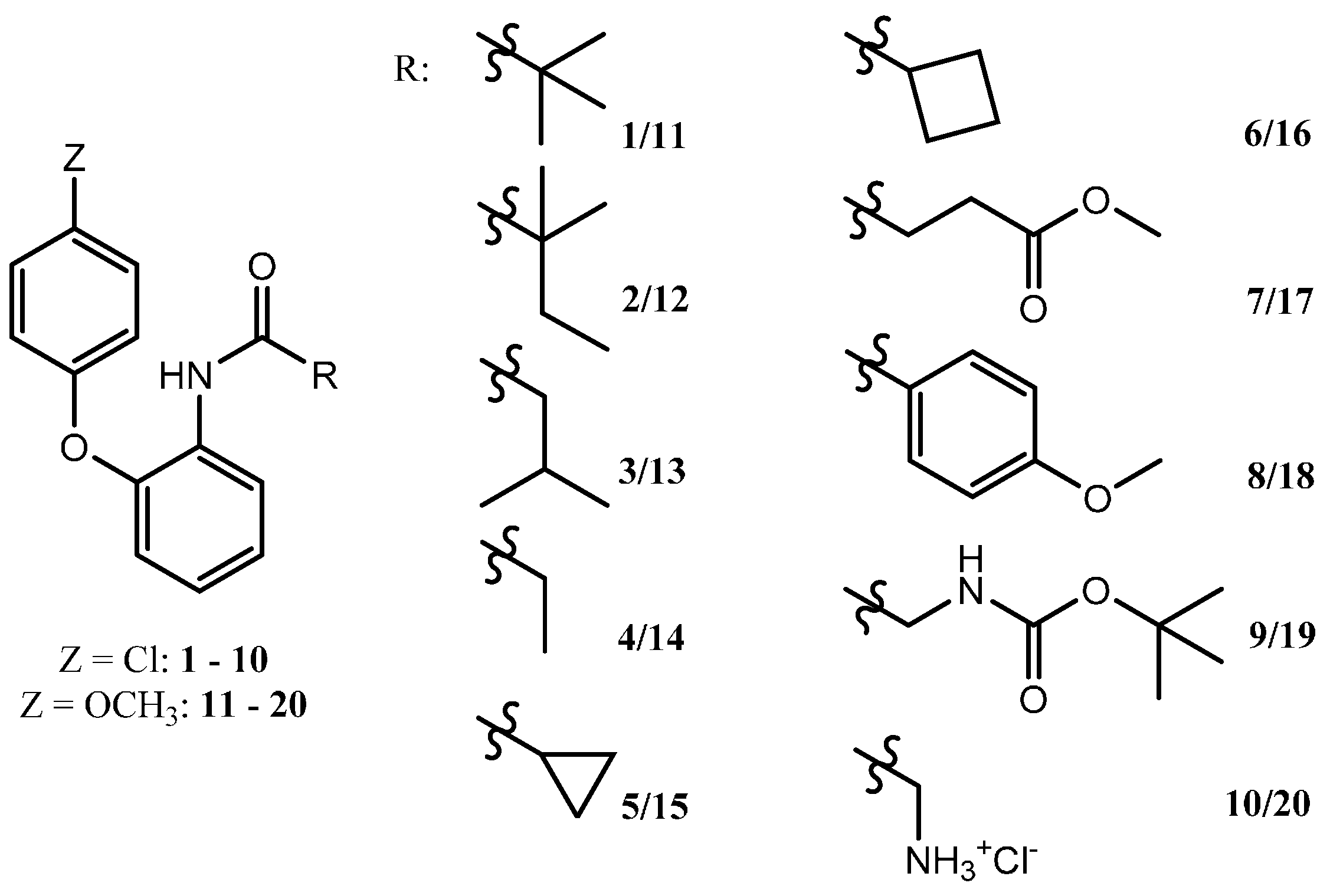
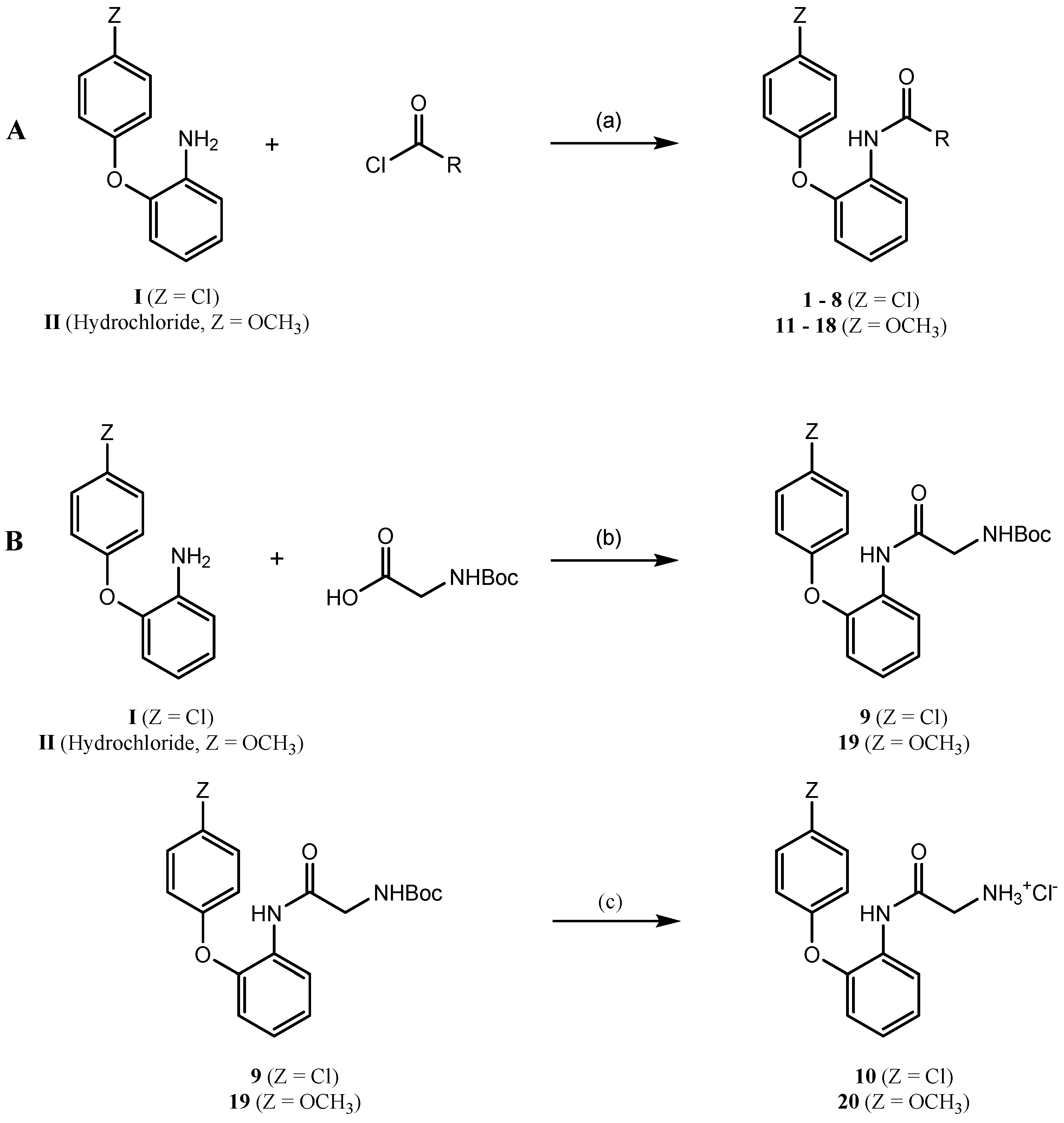
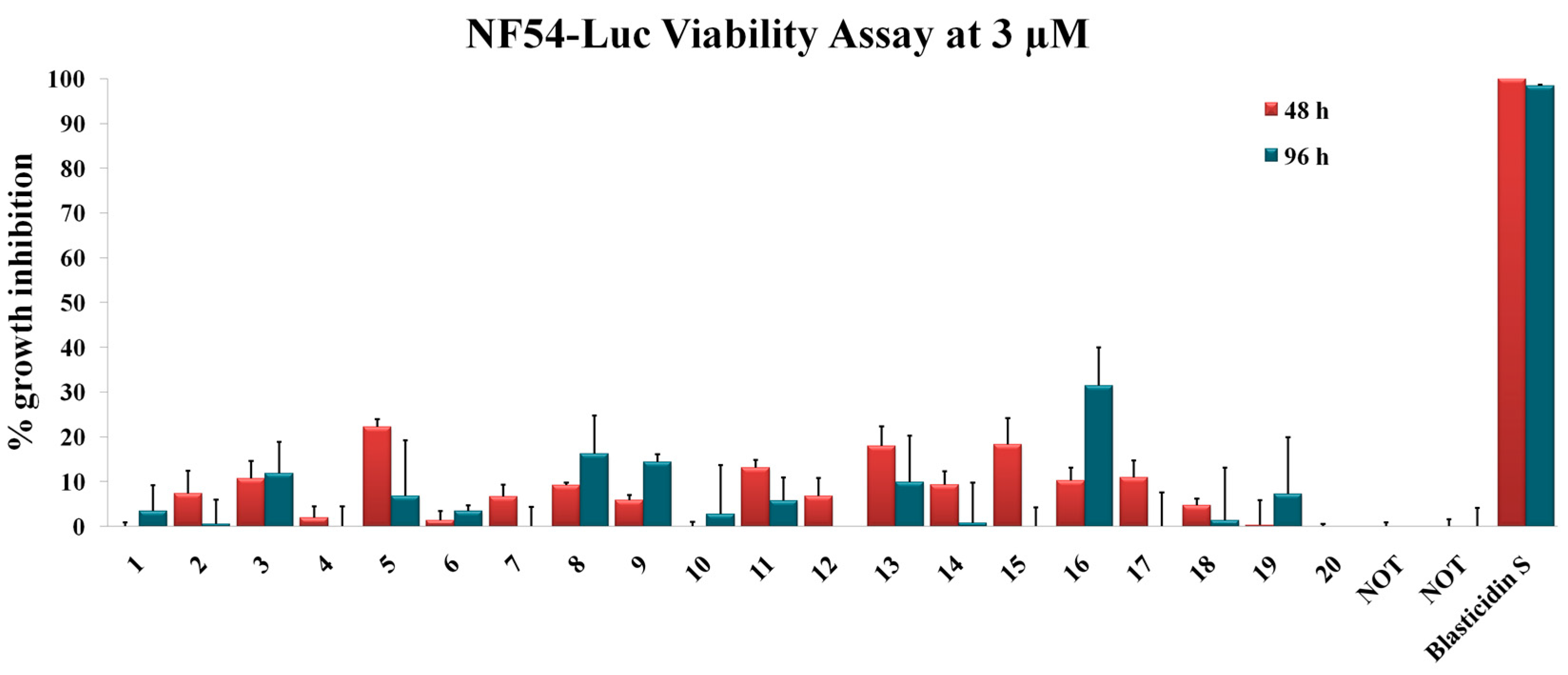
General Procedure for the Synthesis of Compounds 1–8
To a stirred and cooled solution of 2-(4-chlorophenoxy)aniline (330 mg, 1.50 mmol) and triethylamine (230 µL, 1.65 mmol) in toluene (5 mL), the appropriate acid chloride (pivaloyl chloride, 2,2-dimethylbutyryl chloride, 3-methylbutyryl chloride, propyl chloride, cyclopropanecarbonyl chloride, cyclobutanecarbonyl chloride, methyl succinyl chloride, 4-methoxybenzoyl chloride) (1.65 mmol) was added. Subsequently the reaction mixture was allowed to warm to room temperature. The progress of the reaction was monitored by TLC. After 2–9 h the reaction mixture was extracted with a saturated sodium hydrogen carbonate solution, with a hydrogen chloride solution (10%), with brine and finally with water. Afterwards the organic solution was dried over sodium sulfate and evaporated under reduced pressure. The residue was further purified by recrystallization or column chromatography over silica gel.
N-[2-(4-Chlorophenoxy)phenyl]-2,2-dimethylpropanamide (1): Crystallization from ethanol (70%) yielded slightly brown needles (277 mg, 0.92 mmol, 61%); m.p.: 99–100 °C ; IR (KBr): [cm−1] = 3336 (br, N-H), 1663 (s, C=O); 1H-NMR: (400 MHz, DMSO-d6) δ [ppm] = 1.05 (s, 9H, C(CH3)3), 6.80–7.01 (m, 2H, arom. H), 6.99–7.15 (m, 1H, arom. H), 7.15–7.32 (m, 2H, arom. H), 7.27–7.48 (m, 2H, arom. H), 7.56–7.78 (m, 1H, arom. H), 8.72 (s, 1H, NH); 13C-NMR: (101 MHz, DMSO-d6) δ [ppm] = 26.95 (3C, CH3), 118.40 (2C), 120.46, 124.63, 126.13, 126.62, 129.49 (2C) (CH), 38.12, 126.38, 130.28, 147.84, 155.91 (C), 176.13 (C=O); C17H18ClNO2 (303.78): calcd. C 67.21, H 5.97, N 4.61, found C 67.13, H 6.00, N 4.51; EI-MS: m/z (%): 303.1 [M]+ (28), 176.1 [M − 127]+ (100); HPLC: 99.7% at 254 nm, 99.9% at 280 nm; tR = 7.42 min, tM(DMSO) = 1.06 min (ACN/H2O 60:40), λmax [nm] = 230, 275; HPLC-gradient: 99.4%, tR = 13.62 min, tM(DMSO) = 1.28 min.
N-[2-(4-Chlorphenoxy)phenyl]-2,2-dimethylbutanamide (2): Crystallization from methanol/water (33:10) yielded a beige solid (332 mg, 1.05 mmol, 70%); m.p.: 69–70 °C ; IR (KBr): [cm−1] = 3316 (br, N-H), 1657 (s, C=O); 1H-NMR: (400 MHz, DMSO-d6) δ [ppm] = 0.67 (t, J = 7.4 Hz, 3H, CH3), 1.01 (s, 6H, C(CH3)2), 1.47 (q, J = 7.4 Hz, 2H, CH2), 6.87–6.96 (m, 2H, arom. H), 7.03–7.13 (m, 1H, arom. H), 7.17–7.27 (m, 2H, arom. H), 7.34–7.43 (m, 2H, arom. H), 7.59–7.68 (m, 1H, arom. H), 8.70 (s, 1H, NH); 13C-NMR: (101 MHz, DMSO-d6) δ [ppm] = 8.82, 24.54 (2C) (CH3), 32.82 (CH2) 118.49 (2C), 120.46, 124.63, 126.16, 126.74, 129.55 (2C) (CH), 42.42, 126.43, 130.29, 147.98, 155.99 (C), 175.48 (C=O); C18H20ClNO2 (317.81): calcd. C 68.03, H 6.34, N 4.41, found C 68.09, H 6.22, N 4.28; EI-MS: m/z (%): 317.1 [M]+ (28), 190.1 [M − 127]+ (100); HPLC: 98.7% at 254 nm, 99.0% at 280 nm; tR = 9.82 min, tM(DMSO) = 1.06 min (ACN/H2O = 60:40), λmax[nm] = 232, 275; HPLC-Gradient: 97.3%, tR = 14.17 min, tM(DMSO) = 1.28 min.
N-[2-(4-Chlorophenoxy)phenyl]-3-methylbutanamide (3): Purification by column chromatography (dichloromethane/methanol 200:1) yielded a slightly yellow solid (418 mg, 1.38 mmol, 92%); m.p.: 69–71 °C; IR (KBr): [cm−1] = 3291 (m, N-H), 1655 (s, C=O); 1H-NMR: (400 MHz, DMSO-d6): δ [ppm] = 0.81 (d, J = 6.7 Hz, 6H, CH3), 1.92 (hept, J = 6.8 Hz, 1H, CH(CH3)2), 2.14 (d, J = 7.2 Hz, 2H, CH2), 6.79–7.08 (m, 3H, arom. H), 7.08–7.25 (m, 2H, arom. H), 7.28–7.51 (m, 2H, arom. H), 7.88 (dd, J = 7.6 Hz, 2.2 Hz, 1H, arom. H), 9.38 (s, 1H, NH); 13C-NMR (101 MHz, DMSO-d6) δ [ppm] = 22.10 (2C, CH3), 44.93 (CH2), 25.60, 119.28 (2C), 119.83, 124.34, 124.87, 125.25, 129.56 (2C) (CH), 126.67, 130.18, 147.04, 155.98 (C), 170.89 (C=O); C17H18ClNO2 (303.78): calcd. C 67.21, H 5.97, N 4.61, found C 66.95, H 6.02, N 4.39; EI-MS: m/z (%): 303.1 [M]+ (16), 219.0 [M − 84]+ (100); HPLC: 98.1% at 254 nm, 99.0% at 280 nm; tR = 6.03 min, tM(DMSO) = 1.06 min (ACN/H2O = 60:40), λmax [nm] = 249; HPLC-gradient: 95.6%, tR = 13.23 min, tM(DMSO) = 1.28 min.
N-[2-(4-Chlorophenoxy)phenyl]propanamide (4): Purification by column chromatography (petroleum ether/ethyl acetate 4:1) yielded a colorless solid (265 mg, 0.96 mmol, 63%); m.p.: 99–100 °C; IR (KBr): [cm−1] = 3330 (br, N-H), 1675 (s, C=O); 1H-NMR: (400 MHz, DMSO-d6) δ [ppm] = 0.98 (t, J = 7.6 Hz, 3H, CH3), 2.29 (q, J = 7.6 Hz, 2H, CH2), 6.93–7.02 (m, 3H, arom. H), 7.08–7.22 (m, 2H, arom. H), 7.37–7.46 (m, 2H, arom. H), 7.91–7.98 (m, 1H, arom. H), 9.39 (s, 1H, NH); 13C-NMR: (101 MHz, DMSO-d6): δ [ppm] = 9.65 (CH3), 29.03 (CH2), 119.53 (2C), 124.24, 124.43, 125.02, 129.59 (2C) (CH), 126.78, 130.19, 146.93, 155.85 (C), 172.31 (C=O), one signal missing in 13C-NMR; C15H14ClNO2 (275.73): calcd. C 65.34, H 5.12, N 5.08, found C 65.33, N 4.98, H 5.02; EI-MS: m/z (%): 275.1 [M]+ (18), 219.0 [M − 56]+ (100); HPLC: 98.9% at 254 nm, 99.4% at 280 nm; tR = 3.95 min, tM(DMSO) = 1.06 min (ACN/H2O = 60:40), λmax [nm] = 248, 274; HPLC-gradient: 96.0%, tR = 12.23 min, tM(DMSO) = 1.28 min.
N-[2-(4-Chlorophenoxy)phenyl]cyclopropanecarboxyamide (5): Crystallization from ethanol (70%) yielded beige needles (387 mg, 1.34 mmol, 90%); m.p.: 100–102 °C; IR (KBr): [cm−1] = 3371, 3332 (m, N-H), 1663 (s, C=O); 1H-NMR: (400 MHz, DMSO-d6): δ [ppm] = 0.67–0.77 (m, 4H, CH2), 1.95 (td, J = 7.5 Hz, 3.9 Hz, 1H, CH), 6.94 (dd, J = 7.5 Hz, 2.1 Hz, 1H, arom. H), 6.96–7.03 (m, 2H, arom. H), 7.06–7.26 (m, 2H, arom. H), 7.37–7.41 (m, 2H, arom. H), 7.96 (dd, J = 7.7 Hz, J = 2.1 Hz, 1H, arom. H), 9.71 (s, 1H, NH); 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 7.16 (2C, CH2), 13.86, 119.14, 119.70 (2C), 124.01, 124.73, 129.55 (2C) (CH), 126.82, 130.09, 146.66, 155.71 (C), 171.99 (C=O); C16H14ClNO2 (287.74): calcd. C 66.79, H 4.90, N 4.87, found C 66.96, H 4.92, N 4.77; EI-MS: m/z (%): 287.1 [M]+ (21), 219.0 [M − 68]+ (100); HPLC: 98.4% at 254 nm, 98.3% at 280 nm; tR = 4.40 min, tM(DMSO) = 1.06 min (ACN/H2O = 60:40), λmax [nm] = 241; HPLC-gradient: 97.5%, tR = 12.57 min, tM(DMSO) = 1.28 min.
N-[2-(4-Chlorophenoxy)phenyl]cyclobutanecarboxyamide (6): Purification by column chromatography (toluene/petroleum ether 10:1 → 20:1) yielded a slightly brown solid (246, 0.82 mmol, 53%): m.p.: 88–89 °C; IR (KBr): [cm−1] = 3433 (br, N-H), 1657 (s, C=O); 1H-NMR: (400 MHz, DMSO-d6) δ [ppm] = 1.66–2.14 (m, 6H, CH2), 3.19–3.33 (m, 1H, CH), 6.90–7.03 (m, 3H, arom. H), 7.12–7.20 (m, 2H, arom. H), 7.36–7.45 (m, 2H, arom. H), 7.91 (dd, J = 7.4 Hz, 2.1 Hz, 1H, arom. H), 9.22 (s, 1H, NH); 13C-NMR: (101 MHz, DMSO-d6) δ [ppm] = 17.65, 24.47 (2C) (CH2), 38.88, 119.28 (2C), 119.78, 124.36, 124.74, 125.18, 129.55 (2C) (CH), 126.68, 130.18, 147.00, 155.87 (C), 173.09 (C=O); C17H16ClNO2 (301.77): calcd. C 67.66, H 5.34, N 4.64, found C 67.81, H 5.43, N 4.55; EI-MS: m/z (%): 301.1 [M]+ (20), 219.0 [M − 82]+ (100); HPLC: 99.4% at 254 nm, 99.8% at 280 nm; tR = 5.86 min; tM(DMSO) = 1.06 min (ACN/H2O = 60:40), λmax [nm] = 229; HPLC-gradient: 98.4%, tR = 13.15 min; tM(DMSO) = 1.28 min.
Methyl 4-{[2-(4-chlorophenoxy)phenyl]amino}-4-oxobutanoate (7): Crystallization from methanol yielded a beige solid (278 mg, 0.83 mmol, 56%); m.p.: 109–110 °C; IR (KBr): [cm−1] = 3341 (m, N-H),1721 (s, C=O, ester) 1687 (s, C=O, amide); 1H-NMR: (400 MHz, CDCl3): δ [ppm] = 2.35–2.97 (m, 4H, CH2), 3.67 (s, 3H, OCH3), 6.83 (dd, J = 8.1 Hz, 1.4 Hz, 1H, arom. H), 6.93–7.05 (m, 3H, arom. H), 7.12 (td, J = 7.8 Hz, 1.5 Hz, 1H, arom. H), 7.28–7.38 (m, 2H, arom. H), 7.91 (s, 1H, NH), 8.41 (d, J = 7.8 Hz, 1H, arom. H); 13C-NMR (101 MHz, CDCl3) δ [ppm] = 51.93 (CH3) 29.15, 32.27 (CH2), 117.77, 119.84 (2C), 121.14, 124.10, 124.44, 129.95 (2C) (CH), 128.98, 129.78, 145.22, 155.13 (C), 169.69 (C=O), 173.23 (C=O); C17H16ClNO4 (333.77): calcd. C 61.18, H 4.83, N 4.20, found: C 61.15, H 4.83, N 4.21; EI-MS: m/z (%): 333.1 [M]+ (12), 174.1 [M − 159]+ (100); HPLC: 99.3% at 254 nm, 99.7% at 280 nm; tR = 3.44 min, tM(DMSO) = 1.06 min (ACN/H2O = 60:40), λmax [nm] = 228; HPLC-gradient: 98.5%, tR = 11.99 min, tM(DMSO) = 1.28 min.
N-(2-(4-Chlorophenoxy)phenyl)-4-methoxybenzamide (8): Crystallization from methanol yielded colorless needles (346mg,0.98 mol, 65%); m.p.: 101–102 °C; IR (KBr): [cm−1] = 3420 (m, N-H), 1660 (s, C=O); 1H-NMR: (400 MHz, DMSO-d6): δ [ppm] = 3.81 (s, 3H, OCH3), 6.89–7.13 (m, 5H, arom. H), 7.17–7.30 (m, 2H, arom. H), 7.30–7.48 (m, 2H, arom. H), 7.65–7.76 (m, 1H, arom. H), 7.75–7.99 (m, 2H, arom. H), 9.69 (s, 1H, NH); 13C-NMR (101 MHz, DMSO-d6) δ [ppm] = 55.29 (CH3), 113.44 (2C), 119.36 (2C), 119.69, 124.15, 126.32, 126.83, 129.38 (2C), 129.48 (2C) (CH), 126.25, 126.64, 129.79, 149.02, 155.71, 161.80 (C), 164.69 (C=O); C20H16ClNO3 (353.80): calcd. C 67.90, H 4.56, N 3.96, found: C 67.78, H 4.43, N 3.95; EI-MS: m/z (%): 353.1 [M]+ (8), 135.0 [M − 218]+ (100); HPLC: 99.7% at 254 nm, 99.8% at 280 nm; tR = 6.37 min, tM(DMSO) = 1.06 min (ACN/H2O = 60:40), λmax [nm] = 266; HPLC-gradient: 99.0%, tR = 13.37 min, tM(DMSO) = 1.28 min.
General Procedure for the Synthesis of Compounds 11–18
To a stirred and cooled solution of 2-(4-methoxyphenoxy)aniline hydrochloride (1.50 mmol) and triethylamine (4.00 mmol) in toluene (5–8 mL), the appropriate acid chloride (pivaloyl chloride, 2,2-dimethylbutyryl chloride, 3-methylbutyryl chloride, propyl chloride, cyclopropanecarbonyl chloride, cyclobutanecarbonyl chloride, methyl succinyl chloride, 4-methoxybenzoyl chloride) (1.65 mmol) was added. Thereafter the reaction mixture was allowed to warm to room temperature. The progress of the reaction was monitored by TLC. After 4–24 h an aqueous work-up was performed similar to that of compounds 1–8. The resulting residue was further purified by recrystallization or column chromatography over silica gel.
N-[2-(4-Methoxyphenoxy)phenyl]-2,2-dimethylpropanamide (11): Crystallization from ethanol (70%) yielded a colorless solid (401 mg, 1.34 mmol, 89%); m.p.: 70–71 °C; IR (KBr): [cm−1] = 3317 (m, N-H), 1661 (s, C=O); 1H-NMR: (400 MHz, DMSO-d6): δ [ppm] = 1.11 (s, 9H, C(CH3)3), 3.72 (s, 3H, OCH3), 6.85–6.90 (m, 1H, arom. H), 6.92 (s, 4H, arom. H), 7.06–7.16 (m, 2H, arom. H), 7.69–7.78 (m, 1H, arom. H), 8.62 (s, 1H, NH); 13C-NMR (101 MHz, DMSO-d6) δ [ppm] = 27.06 (3C), 55.41 (CH3), 114.86 (2C), 118.35, 119.09 (2C), 123.25, 125.06, 125.40 (CH), 38.90, 129.48, 148.96, 149.97, 155.27 (C), 176.10 (C=O); C18H21NO3 (299.37): calcd. C 72.22, H 7.07, N 4.68, found C 72.21, H 7.25, N 4.73; EI-MS: m/z (%): 299.1 [M]+ (48), 176.1 [M − 123]+(100); HPLC: 99.4% at 254 nm, 99.4% at 280 nm; tR = 5.21 min, tM(DMSO) = 1.06 min (ACN/H2O = 60:40), λmax [nm] = 248; HPLC-Gradient: 99.3%, tR = 12.88 min, tM(DMSO) = 1.28 min.
N-[2-(4-Methoxyphenoxy)phenyl]-2,2-dimethylbutanamide (12): Crystallization from ethanol (70%) yielded a slightly yellow solid (415 mg, 1.32 mmol, 88%): m.p.: 71–72 °C; IR (KBr): [cm−1] = 3433 (m, N-H), 1674 (s, C=O); 1H-NMR: (400 MHz, DMSO-d6): δ [ppm] = 0.72 (t, J = 7.4 Hz, 3H, CH3), 1.07 (s, 6H, C(CH3)2), 1.51 (q, J = 7.4 Hz, 2H, CH2), 3.72 (s, 3H, OCH3), 6.83–6.92 (m, 1H, arom. H), 6.92 (s, 4H, arom. H), 7.05–7.18 (m, 2H, arom. H), 7.66–7.74 (m, 1H, arom. H), 8.60 (s, 1H, NH); 13C-NMR (101 MHz, DMSO-d6) δ [ppm] = 8.90, 32.96 (2C), 55.42 (CH3), 24.61 (CH2), 114.90 (2C), 118.31, 119.17 (2C), 123.21, 125.34, 125.50 (CH), 42.56, 129.43, 149.21, 150.00, 155.28 (C), 175.42 (C=O); C19H23NO3 (313.40): calcd. C 72.84, H 7.40, N 4.47, found C 72.72, H 7.45, N 4.52; EI-MS: m/z (%): 313.1 [M]+ (51), 190.1 [M − 123]+ (100); HPLC: 98.0% at 254 nm, 98.3% at 280 nm; tR = 6.69 min, tM(DMSO) = 1.06 min (ACN/H2O = 60:40), λmax [nm] = 228; HPLC-gradient: 98.1%, tR = 13.43 min, tM(DMSO) = 1.28 min.
N-[2-(4-Methoxyphenoxy)phenyl]-3-methylbutanamide (13): Purification by column chromatography (petroleum ether/ethyl acetate 4:1) yielded a colorless oil (303 mg, 1.01 mmol, 67%); IR (NaCl): [cm−1] = 3426 (m, N-H), 3317 (m, br, N-H), 1679 (s, C=O); 1H-NMR: (600 MHz, DMSO-d6): δ [ppm] = 0.87 (d, J = 6.7 Hz, 6H, CH2), 1.99 (hept, J = 6.8 Hz, 1H, CH), 2.21 (d, J = 7.2 Hz, 2H, CH2), 3.73 (s, 3H, OCH3), 6.72–6.83 (m, 1H, arom. H), 6.89–7.01 (m, 4H, arom. H), 7.01–7.10 (m, 2H, arom. H), 7.77–8.09 (m, 1H, arom. H), 9.35 (s, 1H, NH); 13C-NMR (151 MHz, DMSO-d6) δ [ppm] = 22.12 (2C), 55.32 (CH3), 44.94 (CH2) 25.60, 114.81 (2C), 117.42, 120.01 (2C), 122.71, 123.95, 124.65 (CH), 129.16, 148.63, 149.70, 155.35 (C), 170.87 (C=O); C18H21NO3 (299.37): calcd. C 72.22, H 7.07, N 4.68, found C 71.88, H 7.33, N 4.52; EI-MS: m/z (%): 299.1 [M]+ (48), 176.1 [M − 123]+ (100); HPLC: 98.5% at 254 nm, 98.4% at 280 nm; tR = 4.37 min, tM(DMSO) = 1.06 min (ACN/H2O = 60:40), λmax [nm] = 248; HPLC-gradient: 98.1%, tR = 12.47 min, tM(DMSO) = 1.28 min.
N-[2-(4-Methoxyphenoxy)phenyl]propanamide (14): Crystallization from ethanol (70%) yielded colorless crystals (379 mg, 1.40 mmol, 93%); m.p.: 107–109 °C; IR (KBr): [cm−1] = 3299 (m, br, N-H), 1651 (s, C=O); 1H-NMR: (400 MHz, DMSO-d6): δ [ppm] = 1.02 (t, J = 7.6 Hz, 3H, CH3), 2.35 (q, J = 7.5 Hz, 2H, CH2), 3.74 (s, 3H, OCH3), 6.70–6.79 (m, 1H, arom. H), 6.90–7.09 (m, 6H, arom. H), 7.87–8.10 (m, 1H, arom. H), 9.33 (s, 1H, NH); 13C-NMR (101 MHz, DMSO-d6) δ [ppm] = 9.68, 55.39 (CH3), 29.09 (CH2), 114.89 (2C), 117.23, 120.24 (2C), 122.70, 123.53, 124.45 (CH), 129.26, 148.49, 149.64, 155.48 (C), 172.28 (C=O); C16H17NO3 (271.32): calcd. C 70.83, H 6.32, N 5.16, found C 70.70, H 6.37, N 5.12; EI-MS: m/z (%): 271.1 [M]+ (54), 215.1 [M − 59]+ (100); HPLC: 98.6% at 254 nm, 97.9% at 280 nm; tR = 4.89 min, tM(DMSO) = 1.06 min (ACN/H2O = 50:50), λmax [nm] = 239; HPLC-gradient: 97.5%, tR = 11.41 min, tM(DMSO) = 1.28 min.
N-[2-(4-Methoxyphenoxy)phenyl]cyclopropanecarboxyamide (15): Crystallization from ethanol (70%) yielded a colorless solid (360 mg, 1.27 mmol, 85%); m.p.: 138–139 °C; IR (KBr): [cm−1] = 3312 (m, br, N-H), 1660 (s, C=O); 1H-NMR: (400 MHz, DMSO-d6): δ [ppm] = 0.65–0.87 (m, 4H, CH2), 1.95–2.14 (m, 1H, CH), 3.75 (s, 3H, OCH3), 6.64–6.81 (m, 1H, arom. H), 6.88–7.06 (m, 6H, arom. H), 7.88–8.05 (m, 1H, arom. H), 9.69 (s, 1H, NH); 13C-NMR (101 MHz, DMSO-d6) δ [ppm] = 55.39 (CH3), 7.25 (2C, CH2), 14.02, 114.93 (2C), 116.93, 120.47 (2C), 122.57, 123.38, 124.32 (CH), 129.21, 148.38, 149.54, 155.56 (C), 172.04 (C=O); C17H17NO3 (283.33): calcd. C 72.07, H 6.05, N 4.94, found C 71.86, H 6.20, N 4.93; EI-MS: m/z (%): 283.1 [M]+ (43), 215.1 [M − 68]+ (100); HPLC: 99.2% at 254 nm, 99.4% at 280 nm; tR = 5.79 min, tM(DMSO) = 1.06 min (ACN/H2O = 50:50), λmax [nm] = 247; HPLC-gradient: 98.6%, tR = 11.73 min, tM(DMSO) = 1.28 min.
N-[2-(4-Methoxyphenoxy)phenyl]cyclobutanecarboxyamide (16): Crystallization from ethanol (70%) yielded a colorless solid (378 mg, 1.27 mmol, 85%); m.p.: 72–74 °C; IR (KBr): [cm−1] = 3294 (m, br, N-H), 1665 (s, C=O); 1H-NMR: (400 MHz, CDCl3): δ [ppm] = 1.82–2.07 (m, 2H, CH2), 2.13–2.27 (m, 2H, CH2), 2.28–2.45 (m, 2H, CH2), 3.08–3.29 (m, 1H, CH), 3.81 (s, 3H, OCH3), 6.74 (dd, J = 8.1 Hz, 1.4 Hz, 1H, arom. H), 6.84–7.01 (m, 5H, arom. H), 7.06 (td, J = 7.8 Hz, 1.4 Hz, 1H, arom. H), 7.71 (s, 1H, NH), 8.47 (dd, J = 8.1 Hz, 1.6 Hz, 1H, arom. H); 13C-NMR (101 MHz, CDCl3) δ [ppm] = 55.67 (CH3) 18.02, 25.32 (2C) (CH2), 41.10, 114.99 (2C), 116.31, 120.29 (2C), 120.51, 123.35, 123.54 (CH) 129.29, 146.55, 149.48, 156.21 (C), 173.22 (C=O); C17H17NO3 (283.33): calcd. C 72.07, H 6.05, N 4.94, found C 71.86, H 6.20, N 4.93; EI-MS: m/z (%): 297.1 [M]+ (38) 215.1 [M − 82]+ (100); HPLC: 99.3% at 254 nm, 99.2% at 280 nm; tR = 4.15 min, tM(DMSO) = 1.06 min (ACN/H2O = 60:40), λmax [nm] = 248; HPLC-Gradient: 98.5%, tR = 12.93 min, tM(DMSO) = 1.28 min.
Methyl 4-{[2-(4-methoxyphenoxy)phenyl]amino}-4-oxobutanoate (17): Crystallization from methanol yielded slightly yellow crystals (347 mg, 1.05 mmol, 70%); m.p.: 82–83 °C; IR (KBr): [cm−1] = 3335 (m, br, N-H), 1721 (s, C=O, ester), 1682 (s, C=O, amide); 1H-NMR: (400 MHz, DMSO-d6): δ [ppm] = 2.55 (t, J = 6.8 Hz, 2H, CH2), 2.66 (t, J = 6.8 Hz, 2H, CH2), 3.58 (s, 3H, COOCH3), 3.75 (s, 3H, OCH3), 6.67–6.80 (m, 1H, arom. H), 6.86–7.12 (m, 6H, arom. H), 7.84–8.05 (m, 1H, arom. H), 9.52 (s, 1H, NH); 13C-NMR (101 MHz, DMSO-d6) δ [ppm] = 51.27, 55.39 (CH3), 28.61, 30.62 (CH2), 114.90 (2C), 117.00, 120.43 (2C), 122.60, 123.19, 124.41 (CH), 129.06, 148.39, 149.54, 155.54 (C), 170.19, 172.77 (C=O); C18H19NO5 (329.35): calcd. C 65.64, H 5.82, N 4.25, found C 65.52, H 5.91, N 4.12; EI-MS: m/z (%): 329.1 [M]+ (32), 297.1 [M − 32]+ (100); HPLC: 98.0% at 254 nm, 98.2% at 280 nm; tR = 4.39 min, tM(DMSO) = 1.06 min (ACN/H2O = 50:50), λmax [nm] = 245; HPLC-gradient: 97.4%, tR = 11.20 min, tM(DMSO) = 1.28 min.
N-(2-(4-Methoxyphenoxy)phenyl)-4-methoxybenzamide (18): Crystallization from methanol yielded colorless needles (344 mg, 0.98 mmol, 66%); m.p.: 88–89 °C; IR (KBr): [cm−1] = 3442 (m, N-H), 1674 (s, C=O); 1H-NMR: (400 MHz, DMSO-d6): δ [ppm] = 3.72 (s, 3H, OCH3), 3.82 (s, 3H, OCH33), 6.80–6.88 (m, 1H, arom. H), 6.90–6.96 (m, 2H, arom. H), 6.96–7.04 (m, 4H, arom. H), 7.06–7.20 (m, 2H, arom. H), 7.76 (dd, J = 7.5 Hz, 2.1 Hz, 1H, arom. H), 7.84–7.93 (m, 2H, arom. H), 9.61 (s, 1H, NH); 13C-NMR (101 MHz, DMSO-d6) δ [ppm] = 55.37 (2C, CH3), 113.57 (2C), 114.88 (2C), 117.63, 120.08 (2C), 122.78, 125.89, 126.05, 129.45 (2C) (CH), 126.48, 128.93, 149.69, 150.56, 155.44, 161.87 (C), 164.68 (C=O); C21H19NO4 (349.39): calcd. C 72.19, H 5.48, N 4.01, found C 72.01, H 5.46, N 3.90; EI-MS: m/z (%): 349.1 [M]+ (20), 135.0 [M − 214]+ (100); HPLC: 99.4% at 254 nm, 99.4% at 280 nm; tR = 4.71 min, tM(DMSO) = 1.06 min (ACN/H2O = 60:40), λmax [nm] = 268; HPLC-gradient: 98.8%, tR = 12.78 min, tM(DMSO) = 1.28 min.
tert-Butyl 2-{[2-(4-chlorophenoxy)phenyl]amino}-2-oxoethylcarbamate (9)
A stirred solution of Boc-glycine (1.50 mmol), PyBOP (1.60 mmol) and DIPEA (3.50 mmol) in dichloromethane (10 mL) was cooled to 0 °C with an ice bath. To this solution 2-(4-chlorophenoxy)aniline (1.50 mmol), dissolved in a minimum amount of dichloromethane, was added dropwise. The reaction mixture was allowed to warm to room temperature and was then stirred for 24 h. Thereafter, an aqueous work-up was performed similar to that of compounds 1–8. The resulting oil was further purified by column chromatography over silica gel (dichloromethane/methanol 200:1) to give a slightly orange solid (358 mg, 0.95 mmol, 63%); m.p.: 50–51 °C; IR (KBr): [cm−1] = 3409 (m, N-H), 3342 (s, N-H), 1691 (br, s, C=O); 1H-NMR: (600 MHz, DMSO-d6): δ [ppm] = 1.34 (s, 9H, CH3), 3.71 (d, J = 6.1 Hz, 2H, CH2), 6.87–7.06 (m, 3H, arom. H), 7.05–7.30 (m, 3H, arom. H), 7.35–7.53 (m, 2H, arom. H), 8.12 (d, J = 7.7 Hz, 1H, NH), 9.33 (s, 1H, NH); 13C-NMR (151 MHz, DMSO-d6) δ [ppm] = 28.00 (3C, CH3), 43.92 (CH2), 118.82, 119.90 (2C), 122.35, 124.15, 124.63, 129.70 (2C) (CH), 78.17, 127.17, 129.64, 146.09, 155.47, 155.86, 168.51 (C); EI-MS: m/z (%): 376.1 [M]+ (11), 219.1 [M − 157]+ (100); HREI-MS: calcd. for C19H21ClN2O4 376.11844, found 376.11803; HPLC: 96.3% at 254 nm, 96.3% at 280 nm; tR = 4.84 min, tM(DMSO) = 1.06 min (ACN/H2O = 60:40), λmax [nm] = 230; HPLC-gradient: 95.8%, tR = 12.80 min, tM(DMSO) = 1.28 min.
tert-Butyl 2-{[2-(4-methoxyphenoxy)phenyl]amino}-2-oxoethylcarbamate (19)
A stirred solution of Boc-glycine (2.00 mmol), PyBOP (2.10 mmol) and DIPEA (5.00 mmol) in dichloromethane (10 mL) was cooled to 0 °C with an ice bath. To this solution, 2-(4-methoxyphenoxy)aniline hydrochloride (2.00 mmol), dissolved in dichloromethane (5 mL) together with DIPEA (2.00 mmol), was added dropwise. The reaction mixture was allowed to warm to room temperature and was then stirred for 20 h. Subsequently, an aqueous work-up was performed similar to that of compounds 1–8. The resulting oil was taken up in cold diethyl ether (10 mL) leading to precipitation of a beige solid, which was filtered off and discarded. The ether was evaporated under reduced pressure and the resulting residue was recrystallized from methanol/water (5:1) to give a slightly beige solid(495 mg, 1.33 mmol, 66%); m.p.: 92–93 °C; IR (KBr): [cm−1] = 3393 (w, N-H), 3313 (s, br, N-H), 1696 (s, C=O, carbamate), 1666 (s, C=O, amide); 1H-NMR: (600 MHz, DMSO-d6): δ [ppm] = 1.35 (s, 9H, C(CH3)3), 3.73–3.75 (m, 5H, CH2, OCH3), 6.73 (dd, J = 8.0 Hz, 1.7 Hz, 1H, arom. H), 6.95–7.07 (m, 6H, arom. H), 7.24 (t, J = 5.2 Hz, 1H, arom. H), 8.13 (d, J = 7.0 Hz, 1H, NH), 9.30 (s, 1H, NH); 13C-NMR (151 MHz, DMSO-d6) δ [ppm] = 28.02 (3C), 55.33 (CH3), 44.06 (CH2), 114.92 (2C), 116.55, 120.52 (2C), 121.58, 122.67, 124.19 (CH), 78.22 128.63, 147.69, 149.16, 155.65, 155.90, 168.43 (C); C20H24N2O5 (372.42): calcd. C 64.50, H 6.50, N 7.52, found C 64.10, H 6.68, N 7.61; EI-MS: m/z (%): 372.2 [M]+ (18), 215.1 [M − 157]+ (100); HPLC: 96.5% at 254 nm, 95.5% at 280 nm; tR = 3.41 min, tM(DMSO) = 1.06 min (ACN/H2O = 60:40), λmax [nm] = 241; HPLC-gradient: 95.1%, tR = 11.99 min, tM(DMSO) = 1.28 min.