effective compound could reduce the disease severity to 60%. However, the mechanism for the antifungal activity of these oxylipin mimics needs further investigation.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/2/254/s1.
Acknowledgments
The financial supports (Program No. 2014QC007, 2012BQ029) provided by the Fundamental Research Funds for the Central Universities, China and the National Natural Science Foundation of China (21402056) are gratefully acknowledged.
Author Contributions
Jimei Ma, Zi-Long Li and Hong Jiang conceived and designed the experiments; Yupeng Li and Zhen Zeng performed the experiments; Yupeng Li and Hangwei Chen analyzed the data; Jimei Ma wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Griffiths, G. Biosynthesis and analysis of plant oxylipins. Free Radic. Res. 2015, 49, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; D’Abrosca, B.; DellaGreca, M.; Izzo, A.; Natale, A.; Pascarella, M.T.; Pacifico, S.; Zarrelli, A.; Monaco, P. Chemical characterization of new oxylipins from Cestrum parqui, and their effects on seed germination and early seedling growth. Chem. Biodivers. 2008, 5, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Mosblech, A.; Feussner, I.; Heilmann, I. Oxylipins: Structurally diverse metabolites from fatty acid oxidation. Plant Physiol. Biochem. 2009, 47, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Chehab, E.W.; Perea, J.V.; Gopalan, B.; Theg, S.; Dehesh, K. Oxylipin pathway in rice and arabidopsis. J. Integr. Plant Biol. 2007, 49, 43–51. [Google Scholar] [CrossRef]
- Savchenko, T.V.; Zastrijnaja, O.M.; Klimov, V.V. Oxylipins and plant abiotic stress resistance. Biochemistry 2014, 79, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Schilmiller, A.L. Oxylipin metabolism in response to stress. Curr. Opin. Plant Biol. 2002, 5, 230–236. [Google Scholar] [CrossRef]
- Böttcher, C.; Pollmann, S. Plant oxylipins: Plant responses to 12-oxo-phytodienoic acid are governed by its specific structural and functional properties. FEBS J. 2009, 276, 4693–4704. [Google Scholar] [CrossRef] [PubMed]
- Prost, I.; Dhondt, S.; Rothe, G.; Vicente, J.; Rodriguez, M.J.; Kift, N.; Carbonne, F.; Griffiths, G.; Esquerré-Tugayé, M.-T.; Rosahl, S.; et al. Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiol. 2005, 139, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.L.; Hara-Nishimura, I. Leaf oil bodies are subcellular factories producing antifungal oxylipins. Curr. Opin. Plant Biol. 2015, 25, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.; Raynor, L.; Mitchell, A.; Walker, R.; Walker, K. Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathologia 2004, 157, 87–90. [Google Scholar] [PubMed]
- Christensen, S.A.; Kolomiets, M.V. The lipid language of plant–fungal interactions. Fungal Genet. Biol. 2011, 48, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Santino, A.; Taurino, M.; de Domenico, S.; Bonsegna, S.; Poltronieri, P.; Pastor, V.; Flors, V. Jasmonate signaling in plant development and defense response to multiple (a)biotic stresses. Plant Cell Rep. 2013, 32, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Abdelillah, A.; Houcine, B.; Halima, D.; sari Meriel, C.; Imane, Z.; Eddine, S.D.; Abdallah, M.; sari Daoudi, C. Evaluation of antifungal activity of free fatty acids methyl esters fraction isolated from Algerian Linum usitatissimum L. seeds against toxigenic Aspergillus. Asian Pac. J. Trop. Biomed. 2013, 3, 443–448. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Senthilkumar, A.; Venkatesalu, V. Antibacterial and antifungal efficacy of fatty acid methyl esters from the leaves of Sesuvium portulacastrum L. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 775–780. [Google Scholar] [PubMed]
- Lorente, D.; Escandell-Montero, P.; Cubero, S.; Gómez-Sanchis, J.; Blasco, J. Visible–NIR reflectance spectroscopy and manifold learning methods applied to the detection of fungal infections on citrus fruit. J. Food Eng. 2015, 163, 17–24. [Google Scholar] [CrossRef]
- Chafer, M.; Sanchez-Gonzalez, L.; Gonzalez-Martinez, C.; Chiralt, A. Fungal decay and shelf Life of oranges coated with chitosan and bergamot, thyme, and tea tree essential oils. J. Food Sci. 2012, 77, E182–E187. [Google Scholar] [CrossRef] [PubMed]
- Moscoso-Ramírez, P.; Palou, L. Preventive and curative activity of postharvest potassium silicate treatments to control green and blue molds on orange fruit. Eur. J. Plant Pathol. 2014, 138, 721–732. [Google Scholar] [CrossRef]
- Droby, S.; Eick, A.; Macarisin, D.; Cohen, L.; Rafael, G.; Stange, R.; McColum, G.; Dudai, N.; Nasser, A.; Wisniewski, M.; et al. Role of citrus volatiles in host recognition, germination and growth of Penicillium digitatum and Penicillium italicum. Postharvest Biol. Technol. 2008, 49, 386–396. [Google Scholar] [CrossRef]
- Moscoso-Ramírez, P.; Montesinos-Herrero, C.; Palou, L. Antifungal activity of sodium propylparaben alone or in combination with low doses of imazalil against Penicillium decay on citrus fruit. Eur. J. Plant Pathol. 2014, 140, 145–157. [Google Scholar] [CrossRef]
- Marques, J.P.R.; Amorim, L.; Silva-Junior, G.J.; Spósito, M.B.; Appezzato-da Gloria, B. Structural and biochemical characteristics of citrus flowers associated with defence against a fungal pathogen. AoB Plants 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Vedachalam, S.; Xiang, S.; Liu, X.-W. Direct C-glycosylation of organotrifluoroborates with glycosyl fluorides and its application to the total synthesis of (+)-Varitriol. Org. Lett. 2011, 13, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.L.; Wu, Y.L. Stereoselective synthesis of methyl (11S,12S,13S)-(9Z,15Z)-11-hydroxy-12,13-epoxy octadecadienoate from d-mannose. Tetrahedron 1993, 49, 4665–4670. [Google Scholar] [CrossRef]
- Michieletti, M.; Bracci, A.; Compostella, F.; de Libero, G.; Mori, L.; Fallarini, S.; Lombardi, G.; Panza, L. Synthesis of α-galactosyl ceramide (KRN7000) and analogues thereof via a common precursor and their preliminary biological assessment. J. Org. Chem. 2008, 73, 9192–9195. [Google Scholar] [CrossRef] [PubMed]
- Ohrui, H.; Jones, G.H.; Moffatt, J.G.; Maddox, M.L.; Christensen, A.T.; Byram, S.K. C-Glycosyl nucleosides. V. Some unexpected observations on the relative stabilities of compounds containing fused five-membered rings with epimerizable substituenes. J. Am. Chem. Soc. 1975, 97, 4602–4613. [Google Scholar]
- Yan, S.; Liang, Y.; Zhang, J.; Chen, Z.; Liu, C.-M. Autoxidated linolenic acid inhibits aflatoxin biosynthesis in Aspergillus flavus via oxylipin species. Fungal Genet. Biol. 2015, 81, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.-H.; Hu, K.-D.; Hu, L.-Y.; Li, Y.-H.; Hu, L.-B.; Yan, H.; Liu, Y.-S.; Zhang, H. An antifungal role of hydrogen sulfide on the postharvest pathogens Aspergillus niger and Penicillium italicum. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Liavonchanka, A.; Feussner, I. Lipoxygenases: Occurrence, functions and catalysis. J. Plant Physiol. 2006, 163, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Si, W. The Study on the Biological Characters of Postharvest Pathogen Diplodia natalensis in Ponkan Fruit and Their Control. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2011. [Google Scholar]
- Zhu, Y.; Yu, J.; Brecht, J.K.; Jiang, T.; Zheng, X. Pre-harvest application of oxalic acid increases quality and resistance to Penicillium expansum in kiwifruit during postharvest storage. Food Chem. 2016, 190, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 7 and 8 are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
