Abstract
A series of 3-acylhydrazono-4-hydroxycoumarins were synthesized via condensation of 3-acetyl-4-hydroxycoumarin with appropriate hydrazides. The structures of the newly-synthesized compounds were characterized by spectral and elememental analysis or HRMS measurements. Their antioxidant properties were evaluated by using scavenging effects on 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical as well as inhibition of lipid peroxidation. Moreover, their ability to inhibit in vitro soybean lipoxygenase has been investigated. They were found to be capable of rapid inactivation of alkylperoxy radicals.
1. Introduction
3-Substituted-4-hydroxycoumarins constitute an important class of heterocycles, which occur widely among natural products and have interesting biological properties and importance in medicine. They have been reported to exhibit a variety of pharmacological activity as antibacterial, [1,2] antitumor, [3] activity against HIV virus, [4] antithrombotic, [5,6,7,8], as well as antioxidant activity [9,10]. Coumarins are one of the most important secondary metabolites of plants and are known as naturally occurring benzo-α-pyrone derivatives from metabolism of phenylalanine [11]. More than 1000 different types of coumarins have been isolated from natural sources. Robustic acid, [12] ferulenol and its analogues, [13,14] as well as the two sesquiterpenecoumarins isolated from Ferula pallida [15], are characteristic examples of 4-hydroxycoumarins, which have been isolated as natural products. Furthermore, the 4-hydroxycoumarin moiety has been the molecular template for the synthesis of a variety of analogues with important biological activity. Warfarin is a synthetic coumarin, which is widely used as anticoangulant, [16] whereas aminocoumarin analogues, such as novobiocin, chlorobiocin, coumermycin, and simocyclinone are potent antibiotics [17,18,19]. Furthermore, the importance of hydrazone derivatives is well known because of their use as synthons in organic synthesis [20,21] as well as because of their biological properties. They have been reported to possess among others anticonvulsant, antidepressant, analgestic, anti-inflammatory, antimicrobial, antimalarial, antitumoral, antileukemic, antiviral, antitubercular, as well as antioxidant activity [22,23,24,25,26].
It is well known that the design of biological substrates with antioxidant activity to be used for disease treatment or as food additives, as well as oxidative stress, have attracted many researchers’ interest. The potential activity of both coumarin, as well as hydrazone derivatives, as antioxidant agents prompted us to synthesize a series of new coumarin analogues bearing the 3-acylhydrazono functionality and a 4-hydroxy group on the coumarin ring. The combination of the pharmacophores of two different biologically-active compounds in the same molecule could lead to a new product exhibiting combined activity.
The formation of Reactive Oxygen Species (ROS) is characteristic of aerobic organisms that can normally defend themselves against these highly reactive species. However, in many pathophysiological conditions the excessive production of ROS overwhelms the natural antioxidant defense mechanisms. This imbalance is termed oxidative stress, which has been associated with the inflammation process. ROS, like superoxide radical anion, hydrogen peroxide and hydroxyl radical, are produced during the inflammation process by phagocytic leukocytes. Moreover, these reactive species are involved in the biosynthesis of prostaglandins and in the cycloxygenase- and lipoxygenase-mediated conversion of arachidonic acid. The rates of ROS production are increased in most pathophysiological conditions [27]; therefore, it is evident that the treatment of various diseases could benefit from the use of drugs that combine antioxidant and anti-inflammatory activity.
Thus, based on the above literature findings and on our interest in coumarin [28,29,30,31,32] and hydrazone derivatives [20,21], as well as in the biological activity of small molecules [23,29], we present here the synthesis and structural characterization of a series of 3-acylhydrazono-substituted 4-hydroxycoumarins, as well as their in vitro antioxidant and soybean lipoxygenase inhibitory activity.
2. Results and Discussion
3-Acetyl-4-hydroxycoumarin N-acylhydrazones 2a–l were prepared according to the literature [28] via treatment of 3-acetyl-4-hydroxycoumarins 1 with the appropriate hydrazide in n-propanol, as it is depicted in Scheme 1. The molar ratio of the reactants was 1:1. The reaction was performed under reflux for 24 h to yield hydrazones 2a–l in excellent yields. Products 2b, 2f, 2g, and 2h are new compounds, whereas 2a, 2c–2e, and 2i–2l have been recently synthesized and identified [28]. Compound 2a has also been mentioned in the literature earlier [33] but its spectral data have been only recently reported [28]. New hydrazones 2b, 2f, 2g, and 2h were obtained in 70%–94%, whereas they have been alternatively afforded via reflux of ketone 1 with the appropriate hydrazide for 2 h in very good to excellent yields (69%–94%), comparatively lower to those under 24 h reflux (70%–98%). Hydrazones 2a–l were purified via recrystallization from n-propanol. The mother ketone 1 has been prepared according to the literature by direct acetylation of 4-hydroxy-coumarin with acetyl chloride [34].
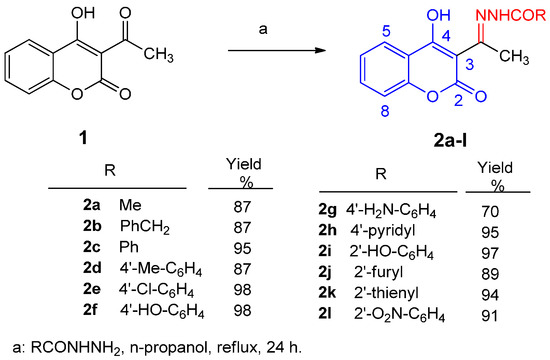
Scheme 1.
Synthesis of 3-acetyl-4-hydroxycoumarin N-acylhydrazones 2a–l.
Scheme 1.
Synthesis of 3-acetyl-4-hydroxycoumarin N-acylhydrazones 2a–l.
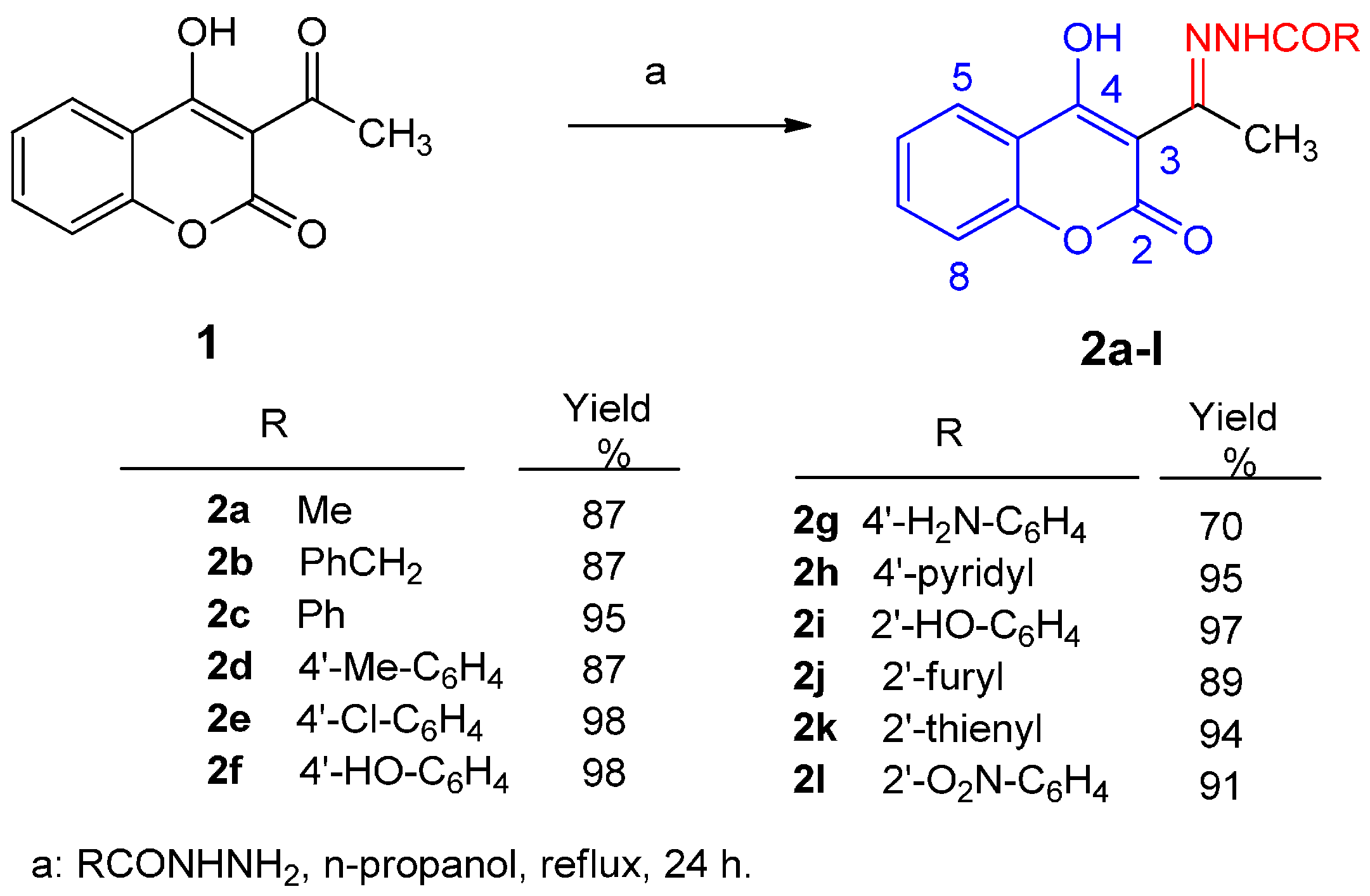
The structure of the new compounds 2b, 2f, 2g, and 2h was identified by their 1H- and 13C-NMR data as well as by their mass spectra and either their elemental analysis or high-resolution exact mass measurement. In 1H-NMR a characteristic singlet at about 2.65–2.77 ppm is assigned to the methyl protons attached at the 3-C=N carbon, whereas the proton at C-5 of the coumarin appears as a doublet of doublets at about 7.95 to 8.02 ppm in accordance with the literature data for other 4-hydroxycoumarin derivatives [29,35]. Furthermore, full assignments of the proton and carbon chemical shifts were based on coupling constants and on analogous coumarin derivatives assigned by detailed study of their 2D NMR data [35,36].
Furthermore, hydrazones 2 show prominent peaks corresponding to the ion [M + 1] in their mass spectra. It should be noted that according to the literature data [35] compound 2 derivatives possibly exist as enols stabilized by hydrogen bond (as shown in Figure 1). Recently, the structure of 3-{N-[(2′-thienylcarbonyl)hydrazono]ethyl}-4-hydroxycoumarin 2k has been confirmed by X-ray analysis [37].
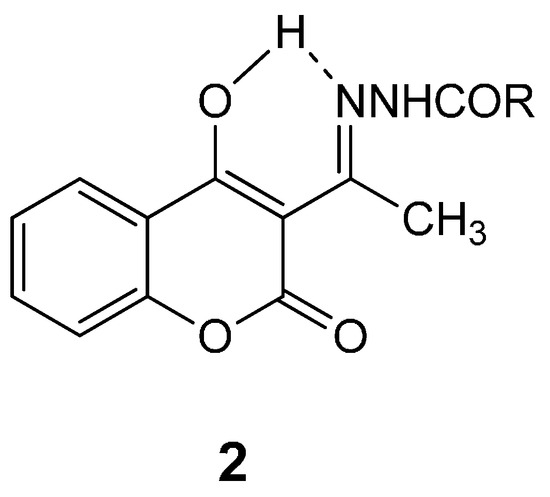
Figure 1.
Hydrogen bond in 3-acetyl-4-hydroxycoumarin N-acylhydrazones.
Figure 1.
Hydrogen bond in 3-acetyl-4-hydroxycoumarin N-acylhydrazones.
2.1. Pharmacology
Antioxidant Activity
Taking the multifactorial character of oxidative stress into account, we decided to evaluate the in vitro antioxidant activity of the synthesized molecules using two different antioxidant assays. Therefore, the radical scavenging ability of the compounds was tested against the 2,2-diphenyl-1-picrylhydrazyl (DPPH) stable free radical and their ability to inhibit lipid peroxidation induced by the thermal free radical producer 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) was evaluated.
It is well known that the interaction of the synthesized 3-acetyl-4-hydroxycoumarin N-acylhydrazones 2 with the stable free radical DPPH indicates their radical scavenging ability in an iron-free system [29]. In the present case, the interaction of the tested 3-acylhydrazono substituted 4-hydroxycoumarins 2 with DPPH was found to be concentration-dependent whereas, the time did not influence the reducing radical scavenging ability. Furthermore, all the tested compounds at 100 μΜ have presented similar radical reducing abilities ranging from 23%–27% to 24%–31% for 20 and 60 min respectively whereas, the interaction was found to be rather limited for the concentration of 50 μM (as shown in Figure 2 and in the collective Table 1). Considering the antioxidant activity of 3-acetyl-4-hydroxy coumarin (1) it seems to be higher than the hydrazone derivatives 2a–l in both concentrations and in relation with the time and it is correlated with the presence of 3-acetyl and 4-hydroxy groups in the lactone ring and the possibility of tautomers (A–D) formation [38,39,40] as shown in Scheme 2. It has been reported that 3-acetyl-4-hydroxycoumarin mainly exists in endocyclic enol form (B) in polar solvents (methanol, ethanol) and it is well known that enols show antioxidant activity e.g., it has been reported that enolic and phenolic hydroxyl groups is beneficial for curcumin to protect erythrocytes against hemin-induced hemolysis and to protect DNA against AAPH-induced oxidation [41]. The lower results of hydrazones 2a–l, are correlated with their stereochemistry which influence their interaction with DPPH.

Table 1.
Inhibition % of DPPH at different concentrations and times, calculated lipophilicity Clog P [41] and % inhibition of LP and (LOX) (IC50) for compound 2.
| Compd. | RA%, 50 μM, 20 min | RA%, 50 μM, 60 min | RA%, 100 µM, 20 min | RA%, 100 μM, 60 min | Clog P | LP a 60 s, 100 μM | LOX b IC50 (μM) |
|---|---|---|---|---|---|---|---|
| 2a | 5 ± 0.2 | 7 ± 0.3 | 23 ± 3.0 | 24 ± 2.0 | 1.85 | 100 ± 9.8 | 62.5 ± 2.3 |
| 2b | 4 ± 0.2 | 7 ± 0.1 | 25 ± 2.0 | 29 ± 1.1 | 3.62 | 100 ± 5.5 | 40 ± 0.5 |
| 2c | 5 ± 0.1 | 7 ± 0.4 | 25 ± 1.2 | 30 ± 2.8 | 3.29 | 98 ± 5.4 | 58 ± 2.7 |
| 2d | 7 ± 0.3 | 10 ± 0.2 | 27 ± 2.2 | 27 ± 1.4 | 3.79 | 100 ± 3.2 | No c |
| 2e | 7 ± 0.2 | 10 ± 0.5 | 27 ± 0.9 | 29 ± 0.8 | 4.20 | 94 ± 4.8 | 55 ± 2.1 |
| 2f | 10 ± 0.5 | 14 ± 1.2 | 24 ± 2.2 | 26 ± 1.2 | 2.96 | 98 ± 2.9 | 70 ± 4.3 |
| 2g | 9 ± 0.3 | 16 ± 0.6 | 27 ± 0.8 | 31 ± 1.4 | 2.38 | 95 ± 7.2 | 46.5 ± 2.3 |
| 2h | 5 ± 0.1 | 8 ± 0.2 | 27 ± 1.5 | 31 ± 1.6 | 2.53 | 99 ± 3.7 | No c |
| 2i | 8 ± 0.5 | 10 ± 0.1 | 27 ± 0.2 | 29 ± 1.7 | 2.96 | 100 ± 8.2 | 49.5 ± 1.2 |
| 2j | 4 ± 0.2 | 8 ± 0.3 | 25 ± 1.8 | 27 ± 0.8 | 2.46 | 95 ± 4.1 | 90 ± 5.1 |
| 2k | 7 ± 0.1 | 9 ± 0.2 | 25 ± 2.1 | 30 ± 2.2 | 3.13 | 98 ± 3.9 | 43.5 ± 3.2 |
| 2l | 6 ± 0.3 | 10 ± 0.2 | 27 ± 2.2 | 29 ± 1.0 | 3.46 | 95 ± 6.2 | 35 ± 0.2 |
| 1 | 29 ± 0.5 | 31± 0.3 | 36± 1.3 | 36 ± 0.8 | 1.91 | 8 ± 0.2 | 44 (± 0.3) d |
| NDGA | 84 ± 2.0 | 83 ± 3.3 | 81 ± 5.2 | 83 ± 4.7 | 5.5 ± 0.1 | ||
| TROLOX | 63 ± 0.2 |
a % inhibition of LP induced by AAPH; b in vitro inhibition of soybean lipoxygenase (LOX); c no action under the reported experimental conditions; d the presented biological response is given as % inhibition. The IC50 value was not be able to be determined.
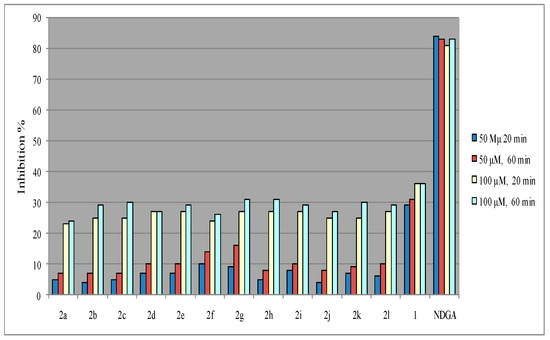
Figure 2.
Effect of compounds 2 towards 2,2-diphenyl-2-picrylhydrazyl (DPPH).
Figure 2.
Effect of compounds 2 towards 2,2-diphenyl-2-picrylhydrazyl (DPPH).
Scheme 2.
Tautomers (A–D) of 3-acetyl-4-hydroxycoumarin 1.
Scheme 2.
Tautomers (A–D) of 3-acetyl-4-hydroxycoumarin 1.
AAPH induced linoleic acid oxidation is based on the inhibition of lipid oxidation and provides a measure of how efficiently antioxidants protect against lipid oxidation in vitro. Oxidation of exogenous linoleic acid by a thermal free radical producer (AAPH) is followed by UV spectrophotometry in a highly-diluted sample [36,42].
In general, all the studied compounds effectively inhibit AAPH induced lipid peroxidation, showing higher activity than the reference compound trolox (63%, Figure 3 and Table 1). 3-Acetyl-4-hydroxy coumarin (1) presents non-significant anti-lipid peroxidation activity. However, all the derivatives exhibit very potent inhibition of lipid peroxidation and almost the same as a result of their combined structural characteristics. Lipophilicity does not seem to play any significant role. For example, methyl derivative 2a, is a good inhibitor of lipid peroxidation (100%), while it presents the lowest Clog P value among all the analogues (Table 1). The tested derivatives possess a favorable electronic distribution for reacting quickly with intermediate lipid peroxy radicals and sufficient lipid solubility to partition effectively in lipid bilayers. Our preliminary results suggest that they are indeed capable to inactivate rapidly alkylperoxy radicals.
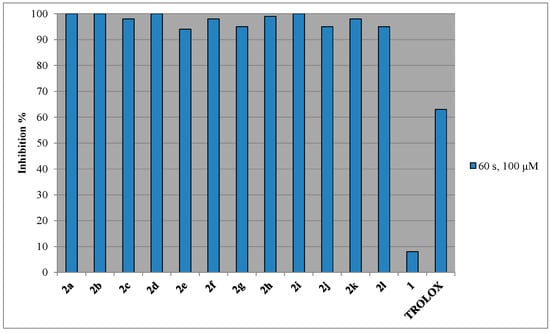
Figure 3.
Effect of compound 2 towards AAPH lipid peroxidation.
Figure 3.
Effect of compound 2 towards AAPH lipid peroxidation.
2.2. In Vitro Inhibition of Soybean Lipoxygenase (LOX)
Coumarins as well as flavonoids are among the most potent 5-lipoxygenase inhibitors. The synthesized coumarins were evaluated for inhibition of soybean lipoxygenase by the UV-absorbance-based enzyme assay [29] and the results are presented in Figure 4 as well as in Table 1. The majority of LOX inhibitors are antioxidants or free radical scavengers [43], since lipoxygenation occurs via a carbon-centered radical. Some studies suggest a relationship between LOX inhibition and the ability of the inhibitors to reduce the Fe3+ at the active site to the catalytically inactive Fe2+. Several LOX inhibitors are excellent ligands for Fe3+ [44,45]. It has been demonstrated that their mechanism of action is presumably related to their coordination with a catalytically crucial Fe3+. 3-Acetyl-4-hydroxy coumarin (1) showed low inhibitory activity at 100 µM and, thus, we did not proceed to determine its IC50 value. In Table 1, its response is given, as a % inhibition value at 100 µM. Among the tested compounds, the 2′-NO2-substituted phenyl (2l) was found to exhibit superior LOX inhibitory activity, followed by the benzyl substituted hydrazine (2b), the 2′-thienyl-substituted derivative (2k), the 4-NH2-phenyl substituted hydrazine (2g) and the 2′-OH-substituted phenyl (2i) (Figure 4, Table 1). No sign for the role of overall lipophilicity is obvious. However, the three most potent derivatives 2l, 2b, and 2k present a mean value of Clog P = 3.4. The 2′-thienyl-substituted derivative (2k) is more potent than the corresponding 2’-furyl derivative (2j), whereas the 4’-pyridyl-analogue 2h and the 4′-CH3-substituted phenyl hydrazone (2d) do not seem to present any activity under the reported experimental conditions. The position of substitution is significant since the 2-substituted derivative, e.g., the 2′-OH-substituted phenyl (2i) is more potent than the corresponding 2f which is a 4′-OH-substituted phenyl hydrazone. Small differences are observed when R is a phenyl or a small alkyl group. Each in vitro experiment was performed at least in triplicate and the standard deviation of absorbance was less than 10% of the mean.
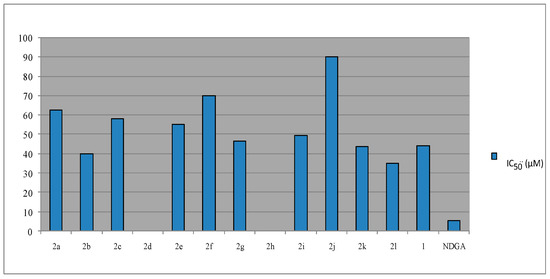
Figure 4.
Effect of compounds 2 towards soybean lipoxygenase (LOX).
Figure 4.
Effect of compounds 2 towards soybean lipoxygenase (LOX).
3. Experimental Section
3.1. General
3-Acetyl-4-hydroxycoumarin 1 was synthesized according to the literature [28,34]. Melting points are uncorrected and were determined on a Fisher-Johns melting point apparatus. 1D-NMR spectra were recorded at room temperature on a Bruker Avance 400 spectrometer (Bruker, Billerica, MA, USA) at 400.15 MHz for 1H-NMR and 100.62 MHz for 13C-NMR in DMSO-d6. The chemical shifts are expressed in δ values (ppm) relative to TMS as internal standard for 1H and relative to TMS (0.00 ppm) or to DMSO-d6 (39.50 ppm) for 13C-NMR spectra. Coupling constants nJ are reported in Hz. Second order 1H spectra, where it was possible, were analyzed by simulation [46]. Either elemental analysis or HRMS has been provided for all new products of 2. All the chemicals used for biological assays were of analytical grade and commercially available by Merck, 2,2-diphenyl-1-picrylhydrazyl (DPPH), nordihydroguairetic acid (NDGA), trolox, and AAPH were purchased from the Aldrich Chemical Co. (Milwaukee, WI, USA). Soybean Lipoxygenase and linoleic acid sodium salt were obtained from Sigma Chemical, Co. (St. Louis, MO, USA).
3.2. Chemistry
Synthesis of 3-[1-(Acyl-hydrazono)ethyl]-4-hydroxycoumarins (2a–l)
To a solution of 3-acetyl-4-hydroxy-coumarin 1 (1 mmol) in n-propanol (15–20 mL) was added the appropriate hydrazide (1 mmol). The mixture was refluxed for 24 h and cooled at room temperature. The precipitate was collected by filtration and dried to give the 3-[1-(acyl-hydrazono)ethyl]-4-hydroxycoumarin (2a–l) as solid and was then recrystallized from n-propanol in very good yields. The following compounds have been prepared according to this procedure:
3-[N-(Acetylhydrazono)ethyl]-4-hydroxycoumarin (2a). Yield: 87% under reflux for 24 h and 81% under reflux for 2 h; light yellow solid; mp 248–249 °C (mp 250–251 °C (from MeOCH2CH2OH/H2O) [30]); 1H-NMR (DMSO-d6, 400 MHz) δ 2.07 (s, 3H, 3-COCH3), 2.66 (s, 3H, 3-CCH3), 7.29 (dd, J = 8.3, 1.0 Hz, 1H, 8-H), 7.32 (ddd, J = 7.9, 7.3, 1.0 Hz, 1H, 6-H), 7.66 (ddd, J = 8.3, 7.3, 1.8 Hz, 1H, 7-H), 7.97 (dd, J = 7.9 Hz, 1.8 Hz, 1H, 5-H), 11.42 (s, 1H, NNH), 15.90 (br s, 1H, 4-OH); 13C-NMR (DMSO-d6, 100 MHz) δ 17.3 (3-CCH3), 20.4 (3-COCH3), 94.9 (C-3), 116.3 (C-8), 119.5 (C-4a), 123.8 (C-5), 125.5 (C-6), 134.2 (C-7), 153.0 (C-8a), 161.8 (C-2), 167.0 (3-C=N), 169.2 (NHCO), 178.7 (C-4); HRMS (ESI+) calcd for C13H12N2O4 m/z: 261.08698 (M + H+); found 261.08688 (M + H+).
3-[N-(Phenylacetylhydrazono)ethyl]-4-hydroxycoumarin (2b). Yield: 87% under reflux for 24 h and 83% under reflux for 2 h; yellow solid; mp 216–217 °C; 1H-NMR (DMSO-d6, 400 MHz) δ 2.65 (s, 3H, 3-CCH3), 3.70 (s, 2H, CH2Ph), 7.28 (dd, J = 8.3, 1.0 Hz, 1H, 8-H), 7.28–7.36 (m, 6H, 6-H, C6H5), 7.64 (ddd, J = 8.3, 7.3, 1.0 Hz, 1H, 7-H), 7.95 (dd, J = 7.8, 1.5 Hz, 1H, 5-H), 11.7 (s, 1H, NNH), 15.9 (br, 1H, 4-OH); 13C-NMR (DMSO-d6, 100 MHz) δ 17.8 (3-CCH3), 40.4 (CH2, masked under the septet of the solvent), 95.5 (C-3), 116.8 (C-8), 119.9 (C-4a), 124.3 (C-5), 126.0 (C-6), 127.3 (C-4′), 128.9 (C-3′,5′), 129.7 (C-2′,6′), 134.7 (C-7), 135.2 (C-1′), 153.5 (C-8a), 161.8 (C-2), 168.4 (3-C=N), 170.3 (NHCO), 179.2 (C-4); HRMS (ESI+) calcd for C19H16N2O4 m/z: 359.10023 (M + Na+), 695.21124 (2M + Na+); found 359.10031 (M + Na+), 695.21160 (2M + Na+).
3-[N-(Benzoylhydrazono)ethyl]-4-hydroxycoumarin (2c). Yield: 95%; white solid; mp 225–226 °C [28]
3-{N-[(4′-Methylbenzoyl)hydrazono]ethyl}-4-hydroxycoumarin (2d). Yield: 87%; white solid; mp 251–252 °C [28].
3-{N-[(4′-Chlorobenzoyl)hydrazono]ethyl}-4-hydroxycoumarin (2e). Yield: 98%; white solid; mp 248–248.5 °C [28].
3-{N-[(4′-Hydroxybenzoyl)hydrazono]ethyl}-4-hydroxycoumarin (2f). Yield: 98% under reflux for 24 h and 94% under reflux for 2 h; white solid; mp 287–288 °C; 1H-NMR (DMSO-d6, 400 MHz) δ 2.75 (s, 3H, 3-CCH3), 6.91 (d, J = 8.7 Hz, 2H, 3′,5′-H), 7.30 (dd, J = 8.3, 1.0 Hz, 1H, 8-H), 7.33 (ddd, J = 7.8, 7.2, 1.0 Hz, 1H, 6-H), 7.67 (ddd, J = 8.3, 7.0, 1.7 Hz, 1H, 7-H), 7.84 (d, J = 8.7 Hz , 2H, 2′,6′-H), 8.00 (dd, J = 7.9, 1.7 Hz, 1H, 5-H), 10.34 (br s, 1H, 4′-OH), 11.55 (s, 1H, NNH), 15.72 (br, 1H, 4-OH); 13C-NMR (DMSO-d6, 100 MHz) δ 18.1 (3-CCH3), 95.7 (C-3), 115.8 (C-3′,5′), 116.8 (C-8), 120.2 (C-4a), 122.0 (C-1′), 124.3 (C-5), 126.2 (C-6), 130.6 (C-2′,6′), 134.8 (C-7), 153.6 (C-8a), 161.98 (C-4′), * 162.04 (C-2), * 165.0 (3-C=N), 172.2 (NHCO), 179.7 (C-4); MS (ESI): m/z 338 (M+). Anal. calcd for C18H14N2O5: C, 63.90; H, 4.17; N, 8.28; found C, 63.70; H, 3.98; N, 8.44. (*: The assignments may be interchanged).
3-{N-[(4′-Aminobenzoyl)hydrazono]ethyl}-4-hydroxycoumarin (2g). Yield: 70% under reflux for 24 h and 69% under reflux for 2 h; light yellow solid; mp 256–257 °C; 1H-NMR (DMSO-d6, 400 MHz) δ 2.74 (s, 3H, 3-CCH3), 6.01 (br, 2H, NH2), 6.63 (d, J = 8.6 Hz, 2H, 3′,5′-H), 7.29 (d, J = 8.4 Hz, 1H, 8-H), 7.33 (dd, J = 7.8, 7.2 Hz, 1H, 6-H), 7.66 (ddd, J = 8.4, 7.2, 1.5 Hz, 1H, 7-H), 7.69 (d, J = 8.6 Hz, 2H, 2′,6′-H), 8.00 (dd, J = 7.8, 1.5 Hz, 1H, 5-H), 11.30 (s, 1H, NNH), 15.74 (br, 1H, 4-OH); 13C-NMR (DMSO-d6, 100 MHz) δ 18.2 (3-CCH3), 95.5 (C-3), 113.2 (C-3′,5′), 116.8 (C-8), 117.2 (C-1′), 120.3 (C-4a), 124.3 (C-5), 126.1 (C-6), 130.3 (C-2′,6′), 134.6 (C-7), 153.6 (C-8a), * 153.8 (C-4′), * 162.0 (C-2), 165.2 (3-C=N), 171.4 (NHCO), 179.6 (C-4); MS (ESI) m/z 337 (M+). Anal. Calcd for C18H15N3O4 C, 64.09; H, 4.48; N, 12.46. Found C, 63.73; H, 4.25; N, 12.46. (*: The assignments may be interchanged).
3-[N-(Isonicotinoylhydrazono)ethyl]-4-hydroxycoumarin (2h). Yield: 95% under reflux for 24 h and 91% under reflux for 2 h; orange solid; mp 274 °C; 1H-NMR (DMSO-d6, 400 MHz) δ 2.78 (s, 3H, 3-CCH3), 7.31 (dd, J = 8.3, 1.0 Hz, 1H, 8-H), 7.34 (ddd, J = 7.8, 7.4, 1.0 Hz, 1H, 6-H), 7.67 (ddd, J = 8.3, 7.4, 1.6 Hz, 1H, 7-H), 7.95 (br d, J = 4.8 Hz, 2H, 2′,6′-H), 8.01 (dd, J = 7.9, 1.6 Hz, 1H, 5-H), 8.84 (br s, 2H, 3′,5′-H), 11.9 (br, 1H, NNH), 15.75 (br s, 1H, 4-OH); 13C-NMR (DMSO-d6, 100 MHz) δ 17.6 (3-CCH3), 95.3 (C-3), 116.3 (C-8), 119.9 (C-4a), 122.0 (C-2′,6′), 123.8 (C-5), 125.6 (C-6), 134.2 (C-7), 140.6 (br, C-1′), 149.3 (br, C-3′,5′), 153.1 (C-8a), 161.50 (C-2), 163.2 (3-C=N), 171.0 (br, NHCO), 178.9 (C-4); HRMS (ESI+) Anal. Calcd for C17H13N3O4 m/z: 324.09788 (M + H+); Found 324.09784 (M+H+).
3-{N-[(2′-Hydroxybenzoyl)hydrazono]ethyl}-4-hydroxycoumarin (2i). Yield: 97%; white solid; mp 271–272 °C [28].
3-{N-[(2′-Furoyl)hydrazono]ethyl}-4-hydroxycoumarin (2j). Yield: 89%; yellow solid; mp 254.5–255.0 °C [28].
3-{N-[(2′-Thienylcarbonyl)hydrazono]ethyl}-4-hydroxycoumarin (2k). Yield: 94%; light yellow solid; mp 228–228.5 °C [28].
3-{N-[(2′-Nitrobenzoyl)hydrazono]ethyl}-4-hydroxycoumarin (2l). Yield: 91%; yellow solid; mp 219 °C [28].
3.3. Pharmacology
3.3.1. Determination of the Reducing Activity of the DPPH (RA%)
To an ethanolic solution of DPPH (0.05 mM) in absolute ethanol the new coumarin derivatives dissolved in DMSO were added (final concentration 50 and 100 µM). The mixture was shaken vigorously and allowed to stand for 20 min or 60 min; absorbance at 517 nm was determined spectrophotometrically against the blank and the percentage of reducing activity (RA) was calculated by the formula: RA% = [(A0 − A1)/A0] × 100 where A0 is the absorbance of blank and A1 is the absorbance of the reaction mixture. All tests were undertaken on three replicates and the results presented in Table 1 were averaged.
3.3.2. Inhibition of Linoleic Acid Lipid Peroxidation
Production of conjugated diene hydroperoxide by oxidation of linoleic acid in an aqueous dispersion is monitored at 234 nm. 2,2′-Azobis(2-amidinopropane) dihydrochloride (AAPH) is used as a free radical initiator. Ten microliters of the 16 mM linoleic acid sodium salt solution was added to the UV cuvette containing 930 µL of 0.05 mM phosphate buffer, pH 7.4 prethermostated at 37 °C. The oxidation reaction was initiated at 37 °C under air by the addition of 50 μL of 40 mM AAPH solution. Oxidation was carried out in the presence of aliquots (10 μL) of the tested coumarins. In the assay without antioxidant, lipid peroxidation was measured in the presence of the same level of DMSO. The rate of oxidation at 37 °C was monitored by recording the increase in absorption at 234 nm caused by conjugated diene hydroperoxides.
3.3.3. Soybean Lipoxygenase Inhibition Study In Vitro
The tested compounds dissolved in DMSO were incubated at room temperature with sodium linoleate (100 µL) and 200 µL of enzyme solution (1/9 × 10−4 w/v in saline) in Tris buffer pH 9. The conversion of sodium linoleate to 13-hydroperoxylinoleic acid at 234 nm was recorded and compared with the appropriate standard inhibitor.
3.3.4. Physicochemical Studies
Since lipophilicity is a significant physicochemical property determining distribution, bioavailability, metabolic activity, and elimination, the theoretically calculated [47] Clog P values in n-octanol-buffer are included in Table 1. For their determination the C-QSAR program of Biobyte Corp. was used.
4. Conclusions
In this study, a series of 3-acylhydrazono substituted 4-hydroxycoumarins have been synthesized and characterized. The antioxidant activity of the synthesized compounds has been studied in vitro using two different assays. Moreover, in an attempt to identify the potential of the compounds as anti-inflammatory agents, their ability to inhibit in vitro soybean lipoxygenase was evaluated. Although the free 4-hydroxy coumarin was not found to present any antioxidant activity [48] its combination with a 3-imino group [49] recently led to antioxidant properties. These results go in parallel to our findings, where the combination of 4-hydroxy coumarin with the 3-acyl-hydrazone group leads to potent inhibitors of lipid peroxidation.
Our study indicates that high LOX inhibitory activity is not accompanied by high DPPH radical scavenging activity. This is in accordance with the finding of Curini et al. [50], who have studied the antioxidant and LOX inhibitory activity of five natural prenyloxy-carboxylic acids and showed that the most efficient LOX inhibitor (boropinic acid) is not the most active DPPH radical scavenger. However, a better correlation exists between LOX inhibitory activity and lipid peroxidation inhibition.
It is of interest that compound 2l, the 2-nitro-substituted-3-acylhydrazono-4-hydroxy-coumarin, is the most potent as it possesses an array of potentially beneficial characteristics: it inhibits lipid peroxidation with satisfactory potency and it inhibits LOX (IC50 = 35 μM). It would, thus, be of special interest to characterize this molecule in terms of its anti-inflammatory profile.
Acknowledgments
The authors are grateful to Royal Society of Chemistry for financial support of the chemical synthesis and to Biobyte Corp. and Hansch and Leo for their support and free access to the C-QSAR program. This research has been done using the above program via Internet. Biobyte Corp., 01 West 4th Street, Suite 204, Claremont, CA 91711, USA.
Author Contributions
A.K. conceived the idea of this piece of research; A.K. and D.J.H. designed the experiments; D.A.N. performed the chemical experiments; C.A.K. performed the pharmacology experiments; P.A.H. performed the spectra analyses and provided HRMS and elemental analysis; A.K., D.J.H., C.A.T., P.A.H., D.A.N. and C.A.K. analyzed the data; A.K., D.J.H., C.A.T. and P.A.H. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Bedair, A.H.; El-Hady, N.A.; Abd El-Latif, M.S.; Fakery, A.H.; El-Agrody, A.M. 4-Hydroxycoumarin in heterocyclic synthesis Part III: Synthesis of some new pyrano[2,3-d]pyrimidine, 2-substituted[1,2,4]triazolo[1,5-c]pyrimidine and pyrimido[1,6-b][1,24]triazine derivatives. Farmaco 2000, 55, 708–714. [Google Scholar] [CrossRef]
- Cravotto, G.; Tagliapietra, S.; Cappello, R.; Palmisano, G.; Curini, M.; Boccalini, M. Long-chain 3-acyl-4-hydroxycoumarins: Structure and antibacterial activity. Arch. Pharm. Chem. Life Sci. 2006, 339, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I. Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem. Anti Cancer Agents 2005, 5, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Kirkiacharian, S.; Thuy, D.T.; Sicsic, S.; Bakhchinian, R.; Kurkjian, R.; Tonnaire, T. Structure-activity relationships of some 3-substituted-4-hydroxycoumarins as HIV-1 protease inhibitors. Farmaco 2002, 57, 703–708. [Google Scholar] [CrossRef]
- Manolov, I.; Danchev, N.D. Synthesis, toxicological and pharmacological assessment of some 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 1995, 30, 531–535. [Google Scholar] [CrossRef]
- Manolov, I.; Maichle-Moessmer, C.; Danchev, N. Synthesis, structure, toxicological and pharmacological investigations of 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2006, 41, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.G.; Sadowski, J.A.; Matschiner, J.T. Mechanism of action of warfarin. Warfarin and metabolism of vitamin K1. Biochemistry 1972, 11, 1959–1961. [Google Scholar] [CrossRef] [PubMed]
- Au, N.; Rettie, A.E. Pharmacogenomics of 4-hydroxycoumarin anticoagulants. Drug Metab. Rev. 2008, 40, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Stanchev, S.V.; Hadjimitova, T.; Traykov, T.; Boyanov, I.; Manolov, I. Investigation of the antioxidant properties of some new 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2009, 44, 3077–3082. [Google Scholar] [CrossRef] [PubMed]
- Al-Ayed, A.S. Synthesis of new substituted chromen[4,3-c]pyrazol-4-ones and their antioxidant activities. Molecules 2011, 10292–10302. [Google Scholar] [CrossRef] [PubMed]
- Tosum, A. Biotechnological production of coumarins. In Biotechnological Production of Plant Secondary Metabolites; Orhan, I.E., Ed.; Bentham e-Books: Sharjah, The United Arab Emirates, 2012; pp. 36–52. [Google Scholar]
- Harper, S. The active principles of leguminous fish-poison plants. Part VI. Robustic acid. J. Chem. Soc. 1942, 181–182. [Google Scholar] [CrossRef]
- Miski, M.; Jakupovic, J. Cyclic farnesyl-coumarin and farnesyl-chromone derivatives from Ferula communis subsp. Communis. Phytochemistry 1990, 29, 1995–1998. [Google Scholar] [CrossRef]
- Lamnaouer, D.; Fraigui, O.; Martin, M.T.; Bodo, B. Structure of ferulenol derivatives from Ferula communis var. genuine. Phytochemistry 1991, 30, 2383–2386. [Google Scholar] [CrossRef]
- Saidkhodzhaev, A.I.; Kushmuradov, A.Y.; Maikov, V.M. Fepaldine-Terpenoid Coumarin from Ferrula-Pallida. Khim. Prip. Soedin. 1980, 6, 716–718. [Google Scholar]
- O’Kennedy, R.; Thornes, R.D. Coumarins: Biology, Applications and Mode of Action; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Galm, U.; Dessoy, M.A.; Schmidt, J.; Wessjohann, L.A.; Heide, I. In vitro and in vivo production of new aminocoumarins by a combined biochemical, genetic, and synthetic approach. Chem. Biol. 2004, 11, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Hu, S.; Pacholec, M.; Walsh, C.T. Synthesis of proposed oxidation-cyclization-methylation intermediates of the coumarin antibiotic biosynthetic pathway. Org. Lett. 2003, 5, 3233–3236. [Google Scholar] [CrossRef] [PubMed]
- Marcu, M.G.; Schulte, T.W.; Neckers, L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J. Natl. Cancer Inst. 2000, 92, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Kotali, A. Transformation of phenolic hydroxyl into acyl group: A new tool in organic synthesis. Arkivoc 2009, 1, 81–96. [Google Scholar]
- Kotali, A.; Kotali, E.; Lafazanis, I.S.; Harris, P.A. Reactions of nitrogen derivatives of carbonyl compounds with phenyliodoso diacetate in organic synthesis. Curr. Org. Synth. 2010, 7, 62–77. [Google Scholar] [CrossRef]
- Rollas, S.; Küçükgüzel, S.G. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Kotali, A.; Lafazanis, I.S.; Papageorgiou, A.; Xrysogelou, E.; Lialiaris, T.; Sinakos, Z. Synthesis, characterization and antileucemic activity of 7-hydroxy-8-acetylcoumarin benzoylhydrazone. Molbank 2008, 2, M574. [Google Scholar] [CrossRef]
- Ponnurengam, M.S.; Malliappan, S.; Sethn, K.G.; Doble, M. QSAR studies on chalcones and flavonoids as anti-tuberculosis agents using genetic function approximation (GFA) method. Chem. Pharm. Bull. 2007, 55, 44–49. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Vanucci-Bacqué, C.; Carayon, C.; Bernis, C.; Camare, C.; Nègre-Salvayre, A.; Bedos-Belval, F.; Baltas, M. Synthesis, antioxidant and cytoprotective evaluation of potential antiatherogenic phenolic hydrazones. A structure-activity relationship insight. Bioorg. Chem. Med. 2014, 22, 4269–4276. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Kotali, A.; Nasiopoulou, D.A.; Harris, P.A.; Helliwell, M.; Joule, J.A. Transformation of a hydroxyl into an acyl group on α-pyrone ring: A novel route to 3,4-diacylcoumarins. Tetrahedron 2012, 68, 761–766. [Google Scholar] [CrossRef]
- Melagraki, G.; Chatzidakis, H.; Afantitis, A.; Igglessi-Markopoulou, O.; Detsi, A.; Koufaki, M.; Kontogiorgis, C.; Hadjipavlou-Litina, D.J. Synthesis and evaluation of the antioxidant and anti-inflammatory activity of novel coumarin-3-aminoamides and their alpha-lipoic acid adducts. J. Eur. J. Med. Chem. 2009, 44, 3020–3026. [Google Scholar] [CrossRef] [PubMed]
- Kotali, A.; Lafazanis, I.S.; Harris, P.A. A novel and facile synthesis of 7,8-diacylcoumarins. Tetrahedron Lett. 2007, 48, 7181–7183. [Google Scholar] [CrossRef]
- Kotali, A.; Lafazanis, I.S.; Harris, P.A. Synthesis of angular 7,8-pyridazinocoumarins via the transformation of a hydroxy group into a carbonyl group. Synthesis 2009, 5, 836–840. [Google Scholar] [CrossRef]
- Kotali, A.; Lafazanis, I.S.; Harris, P.A. Synthesis of 6,7-diacylcoumarins via the transformation of a hydroxy into a carbonyl group. Synth. Commun. 2008, 38, 3996–4006. [Google Scholar] [CrossRef]
- Somogyi, L.; Sohár, P. Tricarbonylmethane acylhydrazones: Reactions under acylating conditions and formation of fused isoxazole derivatives with concomitant N-N bond cleavage. Liebigs Ann. Chem. 1995, 1995, 1903–1906. [Google Scholar] [CrossRef]
- Eisenhauer, H.R.; Link, K.P. Studies on 4-Hydroxycoumarins. XIII. The Mechanism for the reaction of 4-hydroxycoumarin with aliphatic acid chlorides. J. Am. Chem. Soc. 1953, 75, 2044–2045. [Google Scholar] [CrossRef]
- Traven, V.F.; Negrebetsky, V.V.; Vorobjeva, L.I.; Carberry, E.A. Keto-enol tautomerism, NMR spectra and H-D exchange of 4-hydroxycoumarins. Can. J. Chem. 1997, 75, 377–383. [Google Scholar] [CrossRef]
- Gautam, D.R.; Protopappas, J.; Fylaktakidou, K.C.; Litinas, K.E.; Nicolaides, D.N.; Tsoleridis, C.A. Unexpected one-pot synthesis of new polycyclic coumarin[4,3-c]pyridine derivatives via a tandem hetero-Diels–Alder and 1,3-dipolar cycloaddition reaction. Tetrahedron Lett. 2009, 50, 448–451. [Google Scholar] [CrossRef]
- Kotali, A.; Nasiopoulou, D.A.; Harris, P.A.; Helliwell, M.; Joule, J.A. N′-[1-(2,4-Dioxo-3,4-dihydro-2H-1-benzopyran-3-yl-idene)eth-yl]thiophene-2-carbo-hydrazide. Acta Cryst. Sect. E 2010, E67, o1014. [Google Scholar]
- Lyssenko, K.A.; Antipin, M.Y. Hydrogen bond in 3-acetyl-4-hydroxycoumarin: X-ray diffraction study and quantum-chemical calculations. Russ. Chem. Bull. 2001, 50, 418–431. [Google Scholar] [CrossRef]
- Naceur, H.; Fischmeister, C.; Puerta, M.C.; Valerga, P. A rapid access to new coumarinyl chalcone and substituted chromeno [4, 3-c] pyrazol-4 (1H)-ones and their antibacterial and DPPH radical scavenging activities. Med. Chem. Res. 2011, 20, 522–530. [Google Scholar]
- Abdou, M.M. 3-Acetyl-4-hydroxycoumarin: Synthesis, reactions and applications. Arab. J. Chem. 2014. [Google Scholar] [CrossRef]
- Feng, J.Y.; Liu, Z.Q. Phenolic and enolic hydroxyl groups in curcumin: Which plays the major role in scavenging radicals? J. Agric. Food Chem. 2009, 57, 11041–11046. [Google Scholar] [CrossRef] [PubMed]
- Liégeois, C.; Lermusieau, G.; Collin, S. Measuring antioxidant efficiency of wort, malt, and hops against the 2,2′-azobis(2-amidinopropane) dihydrochloride-induced oxidation of an aqueous dispersion of linoleic acid. Agric. Food Chem. 2000, 48, 1129–1134. [Google Scholar] [CrossRef]
- Müller, K. 5-Lipoxygenase and 12-lipoxygenase: Attractive targets for the development of novel antipsoriatic drugs. Arch. Pharm. 1994, 327, 3–19. [Google Scholar] [CrossRef]
- Kemal, C.; Louis-Flamberg, P.; Krupinski-Olsen, R.; Shorter, A.L. Reductive inactivation of soybean lipoxygenase 1 by catechols: A possible mechanism for regulation of lipoxygenase activity. Biochemistry 1987, 26, 7064–7072. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, J.; Eling, T.E.; Mason, R.P. Formation of free radical metabolites in the reaction between soybean lipoxygenase and its inhibitors. An ESR study. Biochemistry 1989, 28, 8363–8367. [Google Scholar] [CrossRef] [PubMed]
- Simulated with SpinWorks Simulation Program, Version 2.5.5. Available online: http://davinci.chem.umanitoba.ca/pub/marat/SpinWorks/ (accessed on 29 November 2006).
- Software used for determination of clog P: Biobyte Corp., C-QSAR Database 201 West 4th Street, Suite 204, Claremont, CA 91711, USA, 2002.
- Payá, M.; Halliwell, B.; Hoult, J.R. Interactions of a series of coumarins with reactive oxygen species. Scavenging of superoxide, hypochlorous acid and hydroxyl radicals. Biochem. Pharm. 1992, 44, 205–214. [Google Scholar] [CrossRef]
- Vukovic, N.; Sukdolak, S.; Solujic, S.; Niciforovic, N. An efficient synthesis and antioxidant properties of novel imino and amino derivatives of 4-hydroxy coumarins. Arch. Pharm. Res. 2010, 33, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Curini, M.; Epifano, F.; Genovese, S.; Menghini, L.; Ricci, D.; Fraternale, D.; Giamperi, L.; Bucchini, A.; Bellacchio, E. Lipoxygenase inhibitory activity of boropinic acid, active principle of boronia pinnata. Nat. Prod. Commun. 2006, 1, 1141–1145. [Google Scholar]
- Sample Availability: Samples of the compounds 2d, 2i and 2l are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
