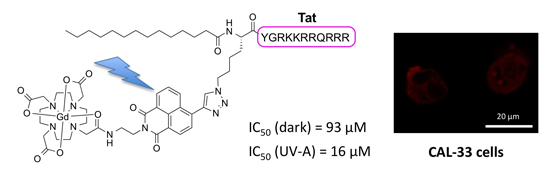
Cellular Uptake and Photo-Cytotoxicity of a Gadolinium(III)-DOTA-Naphthalimide Complex “Clicked” to a Lipidated Tat Peptide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Ligand and Gd(III) Complexes
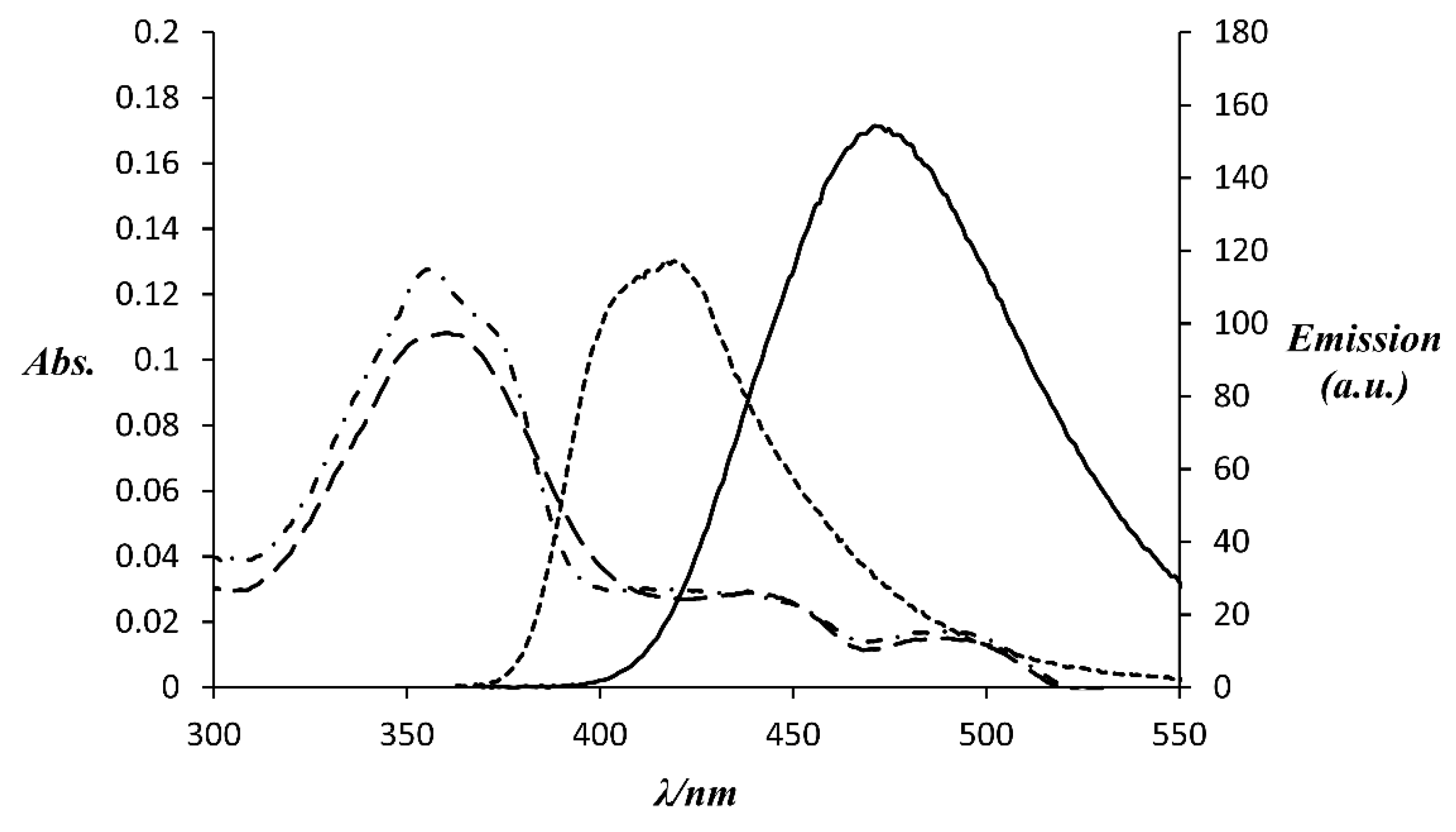
2.2. Photo-Physical Properties of Complexes
| Complex | Absorption λmax (ε (M−1cm−1)) | Emission λmax | Φ a (%) | Brightness (ε × Φ/1000 (M−1cm−1)) |
|---|---|---|---|---|
| Gd-L | 356 (22,500) | 417 | 35% | 7.9 |
| “Clicked” Gd-L | 362 (19,200) | 471 | 59% | 11.3 |
2.3. Conjugation of Complex to Lipidated Tat Peptide

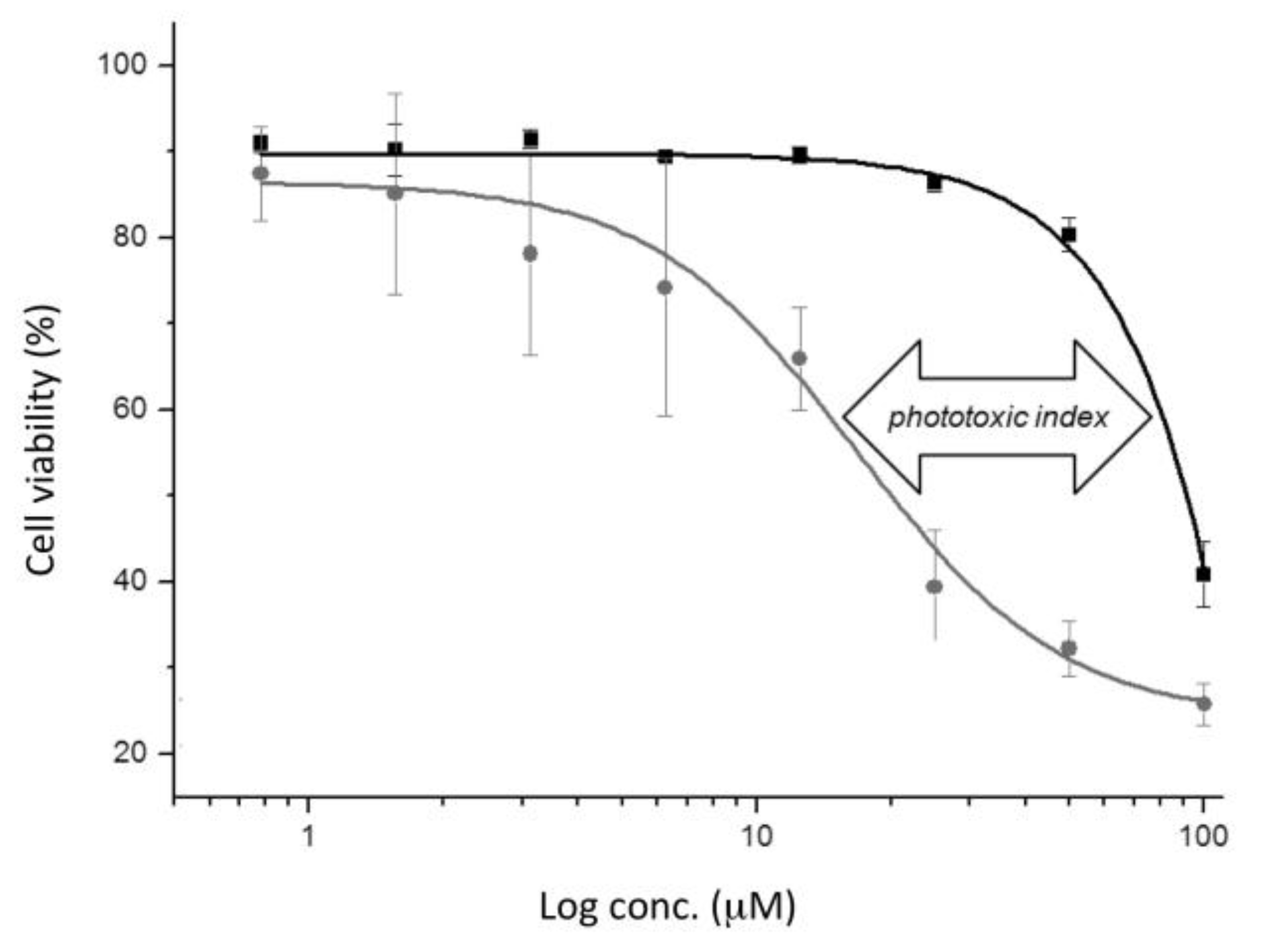
2.4. (Photo-)cytotoxicity of Complexes and Conjugate
| Compound | IC50 (Dark) a (μM) | IC50 (UV-A) a (μM) | PI b (x-Fold) |
|---|---|---|---|
| Gd-L | >100 | >100 | n.a. |
| “clicked” Gd-L | >100 | >100 | n.a. |
| Gd-L-Tat conjugate | 93 ± 3 | 16 ± 6 | 5.8 |
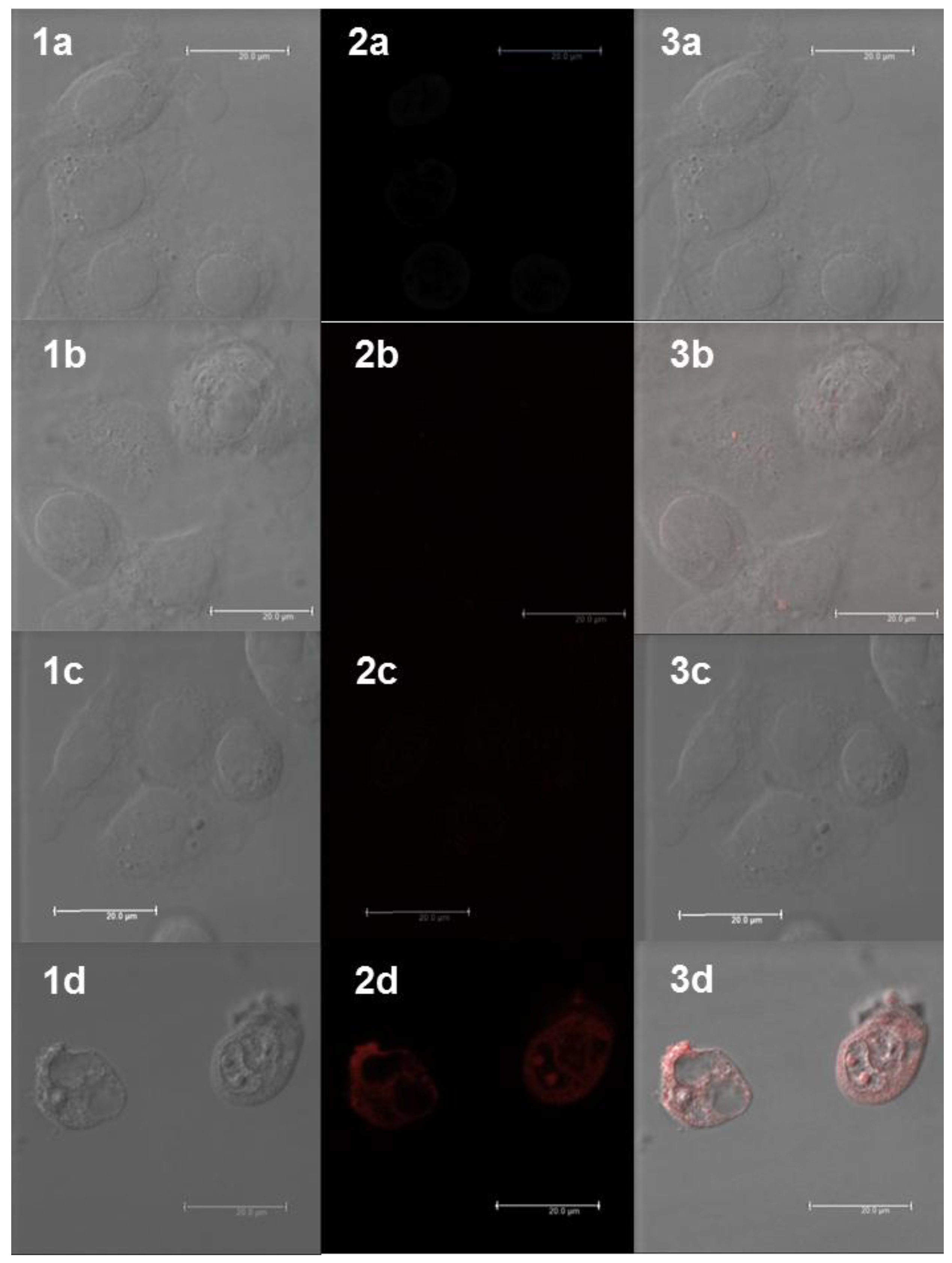
2.5. Cellular Uptake of Complexes and Conjugates
3. Experimental Section
3.1. Materials and Methods
3.2. Synthetic Procedures
3.2.1. tert-Butyl 2-(6-bromo-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)ethylcarbamate (1)
3.2.2. tert-Butyl 2-(1,3-dioxo-6-((trimethylsilyl)ethynyl)-1H-benzo[de]-isoquinolin-2(3H)-yl)ethylcarbamate (2)
3.2.3. 2-(2-Aminoethyl)-6-((trimethylsilyl)ethynyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (3)
3.2.4. 2-Bromo-N-(2-(1,3-dioxo-6-((trimethylsilyl)ethynyl)-1H-benzo[de]isoquinolin-2(3H)-yl)ethyl)acetamide (4)
3.2.5. tri-tert-Butyl 2,2′,2″-(10-(2-((2-(1,3-Dioxo-6-((trimethylsilyl)ethyn-yl)-1H-benzo[de]isoquinolin-2(3H)-yl)ethyl)amino)-2-oxoethyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetate (5)
3.2.6. 2,2′,2″-(10-(2-((2-(6-Ethynyl-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)ethyl)amino)-2-oxoethyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetic acid (L)
3.2.7. Gd-L
3.2.8. “Clicked” Gd-L
3.2.9. Peptide Conjugation
3.3. Quantum Yield Determinations
3.4. Cell Culture
3.5. Cytotoxicity Studies
3.6. Cellular Uptake Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACN: | acetonitrile |
| BOC: | tert-butoxycarbonyl |
| tBu3DO3A: | tert-butyl-protected 1,4,7,10-tetraazacyclodocane-1,4,7,10-tetraacetic acid |
| d: | doublet |
| dd: | doublet of doublets |
| ddd: | doublet of doublet of doublets |
| dt: | doublet of triplets |
| DCM: | dichloromethane |
| DIC: | differential interference contrast |
| DIPEA: | N,N-diisopropylethylamine |
| DMF: | N,N-dimethylformamide |
| DOTA: | 1,4,7,10-tetraazacyclodocane-1,4,7,10-tetraacetic acid |
| ESI: | electrospray ionization |
| HEPES: | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| HPLC: | high-performance liquid chromatography |
| HRMS: | high-resolution mass spectrometry |
| IC50: | half maximal inhibitory concentration |
| J: | coupling constant |
| LC-MS: | liquid chromatography-mass spectrometry |
| m: | multiplet |
| MRI: | magnetic resonance imaging |
| NMR: | nuclear magnetic resonance NMR |
| O/N: | overnight |
| PBS: | phosphate-buffered saline |
| PDT: | photo-dynamic therapy |
| PI: | photo-toxic index |
| RT: | room temperature |
| Rf: | retention factor |
| s: | singlet |
| SPAAC: | strain-promoted azide-alkyne cycloaddition |
| t: | triplet |
| TAT: | trans-activator of transcription |
| THF: | tetrahydrofuran |
| TFA: | trifluoroacetic acid |
| THPTA: | tris(3-hydroxypropyl-triazolylmethyl)amine |
| TLC: | thin layer chromatography |
| TMS: | trimethylsilyl |
| UV-A: | ultraviolet A radiation |
| Φ: | quantum yield |
References
- Lee, S.Y.; Jeon, S.I.; Jung, S.; Chung, I.J.; Ahn, C.-H. Targeted multimodal imaging modalities. Adv. Drug Del. Rev. 2014, 76, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Alafort, L.; Muzzio, P.C.; Rosato, A. Optical and multimodal peptide-based probes for in vivo molecular imaging. Anticancer Agents Med. Chem. 2012, 12, 476–499. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Longmire, M.R.; Ogawa, M.; Choyke, P.L. Rational chemical design of the next generation of molecular imaging probes based on physics and biology: Mixing modalities, colors and signals. Chem. Soc. Rev. 2011, 40, 4626–4648. [Google Scholar] [CrossRef] [PubMed]
- Louie, A. Multimodality imaging probes: Design and challenges. Chem. Rev. 2010, 10, 3146–3195. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.E.; Long, N.J. “Two is better than one”—Probes for dual-modality molecular imaging. Chem. Commun. 2009, 3511–3524. [Google Scholar] [CrossRef] [PubMed]
- Fass, L. Imaging and cancer: A review. Mol. Oncol. 2008, 2, 115–152. [Google Scholar] [CrossRef] [PubMed]
- Glasspool, R.M.; Evans, T.R. Clinical imaging of cancer metastasis. Eur. J. Cancer 2000, 36, 1661–1670. [Google Scholar] [CrossRef]
- Palekar-Shanbhag, P.; Jog, S.V.; Chogale, M.M.; Gaikwad, S.S. Theranostics for cancer therapy. Curr. Drug Deliv. 2013, 10, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Rizzo, L.Y.; Storm, G.; Kiessling, F. Personalized nanomedicine. Clin. Cancer Res. 2012, 18, 4889–4894. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining imaging and therapy. Bioconjugate Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Shin, W.S.; Sunwoo, K.; Kim, W.Y.; Koo, S.; Bhuniya, S.; Kim, J.S. Small conjugate-based theranostic agents: An encouraging approach for cancer therapy. Chem. Soc. Rev. 2015, 44, 6670–6683. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Kim, T.; Paik, S.G.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for theranostics: Recent advances and future challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.-S. Nanotheranostics—Application and further development of nanomedicine strategies for advanced theranostics. Theranostics 2014, 4, 660–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shim, M.S.; Levinson, N.S.; Sung, H.-W.; Xia, Y. Stimuli-responsive materials for controlled release of theranostic agents. Adv. Funct. Metar. 2014, 24, 4206–4220. [Google Scholar] [CrossRef] [PubMed]
- Caldorera-Moore, M.E.; Liechty, W.B.; Peppas, N.A. Responsive theranostic systems: Integration of diagnostic imaging agents and responsive controlled release drug delivery carriers. Acc. Chem. Res. 2011, 44, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Zhang, Y. Photocontrolled nanoparticle delivery systems for biomedical applications. Acc. Chem. Res. 2014, 47, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S. Photoactivated nanomaterials for biomedical release applications. J. Mater. Chem. 2012, 22, 301–318. [Google Scholar]
- Rai, P.; Mallidi, S.; Zheng, X.; Rahmanzadeh, R.; Mir, Y.; Elrington, S.; Khurshid, A.; Hasan, T. Development and applications of photo-triggered theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1094–1124. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Heitz, V.; Sour, A.; Bolze, F.; Kessler, P.; Flamigni, L.; Ventura, B.; Bonnet, C.S.; Tóth, E. A theranostic agent combining a two-photon-absorbing photosensitizer for photodynamic therapy and a gadolinium(III) complex for MRI detection. Chem. Eur. J. 2016. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Novak, T.; Miller, K.; Zhu, Y.; Kenney, M.E.; Broome, A.M. Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale 2015, 7, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Truillet, C.; Lux, F.; Moreau, J.; Four, M.; Sancey, L.; Chevreux, S.; Boeuf, G.; Perriat, P.; Frochot, C.; Antoine, R.; et al. Bifunctional polypyridyl-Ru(II) complex grafted onto gadolinium-based nanoparticles for MR-imaging and photodynamic therapy. Dalton Trans. 2013, 42, 12410–12420. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Pan, R.; Xuan, W.; Wei, Y.; Liu, K.; Zhou, J.; Wang, W. Photo-triggered fluorescent theranostic prodrugs as DNA alkylating agents for mechlorethamine release and spatiotemporal monitoring. Org. Biomol. Chem. 2015, 13, 6742–6748. [Google Scholar] [CrossRef] [PubMed]
- Karaoun, N.; Renfrew, A.K. A luminescent ruthenium(II) complex for light-triggered drug release and live cell imaging. Chem. Commun. 2015, 14038–14041. [Google Scholar] [CrossRef] [PubMed]
- Knoll, J.D.; Turro, C. Control and utilization of ruthenium and rhodium metal complex excited states for photoactivated cancer therapy. Coord. Chem. Rev. 2015, 282, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Mari, C.; Pierroz, V.; Leonidova, A.; Ferrari, S.; Gasser, G. Towards selective light-activated RuII-based prodrug candidates. Eur. J. Inorg. Chem. 2015, 23, 3879–3891. [Google Scholar] [CrossRef]
- Leonidova, A.; Pierroz, V.; Rubbiani, R.; Lan, Y.; Schmitz, A.G.; Kaech, A.; Sigel, R.K.O.; Ferrari, S.; Gasser, G. Photo-induced uncaging of a specific Re(I) organometallic complex in living cells. Chem. Sci. 2014, 5, 4044–4056. [Google Scholar] [CrossRef]
- Dai, Y.; Xiao, H.; Liu, J.; Yuan, Q.; Ma, P.A.; Yang, D.; Li, C.; Cheng, Z.; Hou, Z.; Yang, P.; et al. In vivo multimodality imaging and cancer therapy by near-infrared light-triggered trans-platinum pro-drug-conjugated upconverison nanoparticles. J. Am. Chem. Soc. 2013, 135, 18920–18929. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Monro, S.; Hennigar, R.; Colpitts, J.; Fong, J.; Kasimova, K.; Yin, H.; DeCoste, R.; Spencer, C.; Chamberlain, L.; et al. Ru(II) dyads derived from α-oligothiophenes: A new class of potent and versatile photosensitizers for PDT. Coord. Chem. Rev. 2015, 282, 127–138. [Google Scholar] [CrossRef]
- Ma, D.-L.; He, H.-Z.; Leung, K.-H.; Chan, D.S.-H.; Leung, C.-H. Bioactive luminescent transition-metal complexes for biomedical applications. Angew. Chem. Int. Ed. 2013, 52, 7666–7682. [Google Scholar] [CrossRef] [PubMed]
- Yua, A.; Wu, J.; Tang, X.; Zhao, L.; Xu, F.; Hu, Y. Application of near-infrared dyes for tumor imaging, photothermal, and photodynamic therapies. J. Pharm. Sci. 2013, 102, 6–28. [Google Scholar]
- Meldal, M.; Tornø, C.W. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Jewett, J.C.; Bertozzi, C.R. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 2010, 39, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Prescher, J.A.; Laughlin, S.T.; Agard, N.J.; Chang, P.V.; Miller, I.A.; Lo, A.; Codelli, J.A.; Bertozzi, C.R. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 16793–16797. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Sachin, K.; Jeong, H.J.; Choi, W.; Lee, H.S.; Kim, D.W. F-18 labeled RGD probes based on bioorthogonal strain-promoted click reaction for PET imaging. ACS Med. Chem. Lett. 2015, 6, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xing, Y.; Wang, J.; Conti, P.S.; Chen, K. Near-infrared fluorescence imaging of CD13 receptor expression using a novel Cy5.5-labeled dimeric NGR peptide. Amino Acids 2014, 46, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Zeglis, B.M.; Lewis, J.S.; Anderson, C.J. The growing impact of bioorthogonal click chemistry on the development of radiopharmaceuticals. J. Nucl. Med. 2013, 54, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, X.; Lin, W.-Y.; Shen, C.K.-F.; Yap, L.-P.; Hughes, L.D.; Conti, P.S. Strain-promoted catalyst-free click chemistry for rapid construction of 64Cu-labeled PET imaging probes. ACS Med. Chem. Lett. 2012, 3, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Na, J.H.; Lee, S.; Kang, C.M.; Kim, H.N.; Han, S.J.; Kim, H.; Choe, Y.S.; Jung, K.H.; Lee, K.C.; et al. Facile method to radiolabel glycol chitosan nanoparticles with 64Cu via copper-free click chemistry for MicroPET imaging. Mol. Pharm. 2013, 10, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, S.; Gao, D.; Hu, D.; Gong, P.; Sheng, Z.; Deng, J.; Ma, Y.; Cai, L. Click-functionalized compact quantum dots protected by multidentate-imidazole ligands: Conjugation-ready nanotags for living-virus labeling and imaging. J. Am. Chem. Soc. 2012, 134, 8388–8391. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Bandyopadhyay, D.; Mishra, D.; Datir, S.; Dhak, P.; Jain, S.; Maiti, T.K.; Basak, A.; Pramanik, P. “Clickable”, trifunctional magnetite nanoparticles and their chemoselective biofunctionalization. Bioconjugate Chem. 2011, 22, 1181–1193. [Google Scholar] [CrossRef] [PubMed]
- Beal, D.M.; Albrow, V.E.; Burslem, G.; Hitchen, L.; Fernandes, C.; Lapthorn, C.; Roberts, L.R.; Selby, M.D.; Jones, L.H. Click-enabled heterotrifunctional template for sequential bioconjugations. Org. Biomol. Chem. 2012, 10, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Clavé, G.; Volland, H.; Flaender, M.; Gasparutto, D.; Romieu, A.; Renard, P.-Y. A universal and ready-to-use heterotrifunctional cross-linking reagent for facile synthetic access to sophisticated bioconjugates. Org. Biomol. Chem. 2010, 8, 4329–4345. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, C.; Weck, M. Construction of well-defined multifunctional dendrimers using a trifunctional core. Chem. Commun. 2009, 5710–5712. [Google Scholar] [CrossRef] [PubMed]
- Mari, C.; Pierroz, V.; Ferrari, S.; Gasser, G. Combination of Ru(II) complexes and light: New frontiers in cancer therapy. Chem. Sci. 2015, 6, 2660–2686. [Google Scholar] [CrossRef]
- Antoni, P.M.; Naik, A.; Albert, I.; Rubbiani, R.; Gupta, S.; Ruiz-Sánchez, P.; Munikorn, P.; Mateos, J.M.; Luginbuehl, V.; Thamyongkit, P.; et al. (Metallo) porphyrins as potent phototoxic anti-cancer agents after irradiation with red light. Chem. Eur. J. 2015, 21, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Mion, G.; Gianferrara, T.; Bergamo, A.; Gasser, G.; Pierroz, V.; Rubbiani, R.; Vilar, R.; Leczkowska, A.; Alessio, E. Phototoxic activity and DNA interactions of water-soluble porphyrins and their rhenium(I) conjugates. ChemMedChem 2015, 10, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Gianferrara, T.; Spagnul, C.; Alberto, R.; Gasser, G.; Ferrari, S.; Pierroz, V.; Bergamo, A.; Alessio, E. Towards matched pairs of porphyrin–ReI/99mTcI conjugates that combine photodynamic activity with fluorescence and radio imaging. ChemMedChem 2014, 9, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Frei, A.; Rubbiani, R.; Tubafard, S.; Blacque, O.; Anstaett, P.; Felgenträger, A.; Maisch, T.; Spiccia, L.; Gasser, G. Synthesis, characterization, and biological evaluation of new Ru(II) polypyridyl photosensitizers for photodynamic therapy. J. Med. Chem. 2014, 57, 7280–7292. [Google Scholar] [CrossRef] [PubMed]
- Mari, C.; Pierroz, V.; Rubbiani, R.; Patra, M.; Hess, J.; Spingler, B.; Oehninger, L.; Schur, J.; Ott, I.; Salassa, L.; et al. DNA intercalating RuII polypyridyl complexes as effective photosensitizers in photodynamic therapy. Chem. Eur. J. 2014, 44, 14421–14436. [Google Scholar] [CrossRef] [PubMed]
- Leonidova, A.; Pierroz, V.; Rubbiani, R.; Heier, J.; Ferrari, S.; Gasser, G. Towards cancer cell-specific phototoxic organometallic rhenium(I) complexes. Dalton Trans. 2014, 43, 4287–4294. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Rubbiani, R.; Gasser, G.; Spingler, B. Visible-light-induced annihilation of tumor cells with platinum–porphyrin conjugates. Angew. Chem. Int. Ed. 2014, 53, 6938–6941. [Google Scholar] [CrossRef] [PubMed]
- Gasser, G.; Hüsken, N.; Köster, S.D.; Metzler-Nolte, N. Synthesis of organometallic PNA oligomers by click chemistry. Chem. Commun. 2008, 3675–3677. [Google Scholar] [CrossRef] [PubMed]
- Gasser, G.; Pinto, A.; Neumann, S.; Sosniak, A.M.; Seitz, M.; Merz, K.; Heumann, R.; Metzler-Nolte, N. Synthesis, characterisation and bioimaging of a fluorescent rhenium-containing PNA bioconjugate. Dalton Trans. 2012, 41, 2304–2313. [Google Scholar] [CrossRef] [PubMed]
- Leonidova, A.; Pierroz, V.; Adams, L.A.; Barlow, N.; Ferrari, S.; Graham, B.; Gasser, G. Enhanced cytotoxicity through conjugation of a “clickable” luminescent Re(I) complex to a cell-penetrating lipopeptide. ACS Med. Chem. Lett. 2014, 5, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Viehweger, K.; Barbaro, L.; Pombo García, K.; Joshi, T.; Geipel, G.; Steinbach, J.; Stephan, H.; Spiccia, L.; Graham, B. EGF receptor-targeting peptide conjugate incorporating a near-IR fluorescent dye and a novel 1, 4, 7-triazacyclononane-based 64Cu(II) chelator assembled via click chemistry. Bioconjugate Chem. 2014, 25, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Choy, T.H.; Ozawa, K.; Tuck, K.L.; Barlow, N.; Huber, T.; Otting, G.; Graham, B. Lanthanide tags for site-specific ligation to an unnatural amino acid and generation of pseudocontact shifts in proteins. Bioconjugate Chem. 2013, 24, 260–268. [Google Scholar]
- Loh, C.-T.; Graham, B.; Abdelkader, E.H.; Tuck, K.L.; Otting, G. Generation of pseudocontact shifts in proteins with lanthanides using small “clickable” nitrilotriacetic acid and iminodiacetic acid tags. Chem. Eur. J. 2015, 21, 5084–5092. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, E.H.; Feintuch, A.; Yao, X.; Adams, L.A.; Aurelio, L.; Graham, B.; Goldfarb, D.; Otting, G. Protein conformation by EPR spectroscopy using gadolinium tags clicked to genetically encoded p-azido-l-phenylalanine. Chem. Commun. 2015, 15898–15901. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-T.; Liu, H.-W.; Lo, K.K.-W. Rhenium(I) polypyridine dibenzocyclooctyne complexes as phosphorescent bioorthogonal probes: Synthesis, characterization, emissive behavior, and biolabeling properties. J. Inorg. Biochem. 2015, 148, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Candelon, N.; Hadade, N.D.; Matache, M.; Canet, J.-L.; Cisnetti, F.; Funeriu, D.P.; Nauton, L.; Gautier, A. Luminogenic “clickable” lanthanide complexes for protein labelling. Chem. Commun. 2013, 49, 9206–9208. [Google Scholar] [CrossRef] [PubMed]
- Mastarone, D.J.; Harrison, V.S.R.; Eckermann, A.L.; Parigi, G.; Luchinat, C.; Meade, T.J. A modular system for the synthesis of multiplexed magnetic resonance probes. J. Am. Chem. Soc. 2011, 133, 5329–5337. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.Y.; Holland, J.P.; Lewis, J.S. Clickable bifunctional radiometal chelates for peptide labelling. Chem. Commun. 2010, 46, 1706–1708. [Google Scholar] [CrossRef] [PubMed]
- Mindt, T.L.; Struthers, H.; Brans, L.; Anguelov, T.; Schweinsberg, C.; Maes, V.; Tourwé, D.; Schibli, R. “Click to chelate”: Synthesis and installation of metal chelates into biomolecules in a single step. J. Am. Chem. Soc. 2006, 128, 15096–15097. [Google Scholar] [CrossRef] [PubMed]
- Abraham, B.; Kelly, L.A. Photooxidation of amino acids and proteins mediated by novel 1,8-naphthalimide derivatives. J. Phys. Chem. B 2003, 107, 12534–12541. [Google Scholar] [CrossRef]
- Zhang, J.; Woods, R.J.; Brown, P.B.; Lee, K.D.; Kane, R.R. Synthesis and photochemical protein crosslinking studies of hydrophilic naphthalimides. Bioorg. Med. Chem. Lett. 2002, 12, 853–856. [Google Scholar] [CrossRef]
- Rogers, J.A.; Weiss, S.J.; Kelly, L.A. Photoprocesses of naphthalene imide and diimide derivatives in aqueous solutions of DNA. J. Am. Chem. Soc. 2000, 122, 427–436. [Google Scholar] [CrossRef]
- Aveline, B.A.; Matsugo, S.; Redmond, R.W. Photochemical mechanisms responsible for the versatile application of naphthalimides and naphthaldiimides in biological systems. J. Am. Chem. Soc. 1997, 119, 11785–11795. [Google Scholar] [CrossRef]
- Saito, I.; Takayama, M.; Sugiyama, H.; Nakatani, K. Photoinduced DNA cleavage via electron transfer: Demonstration that guanine residues located 5′ to guanine are the most electron-donating sites. J. Am. Chem. Soc. 1995, 117, 6406–6407. [Google Scholar] [CrossRef]
- Saito, I.; Takayama, M. Photoactivatable DNA-cleaving amino acids: Highly sequence-selective DNA photocleavage by novel L-lysine derivatives. J. Am. Chem. Soc. 1995, 117, 5590–5591. [Google Scholar] [CrossRef]
- Matsugo, S.; Kawanishi, S.; Yamamoto, K.; Sugiyama, H.; Matsuura, T.; Saito, I. Bis(hydroperoxy)naphthaldiimide as a “photo-Fenton reagent”: Sequence-specific photocleavage of DNA. Angew. Chem. Int. Ed. 1991, 30, 1351–1353. [Google Scholar] [CrossRef]
- Stasiuk, G.J.; Long, N.J. The ubiquitous DOTA and its derivatives: The impact of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid on biomedical imaging. Chem. Commun. 2013, 49, 2732–2746. [Google Scholar] [CrossRef] [PubMed]
- Caravan, P.; Ellison, J.; McMurry, T.; Lauffer, R. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef] [PubMed]
- Sherry, A.D.; Caravan, P.; Lenkinski, R.E. Primer on gadolinium chemistry. Magn. Reson. Med. 2009, 30, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Vives, E.; Brodin, P.; Lebleu, B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 1997, 272, 16010–16017. [Google Scholar] [CrossRef] [PubMed]
- Raghunand, N.; Guntle, G.P.; Gokhale, V.; Nichol, G.S.; Mash, E.A.; Jagadish, B. Design, synthesis, and evaluation of 1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid derived, redox-sensitive contrast agents for magnetic resonance imaging. J. Med. Chem. 2010, 53, 6747–6757. [Google Scholar] [CrossRef] [PubMed]
- Cha, T.R.; Hilgraf, R.; Sharpless, K.B.; Fokin, V.V. Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org. Lett. 2004, 6, 2853–2855. [Google Scholar]
- Hong, V.; Presolski, S.I.; Ma, C.; Finn, M.G. Analysis and optimization of copper-catalyzed azide-alkyne cycloaddition for bioconjugation. Angew. Chem. Int. Ed. 2009, 48, 9879–9883. [Google Scholar] [CrossRef] [PubMed]
- Sawa, M.; Hsu, T.-L.; Itoh, T.; Sugiyama, M.; Hanson, S.R.; Vogt, P.K.; Wong, C.-H. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 12371–12376. [Google Scholar] [CrossRef] [PubMed]
- Melhuish, W.H. Quantum efficiencies of fluorescence of organic substances: Effect of solvent and concentration of the fluorescent solute. J. Phys. Chem. 1961, 65, 229–235. [Google Scholar] [CrossRef]
- Sturzu, A.; Klose, U.; Echner, H.; Beck, A.; Gharabaghi, A.; Kalbacher, H.; Heckl, S. Cellular uptake of cationic gadolinium-DOTA peptide conjugates with and without N-terminal myristoylation. Amino Acids 2009, 37, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Malley, W.I.; Rubbiani, R.; Aulsebrook, M.L.; Grace, M.R.; Spiccia, L.; Tuck, K.L.; Gasser, G.; Graham, B. Cellular Uptake and Photo-Cytotoxicity of a Gadolinium(III)-DOTA-Naphthalimide Complex “Clicked” to a Lipidated Tat Peptide. Molecules 2016, 21, 194. https://doi.org/10.3390/molecules21020194
O’Malley WI, Rubbiani R, Aulsebrook ML, Grace MR, Spiccia L, Tuck KL, Gasser G, Graham B. Cellular Uptake and Photo-Cytotoxicity of a Gadolinium(III)-DOTA-Naphthalimide Complex “Clicked” to a Lipidated Tat Peptide. Molecules. 2016; 21(2):194. https://doi.org/10.3390/molecules21020194
Chicago/Turabian StyleO’Malley, William I., Riccardo Rubbiani, Margaret L. Aulsebrook, Michael R. Grace, Leone Spiccia, Kellie L. Tuck, Gilles Gasser, and Bim Graham. 2016. "Cellular Uptake and Photo-Cytotoxicity of a Gadolinium(III)-DOTA-Naphthalimide Complex “Clicked” to a Lipidated Tat Peptide" Molecules 21, no. 2: 194. https://doi.org/10.3390/molecules21020194
APA StyleO’Malley, W. I., Rubbiani, R., Aulsebrook, M. L., Grace, M. R., Spiccia, L., Tuck, K. L., Gasser, G., & Graham, B. (2016). Cellular Uptake and Photo-Cytotoxicity of a Gadolinium(III)-DOTA-Naphthalimide Complex “Clicked” to a Lipidated Tat Peptide. Molecules, 21(2), 194. https://doi.org/10.3390/molecules21020194