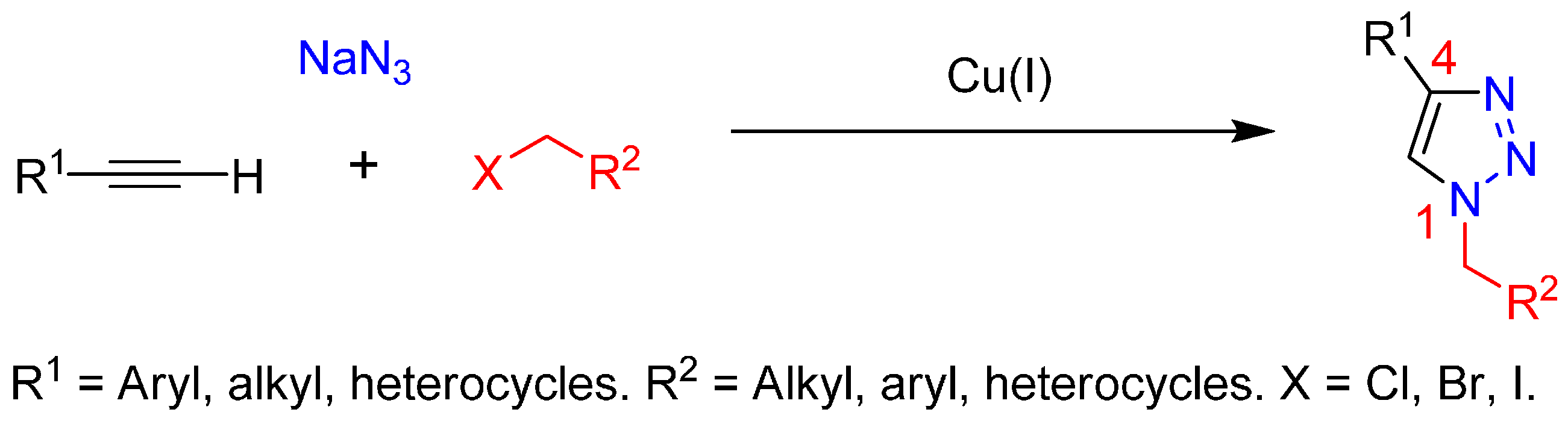Abstract
An efficient one-pot synthesis of 1,2,3-triazole derivatives of dihydropyrimidinones has been developed using two multicomponent reactions. The aldehyde-1,2,3-triazoles were obtained in good yields from in situ-generated organic azides and O-propargylbenzaldehyde. The target heterocycles were synthesized through the Biginelli reaction in which the aldehyde-1,2,3-triazoles reacted with ethyl acetoacetate and urea in the presence of Ce(OTf)3 as the catalyst. The corrosion inhibition of steel grade API 5 L X52 in 1 M HCl by the synthesized compounds was investigated using the electrochemical impedance spectroscopy technique. The measurements revealed that these heterocycles are promising candidates to inhibit acidic corrosion of steel.
1. Introduction
Multicomponent reactions (MCRs) are one-pot processes in which three or more starting materials are combined in a single reaction vessel to give a product that incorporates substantial portions of all the components. MCR processes are of great interest in organic and medicinal chemistry because of several attributes, including selectivity, atom economy, convergence, versatility, and molecular complexity [1,2,3,4,5,6]. In this regard, one example of MCR is the azide-alkyne 1,3-dipolar cycloaddition between benzyl halides, sodium azide and terminal alkyne to afforded 1,2,3-triazoles [7] (Scheme 1). Currently, the copper(I)-catalyzed azide-alkyne cycloaddition (also known as the Click or Huisgen-Meldal-Sharpless reaction) is the most widely used method for the synthesis of 1,4-disubstituted 1,2,3-triazoles from a wide range of organic azides and terminal alkynes [8,9,10].
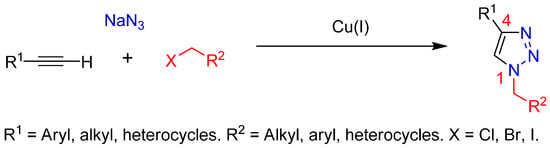
Scheme 1.
Multicomponent click reaction.
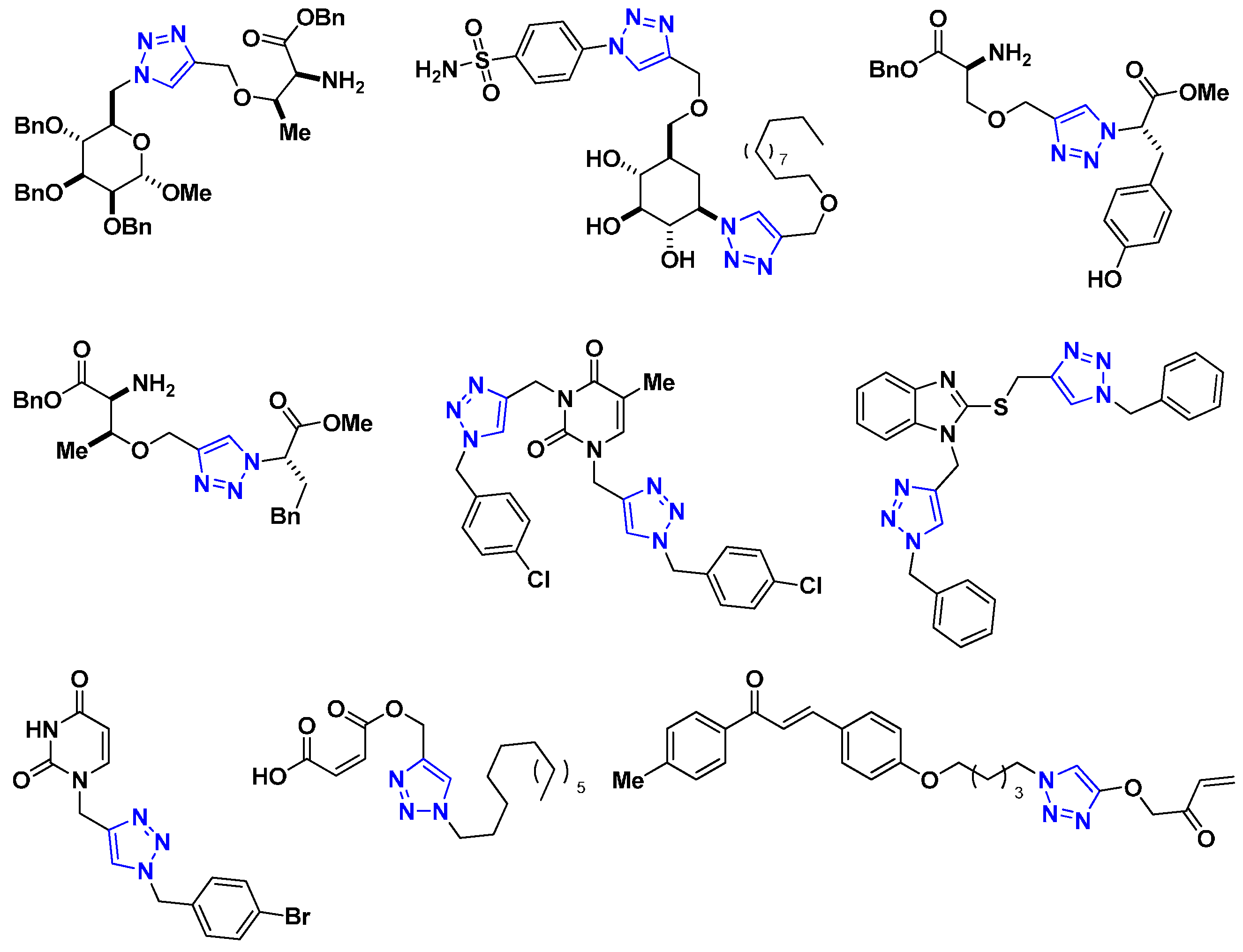
Recently, 1,2,3-triazole derivatives of carbohydrates, amino acids, maleic acid, and chalcones have been studied as effective corrosion inhibitors of steel in acidic media (Figure 1) [11,12,13,14,15,16]. This nitrogen-containing heterocycle is not only used to mitigate corrosion but is also an important pharmacophore because of its presence in many compounds displaying pharmacological activities [17,18,19,20,21,22,23]. Additionally, our group has reported the multicomponent synthesis and corrosion inhibitory activity of 1,2,3-triazole derivatives of pyrimidine nucleobases and 2-mercaptobenzimidazole [24,25,26,27].
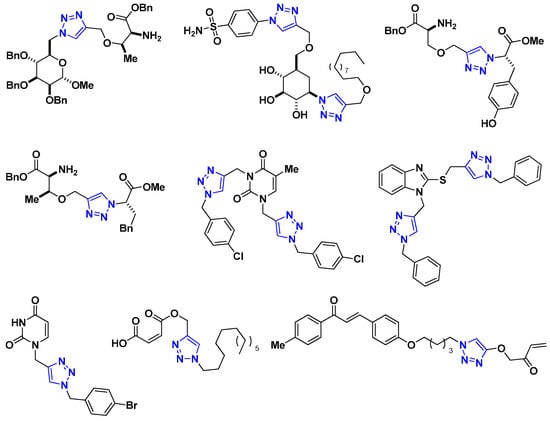
Figure 1.
Representative 1,2,3-triazoles with corrosion inhibition activity.
The corrosion of steels has received a considerable amount of attention as a result of industrial concern. API 5 L X52 steel is typically used in pipelines for gas and fuel conduction in the oil industry [28]. The steel pipelines for hydrocarbon transport are susceptible to corrosion cracking caused by hydrogen embrittlement and contact with fluids with high concentrations of chlorides [29,30]. One of the available methods to fight corrosion is the use of inhibitors that decrease the corrosion rates to an acceptable level. Corrosion inhibitors based on organic compounds containing nitrogen, oxygen, and sulfur atoms as well as π electrons associated with triple bonds, conjugated double bonds or aromatic rings are good inhibitors [31]. In this regard, 3,4-dihydropyrimidin-2(1H)-one (DHPM) is a heterocycle that possesses a wide range of biological activities [32,33,34,35,36,37]; however, its corrosion inhibition properties have hardly been studied [38].
Recently, several groups have introduced MCR strategies for the synthesis of heterocycles containing both dihydropyrimidinone and 1,2,3-triazole rings in their structures [39,40,41,42,43,44,45]. In continuation of our work on the development and study of organic corrosion inhibitors, we report herein a multicomponent synthesis of 1,2,3-triazole derivatives of dihydropyrimidinones and their evaluation as acidic corrosion inhibitors for API 5 L X52 steel.
2. Results and Discussion
2.1. Synthesis
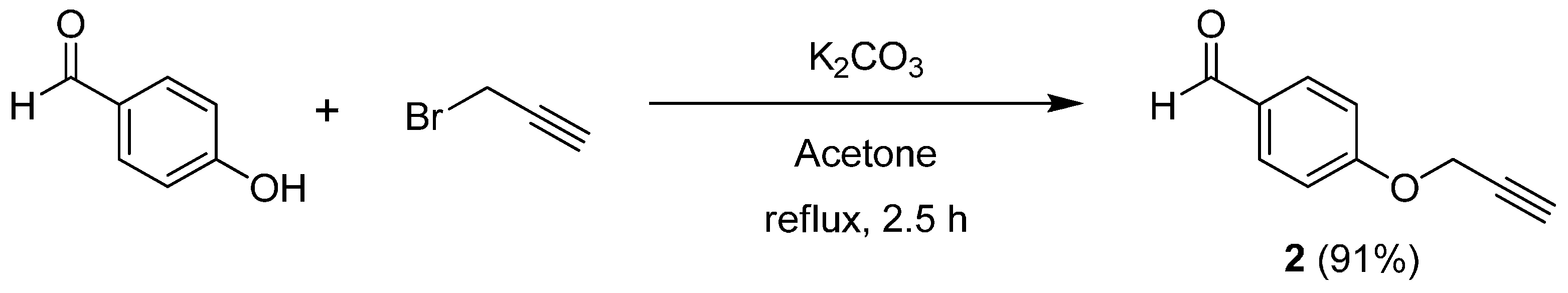
For the synthesis of the title compounds, O-propargylbenzaldehyde (2) was first synthesized from 4-hydroxybenzaldehyde (1) and propargyl bromide in the presence of K2CO3 in acetone under reflux. After 2.5 h, the desired compound 2 was obtained in 91% yield after workup and purification by crystallization (Scheme 2).
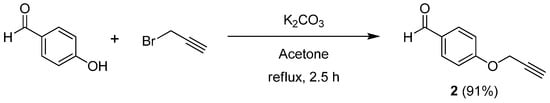
Scheme 2.
Synthesis of O-propargylbenzaldehyde (2).
Based on our previously reported methodology, we then performed the one-pot, three-component 1,3-dipolar cycloaddition reaction between O-propargylbenzaldehyde (2), sodium azide, and benzyl chloride in the presence of Cu(OAc)2·H2O, sodium ascorbate, and 1,10-phenantroline as the catalyst system in EtOH–H2O (2:1, v/v) at room temperature for 12 h [24,25,26]. The desired product 3 was obtained in 78% yield (Table 1, entry 1). As an alternative synthesis for this compound, we also performed the three-component reaction under microwave irradiation. When the reaction between the alkyne 2, sodium azide, and benzyl chloride was performed under microwave irradiation, the aldehyde-triazole 3 was obtained in 82% yield after 15 min at 90 °C (Table 1, entry 2). The aldehyde-triazoles 4–7 were prepared under these established reaction conditions and were obtained in good yields (Table 1, entries 3–6).

Table 1.
One-pot three-component reaction catalyzed by Cu(OAc)2·H2O. 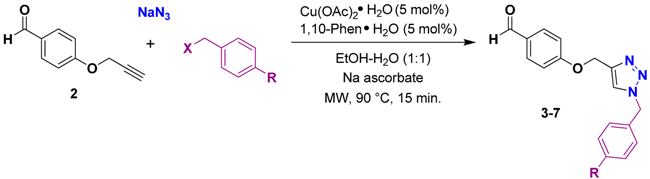

The next step of our synthetic strategy for the production of 1,2,3-triazole-DHPMs 8–12 involved a Biginelli reaction. Many Lewis acids have been reported as efficient catalysts for the Biginelli reaction [36]. We performed the one-pot, three-component reaction between ethyl acetoacetate, the corresponding aldehyde-triazoles 3–7, and urea using cerium trifluoromethanesulfonate [Ce(OTf)3] as a catalyst because of its high efficiency in this multicomponent reaction [46]. The heterocyclic compounds 8–12 were isolated in 80% to 87% yield (Table 2).

Table 2.
Biginelli reaction catalyzed by Ce(OTf)3. 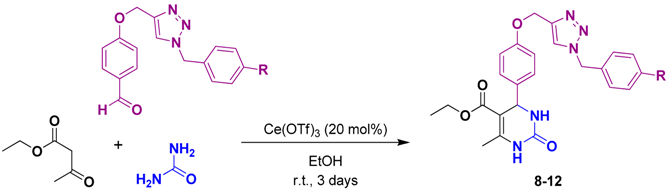

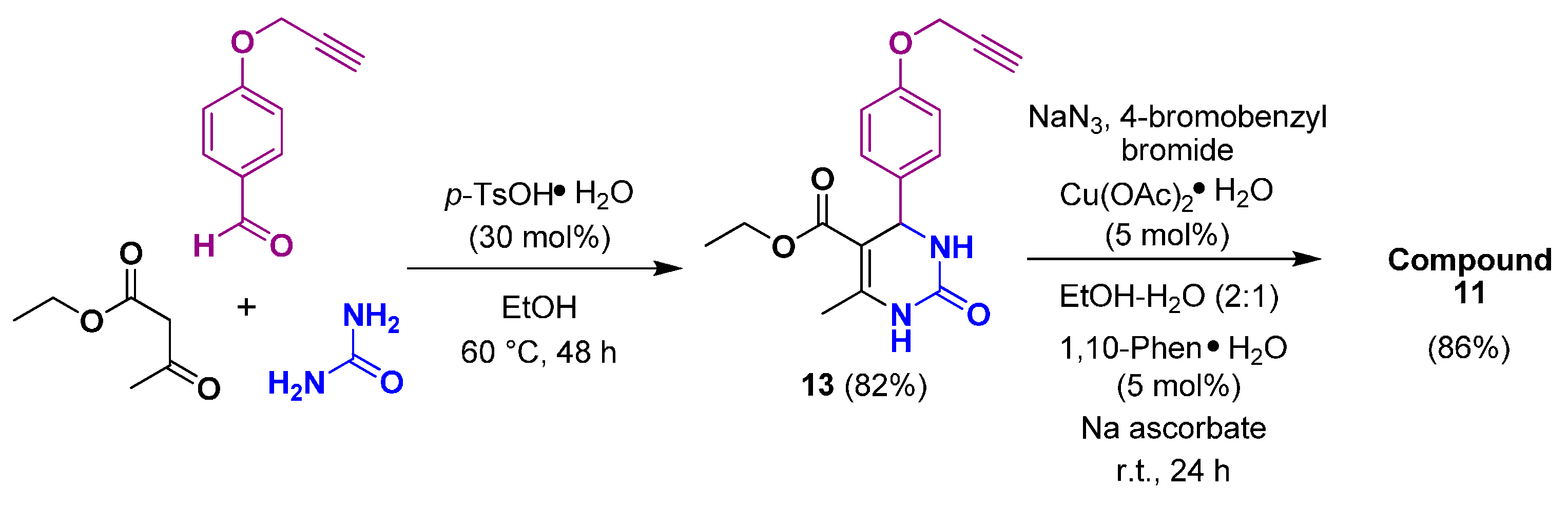
We also investigated another route for the synthesis of the target 1,2,3-triazole-DHPM, which involved the Biginelli/Click sequence. The preparation of DHPM (13) performed with ethyl acetoacetate, O-propargylbenzaldehyde (2), and urea in the presence of p-TsOH·H2O in EtOH at 60 °C for 48 h. The desired DHPM (13) was isolated in 82% yield and was used without purification for the following reaction. The 1H-NMR spectrum of DHPM (13) exhibited a triplet at δ = 3.52 (≡CH) and a doublet at δ = 4.76 (CH2) for the propargyl fragment, whereas signals at δ = 55.9 (CH2), 78.5 (≡CH), and 79.8 (C≡) appeared in the 13C-NMR spectrum (Figure S11). The one-pot, three-component reaction between the alkyne (13), 4-bromobenzyl bromide, and sodium azide was performed in the presence of a copper catalyst to give DHPM-triazole (11) in 86% yield (Scheme 3).
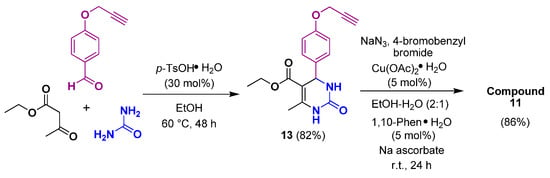
Scheme 3.
Synthesis of 1,2,3-triazole-DHPM through the Biginelli/Click sequence.
It is worth noting that the Biginelli reaction and the Click reaction cannot be performed directly in one step. When all of the reactants—O-propargylbenzaldehyde (2), 4-bromobenzyl bromide, sodium azide, ethyl acetoacetate and urea—were stirred in EOH–H2O (1:1 v/v) at 90 °C for 48 h in the presence of Cu(OAc)2·H2O (10 mol %), sodium ascorbate, and 1,10-phenantroline, only the aldehyde-triazole 6 was observed by thin layer chromatography (CH2Cl2–EtOH 95:5 v/v).
The structures of the synthesized heterocycles 8–12 were confirmed by examination of their 1H- and 13C-NMR and high-resolution mass spectra. The 1H- and 13C-NMR signals for compounds 8–12 were assigned with the help of 2D heteronuclear correlation experiments (HSQC and HMBC). The signals in the 1H-NMR spectra at δ = 8.27–8.28 corresponded to the triazolyl hydrogen, which were supported by the signals in the 13C-NMR spectra at δ = 125.0–125.1. The signals for the quaternary carbon of the triazole ring appeared at δ = 143.6–143.6 in the 13C-NMR spectra. These chemical shift values are consistent with those reported for 1,4-disubstituted 1,2,3-triazoles [24,25,26].
2.2. Corrosion Inhibition Efficiencies
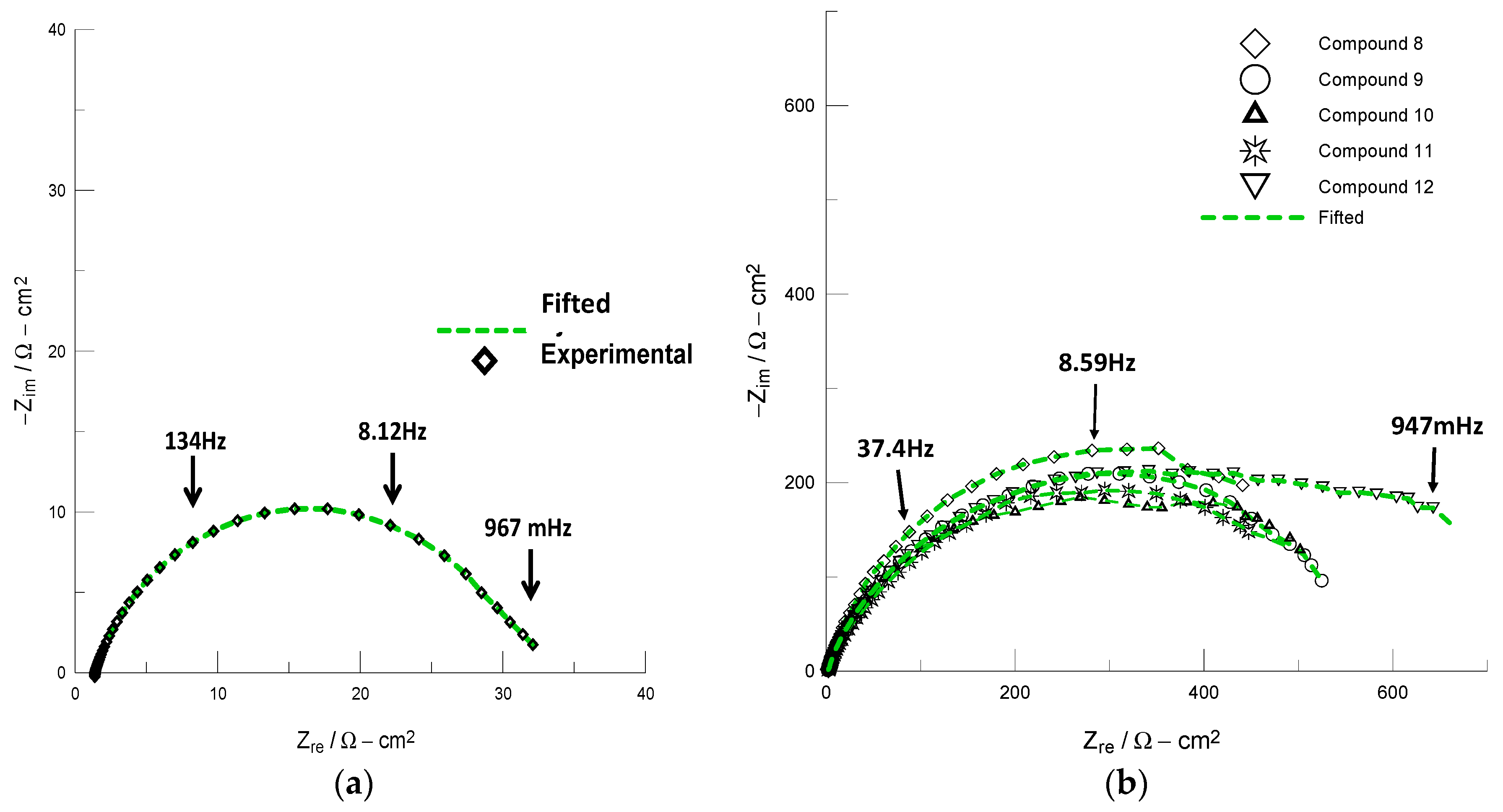
We employed electrochemical impedance spectroscopy (EIS) to evaluate the activity of compounds (8–12) on the corrosion inhibition of API 5 L X52 steel. The Nyquist plots obtained for API 5 L X52 steel in 1 M HCl solution in the absence and presence of the tested compounds 8–12 are shown in Figure 2. Figure 2a clearly shows that the steel in 1 M HCl shows one semicircle (Zre~30 Ωcm2), which indicates that the steel corrosion is mainly controlled by a charge transfer process. In contrast, the corresponding Nyquist plots obtained for the steel in the acid solution in the presence of 8–12 (10 ppm) increased the impedance (Zre) value (Figure 2b), which is generally attributed to the adsorption of the organic compounds onto the metal surface [16,27]. The electrochemical parameters obtained from fitting the recorded EIS data using the appropriate equivalent circuit model (R(Q)R) are listed in Table 3.
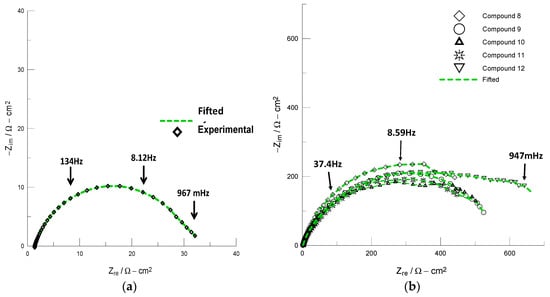
Figure 2.
Nyquist plots recorded in the following systems: (a) API 5 L X52/1 M HCl and (b) API 5 L X52/1 M HCl +10 ppm of compounds 8–12.

Table 3.
Electrochemical parameters obtained from experimental impedance data including the corrosion inhibition efficiencies (IE) at 10 ppm of the organic inhibitor.
The electrochemical data in Table 3 show that the charge transfer resistance (Rct) values increased, whereas the double layer capacitance (Cdl) values decreased with addition of compounds 8–12. A large Rct value is associated with a slower corrosion rate, whereas the decrease in Cdl can be attributed to the formation of a protective layer on the metal surface [14,16,27]. It is worth noting that all of the compounds exhibited corrosion inhibitory activity with inhibition efficiencies (IE) of approximately 95% at relatively low concentration values. These compounds provide excellent inhibition activity under static conditions that is comparable to if not better than those of organic inhibitors that incorporate the 1,2,3-triazole moiety [11,12,13,14,15,16,25,26,27].
3. Experimental Section
3.1. General Information
Commercially available reagents and solvents were used as received. Column chromatography was performed on Kieselgel silica gel 60 (230–400 mesh). Melting points were determined using a Fisher-Johns apparatus and are uncorrected. IR spectra were recorded on an Alpha FT-IR/ATR spectrometer (Bruker, Leipzig, Germany). The NMR spectra (400 MHz for 1H and 100.6 MHz for 13C) were obtained using a Bruker Ascend-400 spectrometer (Billerica, MA, USA). Chemical shifts (δ) are given in ppm and coupling constants (J) are given in hertz (Hz). High-resolution mass spectra (HRMS) were recorded on JMS-SX 102a (JEOL, Tokyo, Japan) and MSD-TOF-1069A (Agilent, Santa Clara, CA, USA) spectrometers. Microwave irradiation experiments were performed using a Discover System (CEM Corporation, Matthews, NC, USA) single-mode microwave with standard sealed microwave glass vials. The reaction temperature was monitored by an IR sensor on the outside wall of the reaction vial. The electrochemical impedance study was performed at room temperature using a Gill-AC electrochemical workstation (ACM Instruments, Cark, Cumbria, UK), with a sinusoidal perturbation signal of ±10 mV around Ecorr, within a range of 10−1 Hz to 104 Hz. A saturated Ag/AgCl electrode was used as the reference, a graphite rod was used as the counter electrode, and the working electrode was the API 5 L X52 steel sample with an exposed area of approximately 1 cm2 and a chemical composition (wt %) of C: 0.080, Mn: 1.06, Si: 0.26, Ti: 0.003, V: 0.054, Nb: 0.041, P: 0.019, S: 0.003, Al: 0.039, Ni: 0.019, Ceq: 0.274, Fe: balance [47], which was prepared prior to each experiment using standard metallographic procedures. The corrosion inhibition efficiency (IE) was evaluated by means of electrochemical impedance spectroscopy (EIS) in the API 5 L X52 steel/1 M HCl system containing 0 (blank) or 10 ppm of the organic inhibitor. A simulation of the impedance data recorded was conducted by means of electrical equivalent circuits and the electrical parameters: charge transfer resistance (Rct) and double layer capacitance (Cdl) were obtained in this way.
3.2. Product Synthesis and Characterization
4-(Prop-2-ynyloxy)benzaldehyde (2). In a 100 mL round bottom flask equipped with a magnetic stirrer and a reflux condenser, 4-hydroxybenzaldehyde (1, 1.00 g, 8.18 mmol) was dissolved in acetone (20 mL). Anhydrous potassium carbonate (1.47 g, 10.63 mmol) was then added and the mixture was heated to reflux for 30 min. After cooling to room temperature, propargyl bromide (1.18 mL, 13.29 mmol, 80 wt.% in toluene) was added slowly and the reaction mixture was heated to reflux for an additional 2 h. The solvent was evaporated under vacuum and the crude reaction mixture was treated with H2O (15 mL) and extracted with EtOAc (3 × 15 mL). The combined organic phases were dried (Na2SO4) and then activated charcoal was added and the combined organics were stirred and heated at 40 °C for 15 min. After the combined organics were filtered over Celite, which was washed with EtOAc (15 mL), the solvent was evaporated under vacuum and the crude product was purified by recrystallization from EtOAc/hexane (1:2 v/v) to afford 1.19 g (91% yield) of 2 as a white solid, m.p. 75–77 °C (Lit. [48] m.p. 75–78 °C). 1H-NMR (CDCl3): δ = 2.59 (t, J = 2.4 Hz, 1H, ≡CH), 4.79 (d, J = 2.4 Hz, 2H, OCH2), 7.10 (d, J = 8.7 Hz, 2H, ArH), 7.87 (d, J = 8.8 Hz, 2H, ArH), 9.91 (s, 1H, CHO). 13C-NMR (CDCl3): δ = 55.9 (OCH2), 76.3 (≡CH), 77.5 (C≡), 115.2 (2 × ArCH), 130.6 (Cipso), 131.9 (2 × ArCH), 162.4 (O-Cipso), 190.7 (C=O). FT-IR/ATR νmax cm−1: 3206 (≡CH), 2831, 2807, 2749, 2121 (C≡C), 1677 (C=O), 1601, 1574, 1504, 1248, 1213, 1168, 1006, 825, 509. HRMS (ESI-TOF) calculated for C10H8O2 + H+: 161.0597; Found: 161.0599.
4-((1-Benzyl-1H-1,2,3-triazol-4-yl)methoxy)benzaldehyde (3). In a 10 mL microwave vial equipped with a magnetic stirrer, Cu(OAc)2·H2O (11.4 mg, 0.063 mmol, 5 mol%), 1,10-phenanthroline monohydrate (12.5 mg, 0.063 mmol, 5 mol%), and sodium l-ascorbate (188 mg, 0.95 mmol) were added in EtOH–H2O (1:1 v/v, 4 mL), followed by stirring for five min at room temperature. Subsequently, alkyne 2 (200 mg, 1.25 mmol), sodium azide (90 mg, 1.38 mmol), and benzyl chloride (0.16 mL, 1.38 mmol) were added to the reaction mixture, which was irradiated (5–6 W) at 90 °C for 15 min. Afterward, H2O (15 mL) was added to the reaction mixture and extracted with CH2Cl2 (3 × 15 mL). The combined organic phases were dried (Na2SO4) and then the solvent was evaporated under vacuum. The crude product was purified by column chromatography (Hexane/EtOAc 1:1, v/v) and recrystallized from EtOAc/hexane (1:2, v/v) to afford 300 mg (82% yield) of 3 as a white solid, m.p. 95–97 °C [Lit. [48] m.p.100–103 °C]. 1H-NMR (CDCl3): δ = 5.26 (s, 2H, OCH2), 5.54 (s, 2H, NCH2), 7.08 (d, J = 8.7 Hz, 2H, ArH), 7.26–7.29 (m, 2H, ArH), 7.36–7.39 (m, 3H, ArH), 7.55 (s, 1H, ArH triazole), 7.82 (d, J = 8.7 Hz, 2H, ArH), 9.88 (s, 1H, CHO). 13C-NMR (CDCl3): δ = 54.3 (NCH2), 62.2 (OCH2), 115.0 (2 × ArCH), 122.8 (ArCH triazole), 128.2 (2 × ArCH), 128.9 (ArCH), 129.2 (2 × ArCH), 130.4 (Cipso), 132.0 (2 × ArCH), 134.3 (Cipso), 143.6 (Cipso triazole), 163.1 (O-Cipso), 190.8 (C=O). FT-IR/ATR νmax cm−1: 3143, 3097, 3064, 2963, 2935, 2842, 2820, 2760, 1673 (C=O), 1601, 1575, 1240, 1216, 1172, 834, 752. HRMS (ESI-TOF) calculated for C17H15N3O2 + H+: 294.1237; Found: 294.1240.
4-((1-(4-Fluorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)benzaldehyde (4). The procedure described above (using the same quantities of Cu(OAc)2·H2O, 1,10-phenanthroline monohydrate, and sodium L-ascorbate) was followed to obtain compound 4, employing alkyne 2 (200 mg, 1.25 mmol), NaN3 (90 mg, 1.38 mmol), and 4-fluorobenzyl chloride (0.16 mL, 1.38 mmol). The crude product was purified by column chromatography (hexane/EtOAc 1:1 v/v) and recrystallized from EtOAc/hexane (1:2 v/v) to afford 313 mg (80% yield) of the desired product 4 as a white solid, m.p. 89–90 °C [Lit. [49] m.p.101–104 °C]. 1H-NMR (CDCl3): δ = 5.26 (s, 2H, OCH2), 5.51 (s, 2H, NCH2), 7.04–7.09 (m, 4H, ArH), 7.26–7.30 (m, 2H, ArH), 7.56 (s, 1H, ArH triazole), 7.83 (d, J = 8.8 Hz, 2H, ArH), 9.88 (s, 1H, CHO). 13C-NMR (CDCl3): δ = 53.6 (NCH2), 62.2 (OCH2), 115.1 (2 × ArCH), 116.2 (d, JCF = 21.8 Hz, 2 × ArCH), 122.6 (ArCH triazole), 130.1 (d, JCF = 8.4 Hz, 2 × ArCH), 130.2 (d, JCF = 3.3 Hz, Cipso), 130.4 (Cipso), 132.0 (2 × ArCH), 143.8 (Cipso triazole), 162.9 (d, JCF = 248.5 Hz, F-Cipso), 163.1 (O-Cipso), 190.7 (C=O). FT-IR/ATR νmax cm−1: 3151, 3068, 3038, 2944, 2841, 2759, 1672 (C=O), 1601, 1574, 1456, 1254, 1217, 1157, 1032, 823, 501. HRMS (ESI-TOF) calculated for C17H14FN3O2 +H+: 312.1143; Found: 312.1146.
4-((1-(4-Chlorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)benzaldehyde (5). The procedure described above (using the same quantities of Cu(OAc)2·H2O, 1,10-phenanthroline monohydrate, and sodium l-ascorbate) was followed to obtain compound 5, employing alkyne 2 (200 mg, 1.25 mmol), NaN3 (90 mg, 1.38 mmol), and 4-chlorobenzyl chloride (222 mg, 1.38 mmol). The crude product was purified by column chromatography (hexane/EtOAc 1:1, v/v) and recrystallized from EtOAc/hexane (1:2, v/v) to afford 350 mg (85% yield) of the desired product 5 as a white solid, m.p. 88–89 °C [Lit. [49] m.p.108–110 °C]. 1H-NMR (CDCl3): δ = 5.26 (s, 2H, OCH2), 5.52 (s, 2H, NCH2), 7.08 (d, J = 8.7 Hz, 2H, ArH), 7.22 (d, J = 8.4 Hz, 2H, ArH), 7.35 (d, J = 8.4 Hz, 2H, ArH), 7.58 (s, 1H, ArH triazole), 7.83 (d, J = 8.7 Hz, 2H, ArH), 9.88 (s, 1H, CHO). 13C-NMR (CDCl3): δ = 53.6 (NCH2), 62.1 (OCH2), 115.1 (2 × ArCH), 122.8 (ArCH triazole), 129.4 (2 × ArCH), 129.5 (2 × ArCH), 130.4 (Cipso), 132.0 (2 × ArCH), 132.8 (Cl-Cipso), 135.0 (Cipso), 143.8 (Cipso triazole), 163.1 (O-Cipso), 190.8 (C=O). FT-IR/ATR νmax cm−1: 3149, 3100, 3055, 2943, 2807, 2744, 1671 (C=O), 1600, 1571, 1160, 992, 833, 806, 772, 501. HRMS (ESI-TOF) calculated for C17H14ClN3O2 + H+: 328.0847; Found: 328.0845.
4-((1-(4-Bromobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)benzaldehyde (6). The procedure described above (using the same quantities of Cu(OAc)2·H2O, 1,10-phenanthroline monohydrate, and sodium l-ascorbate) was followed to obtain compound 6, employing alkyne 2 (200 mg, 1.25 mmol), NaN3 (90 mg, 1.38 mmol), and 4-bromobenzyl bromide (345 mg, 1.38 mmol). The crude product was purified by column chromatography (hexane/EtOAc 1:1, v/v) and recrystallized from EtOAc/hexane (1:2, v/v) to afford 355 mg (76% yield) of the desired product 6 as a white solid, m.p. 82–84 °C. 1H-NMR (CDCl3): δ = 5.26 (s, 2H, OCH2), 5.50 (s, 2H, NCH2), 7.08 (d, J = 8.8 Hz, 2H, ArH), 7.16 (d, J = 8.4 Hz, 2H, ArH), 7.51 (d, J = 8.4 Hz, 2H, ArH), 7.57 (s, 1H, ArH triazole), 7.83 (d, J = 8.8 Hz, 2H, ArH), 9.88 (s, 1H, CHO). 13C-NMR (CDCl3): δ = 53.6 (NCH2), 62.2 (OCH2), 115.1 (2 × ArCH), 122.7 (ArCH triazole), 123.1 (Br-Cipso), 129.7 (2 × ArCH), 130.4 (Cipso), 132.0 (2 × ArCH), 132.4 (2 × ArCH), 133.3 (Cipso), 143.9 (Cipso triazole), 163.1 (O-Cipso), 190.7 (C=O). FT-IR/ATR νmax cm−1: 3149, 3100, 3054, 2943, 2807, 2746, 1670 (C=O), 1600, 1572, 1249, 1212, 1160, 991, 833, 770, 491. HRMS (ESI-TOF) calculated for C17H14BrN3O2 + H+: 372.0342; Found: 372.0347.
4-((1-(4-Iodobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)benzaldehyde (7). The procedure described above was followed to obtain compound 7, employing Cu(OAc)2·H2O (5.6 mg, 0.031 mmol), 1,10-phenanthroline monohydrate (6.1 mg, 0.031 mmol), sodium L-ascorbate (93 mg, 0.47 mmol), alkyne 2 (100 mg, 0.62 mmol), NaN3 (44 mg, 0.68 mmol), and 4-iodobenzyl bromide (214 mg, 0.72 mmol). The crude product was purified by column chromatography (Hexane/EtOAc 1:1 v/v) and recrystallized from EtOAc/hexane (1:2 v/v) to afford 190 mg (73% yield) of the desired product 7 as a white solid, m.p. 85–87 °C. 1H-NMR (CDCl3): δ = 5.27 (s, 2H, OCH2), 5.49 (s, 2H, NCH2), 7.03 (d, J = 8.4 Hz, 2H, ArH), 7.08 (d, J = 8.7 Hz, 2H, ArH), 7.56 (s, 1H, ArH triazole), 7.71 (d, J = 8.4 Hz, 2H, ArH), 7.83 (d, J = 8.8 Hz, 2H, ArH), 9.88 (s, 1H, CHO). 13C-NMR (CDCl3): δ = 53.7 (NCH2), 62.2 (OCH2), 94.7 (I-Cipso), 115.1 (2 × ArCH), 122.7 (ArCH triazole), 129.9 (2 × ArCH), 130.4 (Cipso), 132.0 (2 × ArCH), 134.0 (Cipso), 138.4 (2 × ArCH), 143.9 (Cipso triazole), 163.1 (O-Cipso), 190.7 (C=O). FT-IR/ATR νmax cm−1: 3154, 2953, 2919, 2873, 2820, 2799, 2738, 1682 (C=O), 1604, 1579, 1252, 1210, 1154, 1047, 1008, 811, 768. HRMS (ESI-TOF) calculated for C17H14IN3O2 + H+: 420.0203; Found: 420.0206.
Ethyl 4-(4-((1-benzyl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (8). In a 25 mL round-bottomed flask equipped with a magnetic stirrer, aldehyde-triazole 3 (100 mg, 0.34 mmol), urea (22 mg, 0.37 mmol), ethyl acetoacetate (47 μL, 0.37 mmol), and cerium trifluoromethanesulfonate (40 mg, 0.068 mmol, 20 mol %) were added in EtOH (2 mL). The reaction mixture was stirred at room temperature for 3 days. The solvent was evaporated under vacuum and the crude reaction mixture was purified by column chromatography (CH2Cl2→CH2Cl2/EtOH 98:2 v/v) to afford 122 mg (80% yield) of 8 as a white foam, m.p. 72–75 °C [Lit. [41] m.p.131–133 °C]. 1H-NMR (DMSO-d6): δ = 1.09 (t, J = 7.1 Hz, 3H, CH3), 2.24 (s, 3H, CH3), 3.97 (q, J = 7.1 Hz, 2H, OCH2), 5.09 (s, 1H, CH), 5.10 (s, 2H, OCH2), 5.61 (s, 2H, NCH2), 6.97 (d, J = 8.7 Hz, 2H, ArH), 7.15 (d, J = 8.7 Hz, 2H, ArH), 7.30–7.40 (m, 5H, ArH), 7.70 (br, 1H, NH), 8.28 (s, 1H, ArH triazole), 9.18 (br, 1H, NH). 13C-NMR (DMSO-d6): δ = 14.6 (OCH2CH3), 18.2 (CH3), 53.3 (NCH2), 53.8 (CH), 59.6 (OCH2CH3), 61.5 (OCH2), 100.0 (C=), 114.9 (2 × ArCH), 125.1 (ArCH triazole), 127.9 (2 × ArCH), 128.4 (2 × ArCH), 128.6 (ArCH), 129.2 (2 × ArCH), 136.5 (Cipso), 137.9 (Cipso), 143.5 (Cipso triazole), 148.5 (=CNH), 152.6 (NC=ON), 157.7 (O-Cipso), 165.8 (OC=O). FT-IR/ATR νmax cm−1: 3231, 3098, 2931, 1692 (C=O), 1638, 1507, 1454, 1220, 1174, 1085, 794, 756, 716, 653. HRMS (ESI-TOF) calculated for C24H25N5O4 + H+: 448.1979; Found: 448.1983.
Ethyl 4-(4-((1-(4-fluorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydro-pyrimidine-5-carboxylate (9). The procedure described above was followed to obtain compound 9, employing aldehyde-triazole 4 (100 mg, 0.32 mmol), urea (21 mg, 0.35 mmol), ethyl acetoacetate (44 μL, 0.35 mmol), and cerium trifluoromethanesulfonate (38 mg, 0.064 mmol, 20 mol %). The crude reaction mixture was purified by column chromatography (CH2Cl2→CH2Cl2/EtOH 98:2 v/v) to afford 130 mg (87% yield) of 9 as a white foam, m.p. 77–80 °C. 1H-NMR (DMSO-d6): δ = 1.10 (t, J = 7.1 Hz, 3H, CH3), 2.25 (s, 3H, CH3), 3.98 (q, J = 7.1 Hz, 2H, OCH2), 5.10 (s, 3H, CH, OCH2), 5.60 (s, 2H, NCH2), 6.97 (d, J = 8.6 Hz, 2H, ArH), 7.16 (d, J = 8.1 Hz, 2H, ArH), 7.18–7.23 (m, 2H, ArH), 7.38–7.42 (m, 2H, ArH), 7.67 (br, 1H, NH), 8.27 (s, 1H, ArH triazole), 9.15 (br, 1H, NH). 13C-NMR (DMSO-d6): δ = 14.5 (OCH2CH3), 18.2 (CH3), 52.5 (NCH2), 53.8 (CH), 59.6 (OCH2CH3), 61.6 (OCH2), 100.1 (C=), 115.0 (2 × ArCH), 116.0 (d, JCF = 21.6 Hz, 2 × ArCH), 125.0 (ArCH triazole), 127.9 (2 × ArCH), 130.7 (d, JCF = 8.4 Hz, 2 × ArCH), 132.6 (d, JCF = 3.0 Hz, Cipso), 137.9 (Cipso), 143.6 (Cipso triazole), 148.5 (=CNH), 152.6 (NC=ON), 157.7 (O-Cipso), 162.4 (d, JCF = 244.5 Hz, F-Cipso), 165.9 (OC=O). FT-IR/ATR νmax cm−1: 3228, 3098, 2928, 1692 (C=O), 1639, 1508, 1455, 1220, 1085, 829, 778. HRMS (ESI-TOF) calculated for C24H24FN5O4 + H+: 466.1885; Found: 466.1889.
Ethyl 4-(4-((1-(4-chlorobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-6-methyl-2oxo-1,2,3,4-tetrahydro-pyrimidine-5-carboxylate (10). The procedure described above was followed to obtain compound 10, employing aldehyde-triazole 5 (100 mg, 0.30 mmol), urea (20 mg, 0.33 mmol), ethyl acetoacetate (42 μL, 0.33 mmol), and cerium trifluoromethanesulfonate (35 mg, 0.060 mmol, 20 mol %). The crude reaction mixture was purified by column chromatography (CH2Cl2→CH2Cl2/EtOH 98:2, v/v) to afford 125 mg (86% yield) of 10 as a white solid, m.p. 157–158 °C. 1H-NMR (DMSO-d6): δ = 1.09 (t, J = 7.1 Hz, 3H, CH3), 2.24 (s, 3H, CH3), 3.97 (q, J = 7.1 Hz, 2H, OCH2), 5.09 (s, 1H, CH), 5.10 (s, 2H, OCH2), 5.61 (s, 2H, NCH2), 6.97 (d, J = 8.7 Hz, 2H, ArH), 7.15 (d, J = 8.6 Hz, 2H, ArH), 7.34 (d, J = 8.5 Hz, 2H, ArH), 7.44 (d, J = 8.4 Hz, 2H, ArH), 7.67 (br, 1H, NH), 8.28 (s, 1H, ArH triazole), 9.15 (br, 1H, NH). 13C-NMR (DMSO-d6): δ = 14.6 (OCH2CH3), 18.2 (CH3), 52.5 (NCH2), 53.8 (CH), 59.6 (OCH2CH3), 61.5 (OCH2), 100.0 (C=), 114.9 (2 × ArCH), 125.1 (ArCH triazole), 127.9 (2 × ArCH), 129.2 (2 × ArCH), 130.4 (2 × ArCH), 133.4 (Cl-Cipso), 135.5 (Cipso), 137.9 (Cipso), 143.5 (Cipso triazole), 148.5 (=CNH), 152.6 (NC=ON), 157.7 (O-Cipso), 165.8 (OC=O). FT-IR/ATR νmax cm−1: 3197, 3085, 2926, 1697 (C=O), 1638, 1218, 1172, 1090, 1017, 767. HRMS (ESI-TOF) calculated for C24H24ClN5O4 + H+: 482.1589; Found: 482.1595.
Ethyl 4-(4-((1-(4-bromobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydro-pyrimidine-5-carboxylate (11). The procedure described above was followed to obtain compound 11, employing aldehyde-triazole 6 (100 mg, 0.27 mmol), urea (18 mg, 0.30 mmol), ethyl acetoacetate (38 μL, 0.30 mmol), and cerium trifluoromethanesulfonate (32 mg, 0.054 mmol, 20 mol %). The crude reaction mixture was purified by column chromatography (CH2Cl2→CH2Cl2/EtOH 98:2, v/v) to afford 120 mg (85% yield) of 11 as a white solid, m.p. 188–190 °C. 1H-NMR (DMSO-d6): δ = 1.10 (t, J = 7.1 Hz, 3H, CH3), 2.25 (s, 3H, CH3), 3.98 (q, J = 7.1 Hz, 2H, OCH2), 5.10 (s, 3H, CH, OCH2), 5.60 (s, 2H, NCH2), 6.97 (d, J = 8.6 Hz, 2H, ArH), 7.15 (d, J = 8.6 Hz, 2H, ArH), 7.28 (d, J = 8.3 Hz, 2H, ArH), 7.58 (d, J = 8.4 Hz, 2H, ArH), 7.66 (br, 1H, NH), 8.28 (s, 1H, ArH triazole), 9.14 (br, 1H, NH). 13C-NMR (DMSO-d6): δ = 14.6 (OCH2CH3), 18.2 (CH3), 52.6 (NCH2), 53.8 (CH), 59.6 (OCH2CH3), 61.6 (OCH2), 100.0 (C=), 115.0 (2 × ArCH), 121.9 (Br-Cipso), 125.1 (ArCH triazole), 127.9 (2 × ArCH), 130.7 (2 × ArCH), 132.2 (2 × ArCH), 135.8 (Cipso), 137.9 (Cipso), 143.6 (Cipso triazole), 148.5 (=CNH), 152.6 (NC=ON), 157.7 (O-Cipso), 165.8 (OC=O). FT-IR/ATR νmax cm−1: 3197, 3086, 2925, 1696 (C=O), 1638, 1218, 1089, 826, 766, 656. HRMS (ESI-TOF) calculated for C24H24BrN5O4 + H+: 526.1084; Found: 526.1086.
Ethyl 4-(4-((1-iodobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydro-pyrimidine-5-carboxylate (12). The procedure described above was followed to obtain compound 12, employing aldehyde-triazole 7 (100 mg, 0.24 mmol), urea (16 mg, 0.26 mmol), ethyl acetoacetate (33 μL, 0.26 mmol), and cerium trifluoromethanesulfonate (28 mg, 0.048 mmol, 20 mol %). The crude reaction mixture was purified by column chromatography (CH2Cl2→CH2Cl2/EtOH 98:2, v/v) to afford 115 mg (83% yield) of 12 as a white solid, m.p. 208–210 °C. 1H-NMR (DMSO-d6): δ = 1.10 (t, J = 7.1 Hz, 3H, CH3), 2.24 (s, 3H, CH3), 3.97 (q, J = 7.1 Hz, 2H, OCH2), 5.10 (s, 3H, CH, OCH2), 5.57 (s, 2H, NCH2), 6.97 (d, J = 8.7 Hz, 2H, ArH), 7.11–7.16 (m, 4H, ArH), 7.70 (br, 1H, NH), 7.75 (d, J = 8.2 Hz, 2H, ArH), 8.28 (s, 1H, ArH triazole), 9.18 (br, 1H, NH). 13C-NMR (DMSO-d6): δ = 14.6 (OCH2CH3), 18.2 (CH3), 52.7 (NCH2), 53.8 (CH), 59.6 (OCH2CH3), 61.5 (OCH2), 94.9 (I-Cipso), 100.0 (C=), 114.9 (2 × ArCH), 125.1 (ArCH triazole), 127.9 (2 × ArCH), 130.7 (2 × ArCH), 136.2 (Cipso), 137.9 (Cipso), 138.0 (2 × ArCH), 143.5 (Cipso triazole), 148.5 (=CNH), 152.6 (NC=ON), 157.7 (O-Cipso), 165.8 (OC=O). FT-IR/ATR νmax cm−1: 3212, 3085, 2929, 1692 (C=O), 1638, 1510, 1218, 1090, 765, 658. HRMS (ESI-TOF) calculated for C24H24IN5O4 + H+: 574.0946; Found: 574.0945.
Ethyl 6-methyl-2-oxo-4-(4-(prop-2-ynyloxy)phenyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylate (13). In a 100 mL pressure vessel equipped with a magnetic stirrer, aldehyde 2 (160 mg, 1 mmol), urea (72 mg, 1.2 mmol), ethyl acetoacetate (0.14 mL, 1.1 mmol), and p-toluenesulfonic acid monohydrate (57 mg, 0.3 mmol) were added in EtOH (3 mL). The mixture was heated at 60 °C for 2 days, cooled and poured into ice/water (15 g), and the precipitate was filtered off and dried. The light yellow solid 13 (260 mg, 82% yield) was used directly for the following reaction. A sample for analysis was recrystallized from CH2Cl2-hexane (1:2 v/v), which gave a colorless solid, m.p. 159–160 °C. 1H-NMR (DMSO-d6): δ = 1.10 (t, J = 7.1 Hz, 3H, CH3), 2.24 (s, 3H, CH3), 3.52 (t, J = 2.2 Hz, 1H, ≡CH), 3.99 (q, J = 7.1 Hz, 2H, OCH2), 4.76 (d, J = 2.1 Hz, 2H, OCH2), 5.10 (d, J = 2.8 Hz, 1H, CH), 6.93 (d, J = 8.6 Hz, 2H, ArH), 7.16 (d, J = 8.6 Hz, 2H, ArH), 7.66 (br, 1H, NH), 9.14 (br, 1H, NH). 13C-NMR (DMSO-d6): δ = 14.6 (OCH2CH3), 18.2 (CH3), 53.8 (CH), 55.9 (OCH2), 59.6 (OCH2CH3), 78.5 (≡CH), 79.8 (C≡), 100.0 (C=), 115.2 (2 × ArCH), 127.8 (2 × ArCH), 138.3 (Cipso), 148.5 (=CNH), 152.6 (NC=ON), 156.9 (O-Cipso), 165.8 (OC=O). FT-IR/ATR νmax cm−1: 3276, 3246, 3114, 2981, 2920, 2115 (C≡C), 1704 (C=O), 1650, 1508, 1221, 1175, 1093, 1034, 776. HRMS (ESI-TOF) calculated for C17H18N2O4 + H+: 315.1339; Found: 315.1344.
4. Conclusions
In conclusion, we have developed an efficient synthesis of 1,2,3-triazole derivatives of dihydropyrimidinones by the MCR strategy (Click/Biginelli reactions) in good yields. Consequently, this MCR strategy is a powerful synthetic tool for the construction of new corrosion inhibitors based on 1,2,3-triazoles and dihydropyrimidinones. EIS measurements showed that the inhibitive capacity for API 5 L X52 steel in 1 M HCl of the heterocycles 8–12 was comparable or better than that other organic inhibitors that incorporate the 1,2,3-triazole moiety. Further investigations of these heterocycles as corrosion inhibitors for steel are in progress and will be reported in due course.
Supplementary Materials
Copies of 1H- and 13C-NMR spectra for compounds 3–13 (Figures S1–S11). Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/2/250/s1.
Acknowledgments
The authors would like to thank Consejo Nacional de Ciencia y Tecnología, CONACyT (project 181448) for financial support. R. González-Olvera, G.E. Negrón-Silva, A. Espinoza-Vázquez, F.J. Rodríguez-Gómez and R. Santillan wish to acknowledge the SNI (Sistema Nacional de Investigadores) for the distinction of their membership and the stipend received. We also wish to thank Maria Eugenia Ochoa, Rebeca Yépez for the technical assistance, and Geiser Cuéllar for the mass measurements.
Author Contributions
R.G.-O. conceived, designed, and performed the experiments and prepared the manuscript and the supplementary information; V.R.-R. performed the experiments; G.E.N.-S. provided conceptual guidance, supervised the project, and edited the manuscript; A.E.-V. and F.J.R.-G. designed and performed the electrochemical experiments and analyzed the data; R.S. contributed with analysis tools, discussed the NMR data, and edited the manuscript. All authors read and commented on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dömling, A.; Ugi, I. Multicomponent reactions with Isocianides. Angew. Chem. Int. Ed. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Hulme, C.; Gore, V. Multi-component reactions: Emerging chemistry in drug discovery from Xylocain to Crixivan. Curr. Med. Chem. 2003, 10, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Bienymé, H. (Eds.) Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005.
- Ganem, B. Strategies for innovation in multicomponent reaction design. Acc. Chem. Res. 2009, 42, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, G.; Ruijter, E.; Orru, R.V.A. Efficiency, diversity, and complexity with multicomponent reactions. Synlett 2013, 24, 666–685. [Google Scholar]
- Appkkuttan, P.; Dehaen, W.; Fokin, V.V.; van der Eycken, E. A microwave-assisted click chemistry synthesis of 1,4-disubstituted 1,2,3-triazoles via a copper(I)-catalyzed three-component reaction. Org. Lett. 2004, 6, 4223–4225. [Google Scholar] [CrossRef] [PubMed]
- Bock, V.D.; Hiemstra, H.; van Maarseveen, J.H. CuI-catalyzed alkyne-azide “click” cycloadditions from a mechanistic and synthetic perspective. Eur. J. Org. Chem. 2006. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: New reactivity of Copper(I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Ding, N.-N.; Wei, X.-L.; Cai, L.; He, X.-P.; Long, Y.-T.; Chen, G.-R.; Chen, K. Identification of diverse 1,2,3-triazole-connected benzyl glycoside-serine/threonine conjugates as potent corrosion inhibitors for mild steel in HCl. Corros. Sci. 2012, 64, 64–73. [Google Scholar] [CrossRef]
- Deng, Q.; Shi, H.-W.; Ding, N.-N.; Chen, B.-Q.; He, X.-P.; Liu, G.; Tang, Y.; Long, Y.-T.; Chen, G.-R. Novel triazolyl bis-amino acid derivatives readily synthesized via click chemistry as potential corrosion inhibitors for mild steel in HCl. Corros. Sci. 2012, 57, 220–227. [Google Scholar] [CrossRef]
- Zhang, H.-L.; He, X.-P.; Deng, Q.; Long, Y.-T.; Chen, G.-R.; Chen, K. Research on the structure-surface adsorptive activity relationships of triazolyl glycolipid derivatives for mild steel in HCl. Carbohydr. Res. 2012, 354, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; He, X.-P.; Shi, H.-W.; Chen, B.-Q.; Liu, G.; Tang, Y.; Long, Y.-T.; Chen, G.-R.; Chen, K. Concise Cu(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition reaction ligation remarkably enhances the corrosion inhibitive potency of natural amino acids for mild steel in HCl. Ind. Eng. Chem. Res. 2012, 51, 7160–7169. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, S.; Quan, H.; Huang, Z.; Xu, S. Synthesis and corrosion inhibition performance of alkyl triazole derivatives. Res. Chem. Intermed. 2015, 41, 2709–2724. [Google Scholar] [CrossRef]
- Ramaganthan, B.; Gopiraman, M.; Olasunkanmi, L.O.; Kabanda, M.M.; Yesudass, S.; Bahadur, I.; Adekunle, A.S.; Obot, I.B.; Ebenso, E.E. Synthesized photo-cross-linking chalcones as novel corrosion inhibitors for mild steel in acidic medium: experimental, quantum chemical and Monte Carlo simulation studies. RSC Adv. 2015, 5, 76675–76688. [Google Scholar] [CrossRef]
- Ganesh, A. Potential biological activity of 1,4-sustituted-1H-[1,2,3]triazoles. Int. J. Chem. Sci. 2013, 11, 573–578. [Google Scholar]
- He, X.-P.; Xie, J.; Tang, Y.; Li, J.; Chen, G.-R. CuAAC click chemistry accelerates the discovery of novel chemical scaffolds as promising protein tyrosine phospatases inhibitors. Curr. Med. Chem. 2012, 19, 2399–2405. [Google Scholar] [PubMed]
- Xu, S.; Zhuang, X.; Pan, X.; Zhang, Z.; Duan, L.; Liu, Y.; Zhang, L.; Ren, X.; Ding, K. 1-Phenyl-4-benzoyl-1H-1,2,3-traizoles as orally bioavailable transcriptional function suppressors of estrogen-related receptor α. J. Med. Chem. 2013, 56, 4631–4640. [Google Scholar] [CrossRef] [PubMed]
- Sirivolu, V.R.; Vernekar, S.K.V.; Ilina, T.; Myshakina, N.S.; Parniak, M.A.; Wang, Z. Clicking 3’-azidothymidine into novel potent inhibitors of human immunodeficiency virus. J. Med. Chem. 2013, 56, 8765–8780. [Google Scholar] [CrossRef] [PubMed]
- Pingaew, R.; Prachayasittikul, V.; Mandi, P.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis and molecular docking of 1,2,3-triazole-based sulfonamides as aromatase inhibitors. Bioorg. Med. Chem. 2015, 23, 3472–3480. [Google Scholar] [CrossRef] [PubMed]
- Keck, T.M.; Banal, A.K.; Slack, R.D.; Burzynski, C.; Bonifazi, A.; Okunola-Bakare, O.M.; Moore, M.; Deschamps, J.R.; Rais, R.; Slusher, B.S.; et al. Using click chemistry toward novel 1,2,3-triazole-linked dopamine D3 receptors ligands. Bioorg. Med. Chem. 2015, 23, 4000–4012. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Patil, S.A.; Budagumpi, S.; Nagaraja, B.M. Triazole: A promising antitubercular agent. Chem. Biol. Drug Des. 2015, 86, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Negrón-Silva, G.E.; González-Olvera, R.; Angeles-Beltrán, D.; Maldonado-Carmona, N.; Espinoza-Vázquez, A.; Palomar-Pardavé, M.E.; Romero-Romo, M.A.; Santillan, R. Synthesis of new 1,2,3-triazole derivatives of uracil and thymine with potential inhibitory activity against acidic corrosion of steels. Molecules 2013, 18, 4613–4627. [Google Scholar] [CrossRef] [PubMed]
- González-Olvera, R.; Espinoza-Vázquez, A.; Negrón-Silva, G.E.; Palomar-Pardavé, M.E.; Romero-Romo, M.A.; Santillan, R. Multicomponent click synthesis of new 1,2,3-triazole derivatives of pyrimidine nucleobases: promising acidic corrosion inhibitors for steel. Molecules 2013, 18, 15064–15079. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gonzalez, D.Y.; González-Olvera, R.; Negrón-Silva, G.E.; Lomas-Romero, L.; Gutiérrez-Carrillo, A.; Palomar-Pardavé, M.E.; Romero-Romo, M.A.; Santillan, R.; Uruchurtu, J. One-pot three-component synthesis of new mono- and bis-1,2,3-triazole derivatives of 2-benzimidazolethiol with a promising inhibitory activity against acidic corrosion of steel. Synthesis 2014, 46, 1217–1223. [Google Scholar] [CrossRef]
- Espinoza-Vázquez, A.; Negrón-Silva, G.E.; González-Olvera, R.; Angeles-Beltrán, D.; Herrera-Hernández, H.; Romero-Romo, M.; Palomar-Pardavé, M. Mild steel corrosion inhibition in HCl by di-alkyl and di-1,2,3-triazole derivatives of uracil and thymine. Mater. Chem. Phys. 2014, 145, 407–417. [Google Scholar] [CrossRef]
- Vargas-Arista, B.; Balvantin, A.; Baltazar, A.; Garcia Vazquez, F. On the use of ultrasonic spectra analysis for the characterization of artificially degrade API 5L X52 steel pipeline welded joints. Mater. Sci. Eng. A 2012, 550, 227–234. [Google Scholar] [CrossRef]
- Javidi, M.; Bahalaou Horeh, S. Investigating the mechanism of stress corrosion craking in near-neutral and high pH enviroments for API 5L X52 steel. Corros. Sci. 2014, 80, 213–220. [Google Scholar] [CrossRef]
- Heydari, M.; Javidi, M. Corrosion inhibition and adsorption behaviour of an amido-imidazoline derivative on API 5L X52steel in CO2-saturated solution and synergistic effect of iodide ions. Corros. Sci. 2012, 61, 148–155. [Google Scholar]
- Sastri, V.S. Green Corrosion Inhibitors: Theory and Practice; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Kappe, C.O. 100 Years of the Biginelli Dihydropyrimidine Synthesis. Tetrahedron 1993, 49, 6937–6963. [Google Scholar] [CrossRef]
- Kappe, C.O. Biologically active dihydropirimidones of the Biginelli-type—A literature survey. Eur. J. Med. Chem. 2000, 35, 1043–1052. [Google Scholar] [CrossRef]
- Kappe, C.O.; Stadler, A. The Biginelli dihydropyrimidine synthesis. Org. React. 2004, 63, 1–116. [Google Scholar]
- Jadhav, V.B.; Holla, H.V.; Tekale, S.U.; Pawar, R.P. Bioactive dihydropyrimidines: An overview. Chem. Sin. 2012, 3, 1213–1228. [Google Scholar]
- Suresh; Sandhu, J.S. Past, present and future of the Biginelli reaction: A critical perspective. ARKIVOC 2012, 1, 66–133. [Google Scholar]
- De Fátima, A.; Braga, T.C.; Neto, L.S.; Terra, B.S.; Oliveira, B.G.F.; da Silva, D.L.; Modolo, L.V. A mini-review on Biginelli adducts with notable pharmacological properties. J. Adv. Res. 2015, 6, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, N.; Akbas, E. The inhibition effect of some pyrimidine derivatives on austenitic stainless steel in acidic media. Mater. Chem. Phys. 2011, 126, 983–988. [Google Scholar] [CrossRef]
- Khanetskyy, B.; Dallinger, D.; Kappe, C.O. Combining Biginelli multicomponent and click chemistry: Generation of 6-(1,2,3-triazol-1-yl)-dihydropyrimidone libraries. J. Comb. Chem. 2004, 6, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J.; Wang, J.-D. Ultrasound-assisted synthesis of novel 4-(2-phenyl-1,2,3-Triazol-4-yl)-3,4-dihydropyrimidin-2(1H)-(thio)ones Catalyzed by Sm(ClO4)3. Molecules 2010, 15, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Salehi, P.; Dabiri, M.; Koohshari, M.; Movahed, S.K.; Bararjanian, M. One-pot synthesis of 1,2,3-triazole linked dihydropyrimidinones via Huisgen 1,3-dipolar/Biginelli cycloaddition. Mol. Divers. 2011, 15, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, X.; Li, Y. An efficient protocol for the one-pot synthesis of 4-(2-(4-bromophenyl)-1,2,3-triazol-4-yl)-3,4-dihydropyrimidin-2(1H)-ones/thiones catalyzed by Mg(NO3)2. J. Heterocycl. Chem. 2011, 48, 92–95. [Google Scholar] [CrossRef]
- Quan, Z.-J.; Xu, Q.; Zhang, Z.; Da, Y.-X.; Wang, X.-C. Copper-catalyzed click synthesis of functionalized 1,2,3-triazoles with 3,4-dihydropyrimidinone or amide group via a one-pot four-component reaction. Tetrahedron 2013, 69, 881–887. [Google Scholar] [CrossRef]
- Dharma Rao, G.B.; Anjaneyulu, B.; Kaushik, M.P. A facile one-pot five-component synthesis of glycoside annulated dihydropyrimidinone derivatives with 1,2,3-triazol linkage via transesterification/Biginelli/click reactions in aqueous medium. Tetrahedron Lett. 2014, 55, 19–22. [Google Scholar] [CrossRef]
- Balan, B.; Bahulayan, D. A novel green synthesis of α/β-amino acid functionalized pyrimidinone peptidomimetics using triazole ligation through click-multi-component reactions. Tetrahedron Lett. 2014, 55, 227–231. [Google Scholar] [CrossRef]
- Sun, Z.-L.; Jiang, H. Synthesis and characterization of transition metal methanosulfonates and their catalytic behavior in Biginelli reactions. Trans. Metal. Chem. 2005, 30, 792–796. [Google Scholar]
- American Petroleum Institute. API 5L: Specification for Line Pipe; American Petroleum Institute: Washington, DC, USA, 2004; pp. 8–17. [Google Scholar]
- Kinfe, H.H.; Belay, Y.H. Synthesis and biological evaluation of novel thiosemicarbazone-triazole hybrid compounds as antimalarial agents. S. Afr. J. Chem. 2013, 66, 130–135. [Google Scholar]
- Chavan, P.V.; Pandit, K.S.; Desai, U.V.; Kulkarni, M.A.; Wadgaonkar, P.P. Cellulose supported cuprous iodide nanoparticles (Cell-CuI NPs): A new heterogeneous and recyclable catalyst for the one pot synthesis of 1,4-disubstituted-1,2,3-triazoles in water. RSC Adv. 2014, 4, 42137–42146. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compounds 3–13 are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).