Abstract
Xanthoceras sorbifolia Bunge. is used in traditional medicine in North China. To evaluate the anti-tumor and radical-scavenging activities of X. sorbifolia husks polyphenols and determine their structure-activity relationships, 37 polyphenols 1–37 were obtained by bioassay-guided fractionation. Two new compounds 1–2, and compounds 5, 6, 8, 9, 11, 14–17, 21–25, 27–29, 31, 33, 34, 36, and 37 were isolated from the genus Xanthoceras for the first time. Compounds 1–37 did not show strong cytotoxicity against the four tested tumor cell lines (A549, HepG2, MGC-803, and MFC) compared to paclitaxel and under the conditions tested in the anti-tumor assay, but compounds 3, 4, 7, 8, 10, 18–20, 25, 26, 29, 30, 32, and 35 exhibited stronger radical-scavenging activity than ascorbic acid in a 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt assay. This was the first report on the anti-tumor and radical-scavenging activities of the polyphenols isolated from X. sorbifolia husks. Overall, the present study contributed valuable information concerning X. sorbifolia husks use in medicine and pharmacology.
1. Introduction
Xanthoceras sorbifolia Bunge. is the only species in the genus Xanthoceras (family Sapindaceae) and is distributed in North China [1]. This species is a kind of woody oil-bearing crop, traditionally used in herbal medicine for curing atherosclerosis, rheumatism, hyperpiesia, chronic hepatitis, and child enuresis [2], and it has been included in the 1977 edition of the China Pharmacopoeia [3]. Chemical studies of X. sorbifolia husks, which are considered byproducts, showed that the husks contained a variety of compounds, including polyphenols [4], triterpenoids [5], and sterols [6]. Medical research showed that X. sorbifolia husk components improved learning ability and memory [7], had anti-cancer effects [8], inhibited tyrosinase [9], cured cardiovascular diseases [10], had anti-oxidant properties [11], and inhibited pancreatic lipase activity [12]. However, anti-tumor and radical-scavenging activities have not yet been reported for the polyphenols isolated from X. sorbifolia husks.
Cancer is a multi-step disease that often involves the activation of oncogenes or the inactivation of tumor-suppressor genes. In addition to genetic mutations, different free radicals interfering with enzyme structure or activity are also responsible for cancer development [13]. In particular, growth factors, such as the platelet-derived growth factor and the epidermal growth factor, which can be activated by cancer cells to sustain cellular growth and proliferation, could rapidly, and transiently, increase reactive oxygen species (ROS) generation through nicotinamide adenine dinucleotide phosphate oxidases [14]. ROS are often associated with oxidative stress, which has been related to the progression of many diseases, including cancer and cardiovascular diseases (e.g., atherosclerosis) [15]. Thus, it is necessary to develop and utilize natural radical-scavenging and antitumor agent with low cytotoxicity so that they can help human get rid of over-produced ROS and also reduce the risks of suffering from cancer. Polyphenols, including flavonoids and phenolic acids, have been associated with protection against oxidative stress and cancer risk reduction [16]. In the present study, 37 polyphenols were isolated from X. sorbifolia husks, and their anti-tumor and radical-scavenging capacities were analyzed, together with those of the husks’ 70% aqueous ethanol extract, chloroform fraction, n-butanol fraction, and water fraction. The SAR of the polyphenols were also discussed.
2. Results and Discussion
2.1. Structure Elucidation
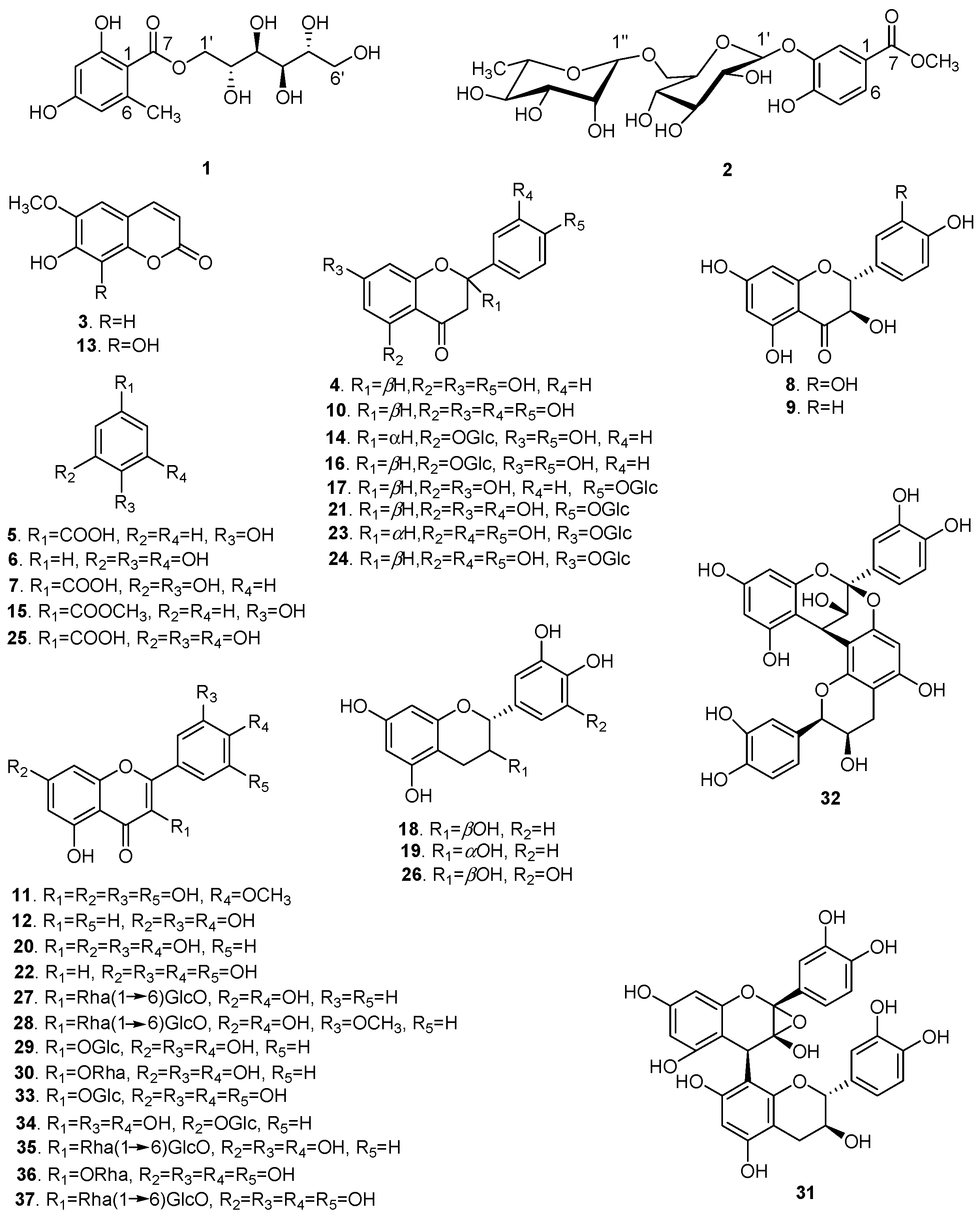
Repeated column chromatography using silica gel, Sephadex LH-20, and preparative high performance liquid chromatography (prep-HPLC) of the 70% aqueous ethanol extract of XS husks resulted in the isolation of 37 polyphenols with purities over 98% (Figure 1). Compound 1 was obtained as a yellowish gum ([α] +2.041°; c 0.49, MeOH). Its UV spectrum revealed absorption at λmax 289 and 263 nm and the infrared (IR) spectrum suggested the presence of hydroxyl (3356.46 cm−1), alkyl (2919.23 cm−1), and olefin (1617.76 cm−1) groups. The molecular formula was determined as C14H20O9 by high resolution electrospray ionization mass spectrometry (HR-ESI-MS) at m/z 355.1003 [M + Na]+ (calcd. for C14H20O9Na, 355.1000), with five degrees of unsaturation. In the proton nuclear magnetic resonance (1H-NMR; Table 1), one tetrasubstituted phenyl [δH 6.18 (1H, br s) and 6.15 (1H, br s)] and one methyl (δH 2.37 (3H, s)) were easily recognized.
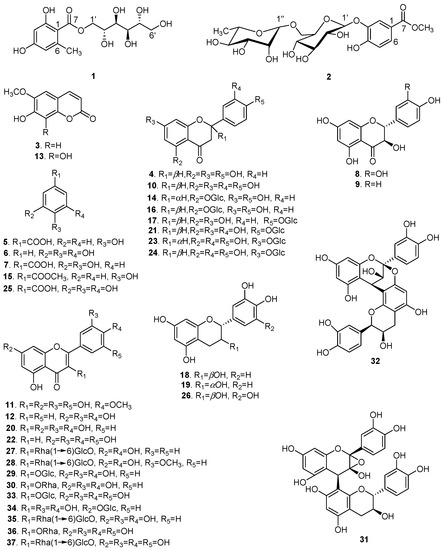
Figure 1.
The chemical structures of polyphenols isolated from X. sorbifolia husks.

Table 1.
1H- and 13C-NMR data of the new compound 1 (in DMSO-d6, 400 MHz).
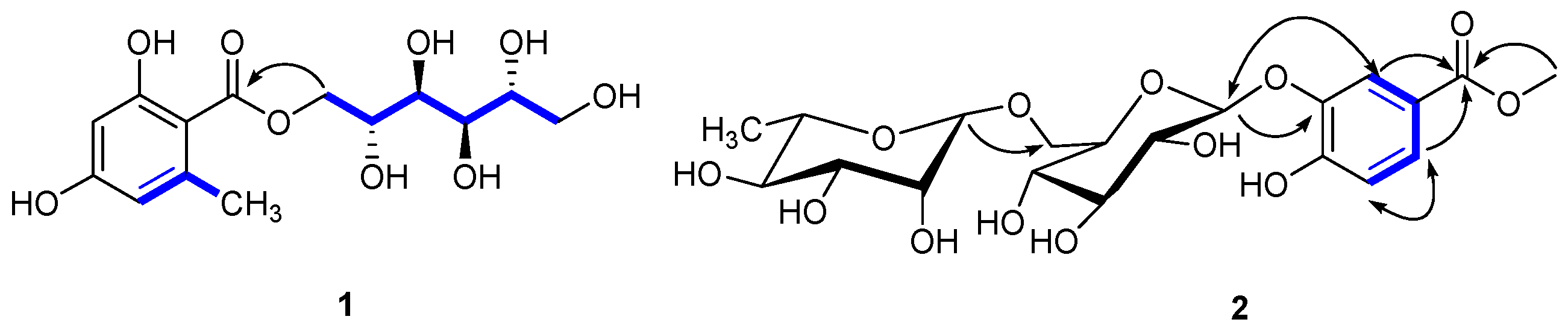
The carbon nuclear magnetic resonance (13C-NMR) spectrum also showed six carbon signals at δc 67.7 (C-1′), 68.1 (C-2′), 69.7 (C-3′), 69.3 (C-4′), 71.2 (C-5′), and 63.8 (C-6′), suggesting the presence of a d-mannitol moiety [17]. The hetero-nuclear single quantum coherence (HSQC) spectroscopy identified one carbonyl (δc 170.2), one phenyl (δc 162.1, 161.5, 141.9, 110.6, 106.5, and 100.5), two oxygenated methylenes (δc 67.7, 63.8), and four oxygenated methines (δc 71.2, 69.7, 69.3, and 68.1). These data suggested that 1 was an orsellinic acid analog. The hetero-nuclear multiple bond correlation (HMBC) confirmed the presence of an orsellinic acid scaffold: H-3/C-1 (δc 106.5), C-2 (δc 162.1), C-5 (δc 110.6), H-5/C-4 (δc 161.5), C-1 (δc 106.5), C-3 (δc 100.5), CH3-6/C-6 (δc 141.9), C-5 (δc 110.6), C-1 (δc 106.5), and C-3 (δc 100.5) (Figure 2). The nuclear Overhauser effect spectroscopy (NOESY) correlation between CH3-6/H-5 and the 1H-NMR [δH 6.18 (1H, br s) and 6.15 (1H, br s)] results were in agreement with the substitution patterns of orsellinic acid. The location of the mannitol moiety at C-7 was deduced from the H-1′/C-7 HMBC correlation. Accordingly, the structure of 1 was determined as d-mannitol orsellinate, trivially named xspolyphenol A.
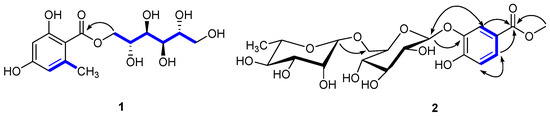
Figure 2.
Key HMBC (H⟶C), 1H-1H COSY (−), and NOESY (⟷) of new compounds 1, 2 isolated from X. sorbifolia husks.
Compound 2 was obtained as a yellowish gum ([α] −52.055°; c 0.73, MeOH). The UV spectrum suggested absorption at λmax 320 nm and the IR spectrum suggested the presence of hydroxyl (3356.07 cm−1), methyl (2918.26 cm−1), carbonyl (1676.87 cm−1), and aryl (1617.71, 1578.08 cm−1) groups. The molecular formula of 2 was determined as C20H28O13 by HR-ESI-MS at m/z 499.1418 [M + Na]+ (calcd, 499.1422), suggesting seven degrees of unsaturation. The 1H-NMR (Table 2) of compound 2 showed one ABX coupling system at: δH 7.39 (d, J = 2.8 Hz), 6.94 (d, J = 9.2 Hz) and 7.25 (dd, J = 2.8, 8.8 Hz) for H-2, H-5, and H-6 of a benzene ring, respectively, indicating the presence of a C-1,3,4 trisubstituted benzene moiety. In the 1H-NMR, the sharp signal at δH 3.90 (3H, s) suggested a –OCH3. In the HMBC spectrum (Figure 2), –OCH3 was assigned at C-7, due to the long-range coupling of C-7 and –OCH3. After the acidic hydrolysis of compound 2, the aqueous layer was separated by thin-layer chromatography (TLC) to yield two glycosides: rhamnose and glucose. The position of the sugar linkage was assigned at C-3 by HMBC correlations (Figure 2) and confirmed by the positive NOESY between H-2 (δH 7.39) and H-1′ (δH 4.68). The configurations of the anomeric protons of 2 were deduced to be α and β forms based on the 3JH1,H2 coupling conditions (H-1′′ (brs) and H-1′′′′′ (J = 7.6 Hz)). Accordingly, the chemical structure of 2 was unambiguously established as methyl 4-hydroxylbenzoate 3-O-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside, trivially named xspolyphenol B.

Table 2.
1H- and 13C-NMR data of the new compound 2 (in DMSO-d6, 400 MHz).
Based on spectroscopic data and by comparison to previously reported compounds, Compounds 3–37 were identified as: scopoletin (3) [18], naringenin (4) [19], p-hydroxybenzoic acid (5) [20], pyrogallol (6) [21], protocatechuic acid (7) [22], taxifolin (8) [23], aromadendrin (9) [24], eriodictyol (10) [25], mearnsetin (11) [26], luteolin (12) [27], fraxetin (13) [28], naringenin 5-O-β-d-glucopyranoside (14) [29], methyl 4-hydroxylbenzoate (15) [30], (−)-salipurposide (16) [31], naringenin 4′-O-β-d-glucopyranoside (17) [32], (+)-catechin (18) [33], epicatechin (19) [34], quercetin (20) [34], eriodictyol 4′-O-β-d-glucopyranoside (21) [35], tricetin (22) [36], (2S)-eriodictyol 7-O-β-d-glucopyranoside (23) [37], (2R)-eriodictyol 7-O-β-glucopyranoside (24) [38], gallic acid (25) [39], gallocatechin (26) [40], kaempferol 3-O-rutinoside (27) [41], isorhamnetin 3-O-rutinoside (28) [42], isoquercitrin (29) [43], quercitrin (30) [44], 2α,3α-epoxy-5,7,3′,4′-tetrahydroxyflavan-(4β-8-catechin) (31) [45], proanthocyanidin A2 (32) [46], isomericitrin (33) [47], quercimetrin (34) [48], rutin (35) [34], myricetrin (36) [44], and myricetin 3-O-rutinoside (37) [49].
2.2. Chemotaxonomic Significance
The polyphenols identified from X. sorbifolia husks provided an image concerning the chemotaxonomic situation of the genus Xanthoceras within the family Sapindaceae. The main polyphenols isolated from X. sorbifolia husks were protocatechuic acid (6.01 mg/100 g husks), epicatechin (5.24 mg/100 g husks), catechin (3.34 mg/100 g husks), rutin (2.81 mg/100 g husks), myricetin-3-O-rutinoside (1.37 mg/100 g husks), quercetin (1.19 mg/100 g husks), and quercitrin (1.12 mg/100 g husks); quercetin and myricetin were the major aglycons in X. sorbifolia husks. Previous phytochemical studies of the genus showed that their polyphenolic pool comprised mostly of flavonoids and phenolic acids [4], but these compounds were not quantified. The present study is the first to isolate and quantify the polyphenolic compounds from X. sorbifolia husks, which might be important for the chemotaxonomy of the genus and family.
2.3. Anti-Tumor Effects of X. sorbifolia Polyphenols
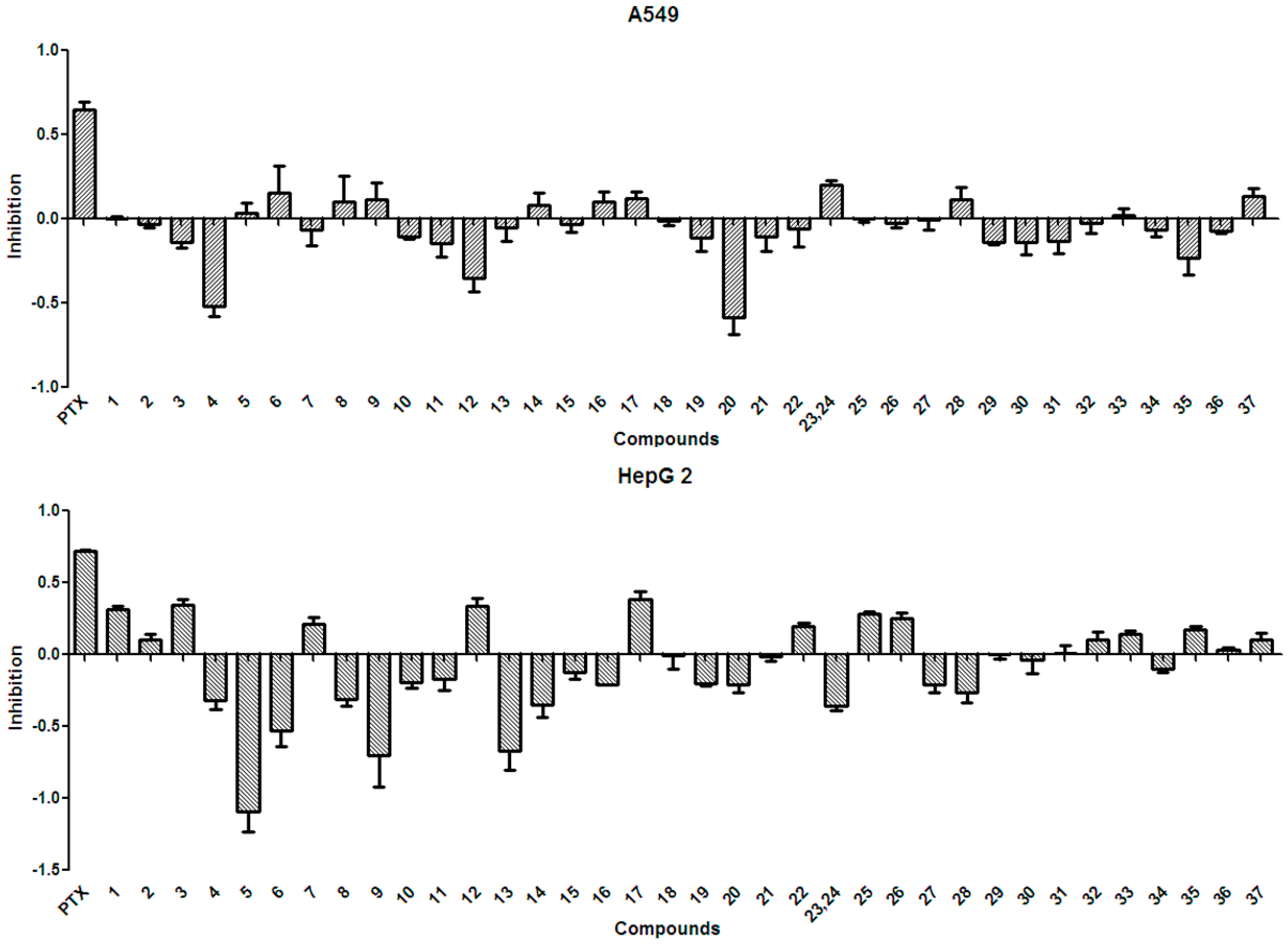
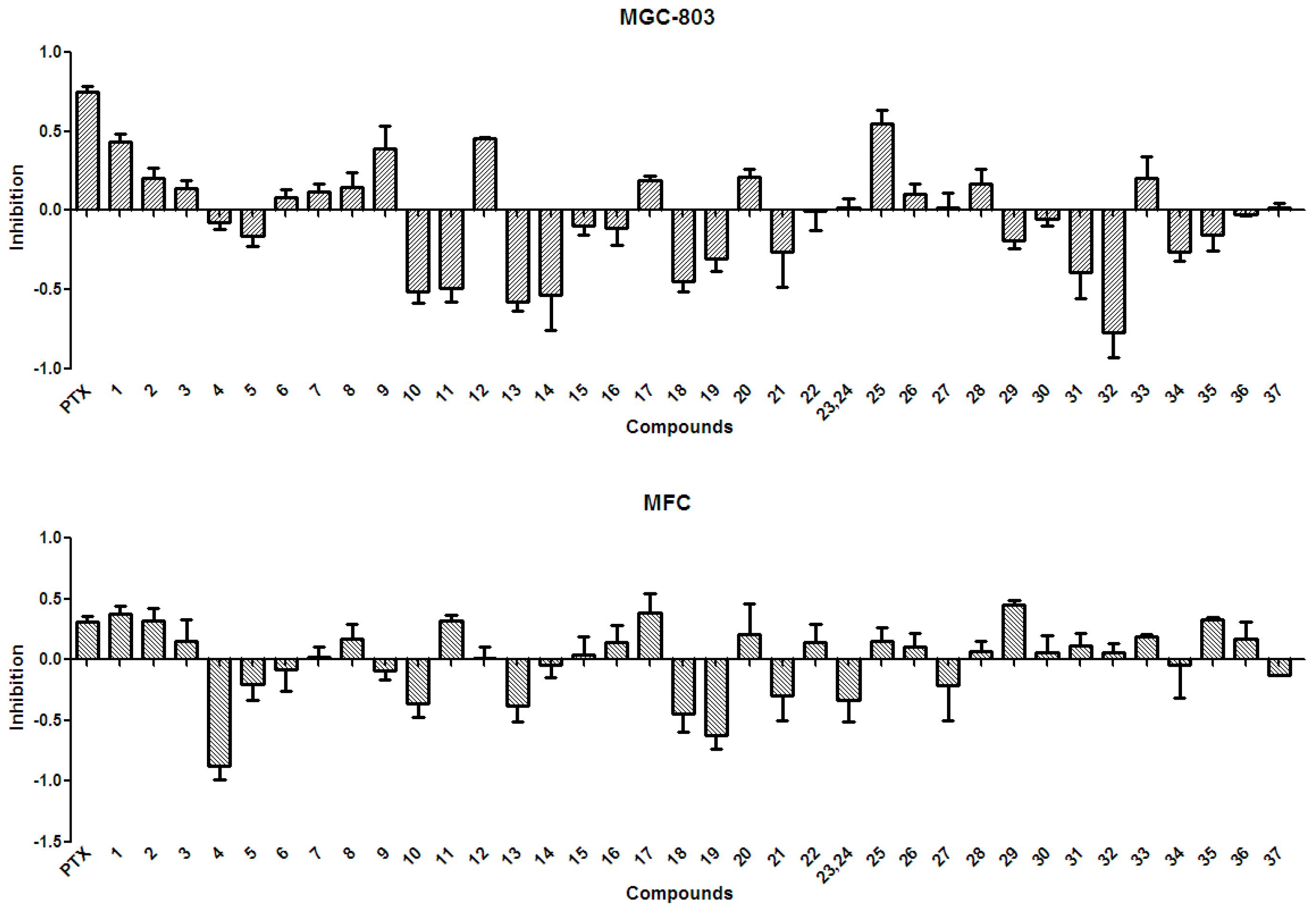
In general, the anti-tumor activities of natural products are evaluated by testing their ability to directly inhibit the proliferation of tumor cells, or their capacity to induce immune cells to secrete cytokines that could act on tumor cells [15]. Tests for the anti-tumor activity of the polyphenols isolated from the husks (1–37) at a concentration of 50 µg/mL, revealed that all compounds had null or weak cytotoxicity, when compared to that of paclitaxel and under the conditions described in the present study. The percentage inhibition of compounds 1–37 at 50 µg/mL against four tumor cell lines is summarized in Figure 3 and Figure 4. Protocatechuic acid (7) showed no effect on the lung adenocarcinoma (A549) cell line, similarly to that described in a previous study [50]; however, in that study, protocatechuic acid (7) exhibited anti-tumor activity against other cancer cell lines, at high concentrations. Previous studies [34,51] also reported that epicatechin (19) had no effect or a weak effect on the A549, liver cancer (HepG2), and gastric carcinoma (MGC-803) cell lines, supporting the results found in the present study. It has also been previously reported [52] that catechin (18) had a weak effect on HepG2 and MGC-803 cell lines. Figure 3 and Figure 4 evidence that rutin (35) exhibited no or weak effects on A549, HepG2, and MGC-803 cell lines, in agreement with previous reports [13,34,53]. Although myricetin 3-O-rutinoside (37) showed no or weak anti-tumor activity against A549, HepG2, MGC-803, and murine foregastric carcinoma (MFC) cell lines (as shown in Figure 3 and Figure 4), it was firstly investigated in the present study. Similar to that found in a previous study [33], quercetin (20) had no effect on the MGC-803 cell line. As there were few reports on the in vitro anti-tumor activity of polyphenols on the MFC cell line, the present study represents a major addition to the knowledge on this subject. Overall, polyphenols isolated from X. sorbifolia husks did not strongly inhibit the proliferation of some cancer cell lines, compared to paclitaxel, under the conditions examined in this research. Interestingly, we found that almost half of the compounds exhibited negative inhibition effects, the mechanism was worthy of further study and could be studied by other tumor cell lines or even animal models.
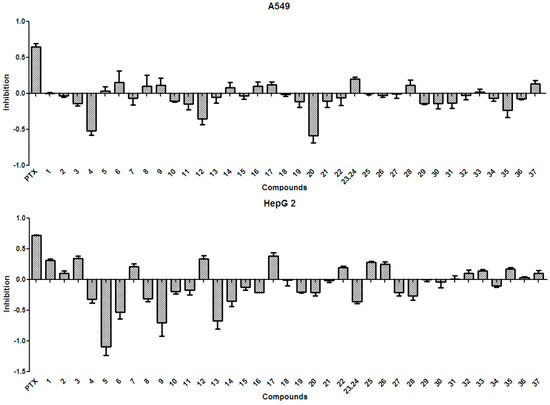
Figure 3.
The anti-tumor activity against A549 and HepG2 of the polyphenols (1–37) isolated from X. sorbifolia husks. MTT 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide was dissolved in PBS at 5 mg/mL. After the formazans were dissolved in DMSO for 10 min, the absorbance values at 490 nm were measured, and the percentage inhibitions were calculated. PTX, Paclitaxel.
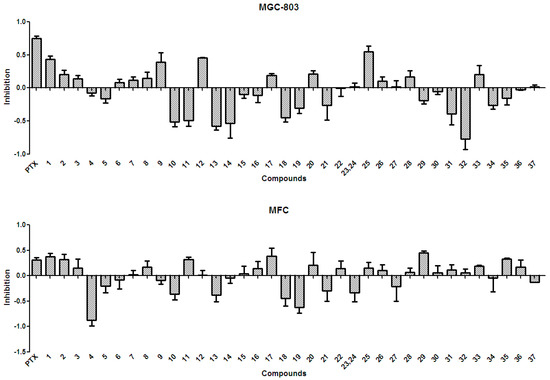
Figure 4.
The anti-tumor activity against MGC-803 and MFC of the polyphenols (1–37) isolated from X. sorbifolia husks. MTT 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide was dissolved in PBS at 5 mg/mL. After the formazans were dissolved in DMSO for 10 min, the absorbance values at 490 nm were measured, and the percentage inhibitions were calculated. PTX, Paclitaxel.
2.4. Radical-Scavenging Activity of Extracts and Polyphenols
2.4.1. Radical-Scavenging Activity
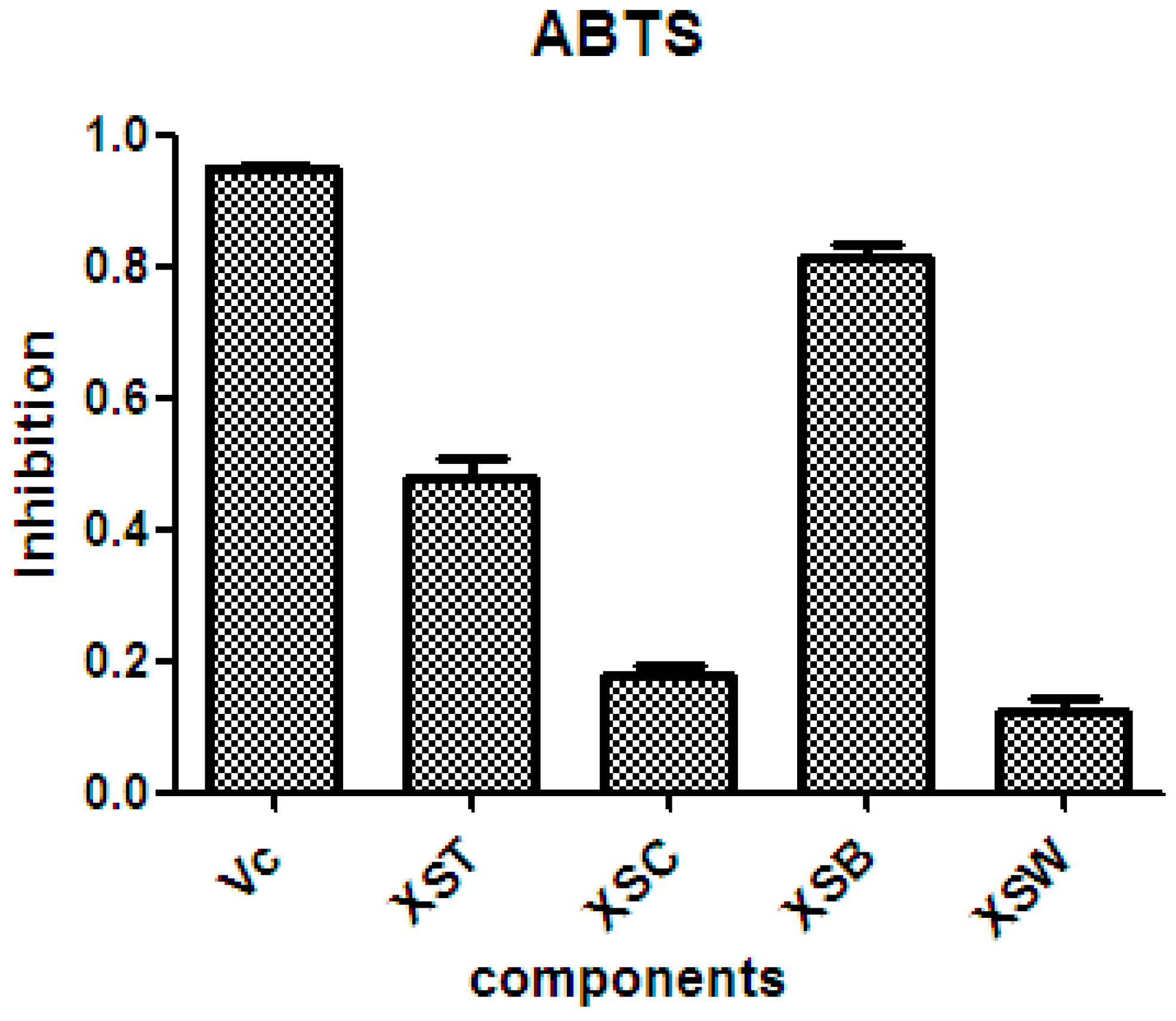
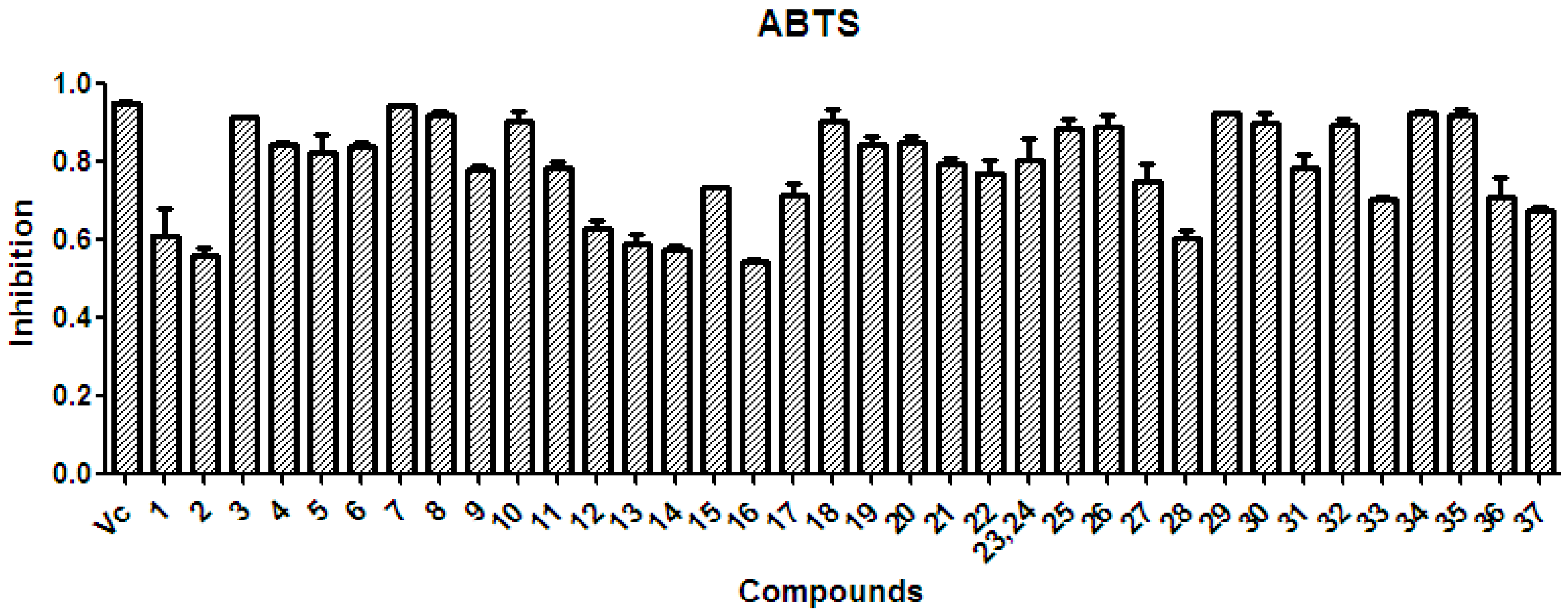
There is considerable evidence that free radicals induce oxidative damage to biomolecules and play an important role in cancer and cardiovascular diseases [54]. The 2,2′-azino-bis(3-ethyl-benzothiazoline-6-sulfonic acid) diammonium salt (ABTS) assay has been a popular radical-scavenging test for natural components [55] and, therefore, it was used in the present study. The XSB fraction presented the highest radical-scavenging activity among X. sorbifolia husk extracts (Figure 5). Then the bioassay-guided fractionation of X. sorbifolia husks led to the isolation of 37 polyphenols. The evaluation on the radical-scavenging activity of the polyphenols at a concentration of 50 µg/mL showed that compounds 1–37 had strong radical-scavenging activity (Figure 6), and, therefore, all compounds were subject to ABTS assay to determine their scavenging capability. As shown in Figure 6 and Table 3, the main polyphenols (protocatechuic acid, epicatechin, (+)-catechin, rutin, myricetin-3-O-rutinoside, quercetin, and quercitrin) exhibited strong radical-scavenging activities, compared to ascorbic acid. Thus, the husks’ main compounds strongly contributed to the striking radical-scavenging activity of the XSB fraction. As free radical damage is indicated to be the main cause of cancer [56], although X. sorbifolia polyphenols did not strongly inhibited the proliferation of cancer cell lines under the conditions described in the present research (Figure 3 and Figure 4), they might indirectly present anti-tumor activity by reducing oxidative damage [57].
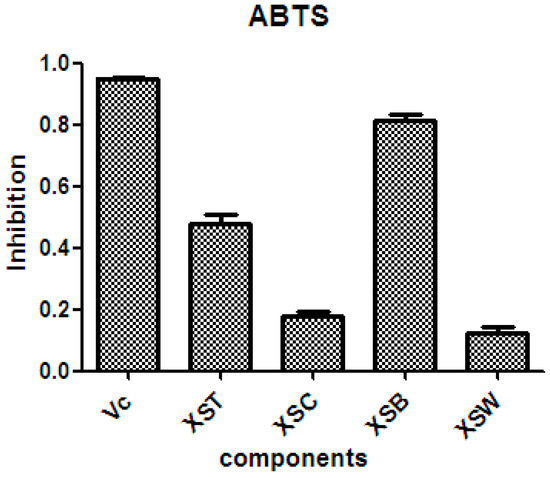
Figure 5.
The radical-scavenging activity of the crude samples from X. sorbifolia husks. ABTS was prepared daily and diluted to an absorbance of 0.70 ± 0.02 at 734 nm. After the crude samples (50 µg/mL) reacted with the ABTS radical solution for 10 min, the absorbance values (Ai) at 734 nm were measured, and the percentage inhibitions were calculated. Vc, ascorbic acid; XST, 70% aqueous ethanol extract; XSC, chloroform soluble fraction; XSB, n-butanol soluble fraction; XSW, water soluble fraction. PTX, Paclitaxel.
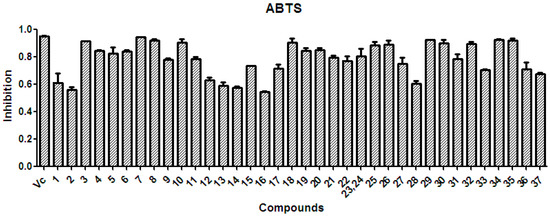
Figure 6.
The radical-scavenging activity of the polyphenols (1–37) isolated from X. sorbifolia husks. ABTS was prepared daily and diluted to an absorbance of 0.70 ± 0.02 at 734 nm. After the crude samples (50 µg/mL) reacted with the ABTS radical solution for 10 min, the absorbance values (Ai) at 734 nm were measured, and the percentage inhibitions were calculated. Vc, ascorbic acid.

Table 3.
Radical-scavenging activity of the compounds (1–37) isolated from X. sorbifolia husks.
2.4.2. Discussion on the SAR of Polyphenols
The polyphenols isolated from X. sorbifolia husks can be divided into three categories (Figure 1): flavonoids, phenolic acids, and coumarins. The twenty-eight flavonoids can be further classified into flavonols, flavones, flavanones, flavan-3-ols, and flavanonols. Flavonoids with vicinal phenolic hydroxyls presented strong radical-scavenging activity, in agreement with that previously reported [58]. In comparison with the flavonoids bearing saccharide groups, different characteristics of the sugar side chain also play important roles in their radical–scavenging effect. The flavonoids 36, 37 possess the same aglycone, but flavonoid glycoside 37 with a disaccharide chain exhibited weaker radical–scavenging effect than 36 with a monosaccharide chain, which suggested that the presence of a disaccharide chain might reduce the radical–scavenging effect. Thus, the antioxidant activities of these flavonoids would depend on not only the substituent groups on the aglycone, but also the sugar moieties. Phenolic acids with both carboxyl and vicinal phenolic hydroxyls also showed strong radical-scavenging activity. However, coumarins with a single phenolic hydroxyl exhibited stronger radical-scavenging activity than coumarins with vicinal phenolic hydroxyls.
3. Materials and Methods
3.1. General Experimental Procedures
Column chromatography was conducted using silica gel (SiO2, 200–300 µm mesh; Qingdao Haiyang Chemical Co., Ltd., Qingdao, China) and Sephadex LH-20 (20–100 µm; Pharmacia, Uppsala, Sweden) as packing materials. Silica GF254 (10–40 mm) for TLC was supplied by the Qingdao Marine Chemical Factory, Qingdao, China. All TLC spots were visualized under UV light (254 nm) and stained with a 10% H2SO4 solution in ethanol, followed by heating. Optical rotations were recorded with a 341 polarimeter (Perkin-Elmer, Waltham, MA, USA) in a 1 dm cell. The UV spectra were measured on a UV-260 spectrophotometer (Shimadzu, Nishinokyo Kuwabara-cho, Nakagyo-ku, Kyoto, Japan) and the IR spectra were obtained on a NEXUS 670 FT-IR spectrometer (Nicolet, Madison, WI, USA). Nuclear magnetic resonance spectra were recorded on INOVA-400 (Varian, Palo Alto, CA, USA) and AVANCE III-400 (Bruker, Billerica, MA, USA) spectrometers. Chemical shifts were given on a δ (ppm) scale using tetramethylsilane as the internal standard. High-resolution electrospray ionization (ESI) mass spectrometry (MS) was carried out on a APEX II mass spectrometer (Bruker Daltonics, Billerica, MA, USA) and ESI-MS spectra were determined on a Bruker Daltonics Esquire 6000 spectrometer. The ABTS and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) used were obtained from Aladdin Industrial Co. (Shanghai, China).
3.2. Plant Material and Reagents
X. sorbifolia husks (Sapindaceae) used in the present study were collected in Gansu Province, China, in 2013, and authenticated by Associate Prof. Huanyang Qi (Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou, China). A voucher specimen (No. 20131106XSB) was deposited in the Key Laboratory of Chemistry of Northwestern Plant Resources and Key Laboratory for Natural Medicine of Gansu Province, Chinese Academy of Sciences. All chemicals were of industrial or analytical grade and used after redistillation.
3.3. Extraction and Isolation
After air-drying, X. sorbifolia husks (10.0 kg) were pulverized and refluxed with 70% aqueous ethanol at 65 °C (four times, using 100 L, for 2 h). Extracts were filtered and then concentrated under reduced pressure to yield the ethanol extract (XST; 2.0 kg). Most XST (1.95 kg) was then suspended in distilled water (18 L) and successively partitioned with chloroform (four times, using 6 L), and water-saturated n-butanol (four times, using 6 L) to yield the chloroform soluble fraction (XSC; 86.0 g), the n-butanol soluble fraction (XSB; 560.0 g), and the aqueous fraction (XSW; 1.3 kg). As XSB exhibited a marked radical-scavenging activity (Figure 6), this active fraction was subjected to sequential silica-gel chromatography, Sephadex LH-20, and prep-HPLC to obtain compounds 1–37 (see Supplementary Materials). Most XSB (500.0 g) was loaded into an ordinary-phase silica gel column (5 kg, 15 cm × 35 cm) with a CHCl3:MeOH elution gradient of 10:1, 5:1, 2:1, 1:1, and 1:1 (water saturated). The eluate was collected in 108 portions of 3000 mL, and eluates containing similar components according to the TLC results were combined into 11 fractions (Fr. 1 to Fr. 11).
Fr. 1 was chromatographed on a Sephadex LH-20 column eluted with CHCl3:MeOH (1:1) to obtain compounds 3 (10.0 mg) and 4 (8.0 mg).
Fr. 2 was chromatographed on a Sephadex LH-20 column eluted with CHCl3:MeOH (1:1), and four subfractions I–IV were obtained. Subfraction II was purified by prep-HPLC eluted with MeOH:H2O (42:58) to obtain compound 5 (tR 10.0 min, 8.0 mg). Subfraction III was firstly purified by prep-HPLC eluted with MeOH:H2O (34:66) to obtain compounds 6 (tR 7.3 min, 4.2 mg) and 7 (tR 8.5 min, 601.3 mg), and then eluted with MeOH:H2O (43:57) to obtain compounds 8 (tR 12.7 min, 8.4 mg), 9 (tR 20.3 min, 10.0 mg), and 10 (tR 31.2 min, 83.0 mg). Subfraction IV was purified by prep-HPLC eluted with MeOH:H2O (45:55) to obtain compounds 11 (tR 55.0 min, 2.6 mg) and 12 (tR 66.7 min, 3.4 mg).
Fr. 3 was chromatographed on a Sephadex LH-20 column eluted with CHCl3:MeOH (1:1), and five sub fractions I–V were obtained. Subfraction III was first purified by prep-HPLC eluted with MeOH:H2O (30:70) to obtain compounds 13 (tR 20.8 min, 15.3 mg), 14 (tR 34.2 min, 9.3 mg), 15 (tR 38.4 min, 5.2 mg), and 16 (tR 41.1 min, 9.2 mg); and then eluted with MeOH:H2O (36:64) to obtain compound 17 (tR 39.9 min, 19.8 mg). Subfraction IV was purified by prep-HPLC eluted with MeOH:H2O (13:87) to obtain compounds 18 (tR 21.6 min, 334.4 mg) and 19 (tR 49.3 min, 523.8 mg). Subfraction V was purified by prep-HPLC eluted with MeOH:H2O (42:58) to obtain compound 20 (tR 74.8 min, 118.5 mg).
Fr. 4 was chromatographed on a Sephadex LH-20 column eluted with CHCl3:MeOH (1:1), and four sub fractions I–IV were obtained. Subfraction III was purified by prep-HPLC eluted with MeOH:H2O (38:62) to obtain compound 21 (tR 28.0 min, 10.0 mg) and subfraction IV was purified by prep-HPLC eluted with MeOH: H2O (43:57) to obtain compound 22 (tR 60.8 min, 2.3 mg).
Fr. 5 was chromatographed on a Sephadex LH-20 column eluted with CHCl3:MeOH (1:1), and five sub fractions I–V were obtained. Subfraction III was first purified by prep-HPLC eluted with MeOH:H2O (33:67) to obtain compound 1 (tR 13.5 min, 4.9 mg), and then eluted with MeOH:H2O (40:60) to obtain the isomers of 23 and 24 at a ratio of approximately 1:1 (tR 18.0 min, 36.3 mg). Sub- fraction V was purified by prep-HPLC eluted with MeOH:H2O (10:90) to obtain compounds 25 (tR 23.0 min, 38.7 mg) and 26 (tR 37.7 min, 8.6 mg).
Fr. 6 was chromatographed on a Sephadex LH-20 column eluted with CHCl3:MeOH (1:1), and six subfractions I–VI were obtained. Subfraction III was first purified by prep-HPLC eluted with MeOH:H2O (30:70) to obtain compound 2 (tR 27.0 min, 7.3 mg), and then eluted with MeOH:H2O (39:61) to obtain compounds 27 (tR 64.9 min, 38.5 mg) and 28 (tR 71.6 min, 17.4 mg). Subfraction IV was purified by prep-HPLC eluted with MeOH:H2O (35:65) to give compounds 29 (tR 48.4 min, 35.8 mg) and 30 (tR 70.2 min, 119.9 mg).
Fr. 7 was chromatographed on a Sephadex LH-20 column eluted with CHCl3:MeOH (1:1), and four subfractions I–IV were obtained. Subfraction III was purified by prep-HPLC eluted with MeOH:H2O (20:80) to obtain compound 31 (tR 45.4 min, 4.0 mg) whereas subfraction IV was purified by prep-HPLC eluted with MeOH:H2O (30:70) to obtain compound 32 (tR 66.0 min, 5.0 mg).
Fr. 8 was chromatographed on a Sephadex LH-20 column eluted with CHCl3:MeOH (1:1), and four sub fractions I–IV were obtained. Subfraction III was purified by prep-HPLC eluted with MeOH:H2O (38.5:61.5) to obtain compounds 33 (tR 31.6 min, 14.5 mg) and 34 (tR 36.4 min, 4.1 mg).
Fr. 9 was chromatographed on a Sephadex LH-20 column eluted with CHCl3:MeOH (1:1), and five subfractions I–V were obtained. Subfraction III was purified by prep-HPLC eluted with MeOH:H2O (40:60) to obtain compound 35 (tR 29.0 min, 280.5 mg).
Fr. 10 was chromatographed on a Sephadex LH-20 column eluted with CHCl3:MeOH (1:1), and six subfractions I–VI were obtained. Subfraction III was purified by prep-HPLC eluted with MeOH:H2O (34.3:65.7) to obtain compound 36 (tR 53.7 min, 7.8 mg).
Fr. 11 was chromatographed on a Sephadex LH-20 column eluted with CHCl3:MeOH (1:1), and six subfractions I–VI were obtained. Subfraction IV was purified by prep-HPLC eluted with MeOH:H2O (24:76) to give compound 37 (tR 54.7 min, 137.1 mg).
3.4. Acidic Hydrolysis of the New Compound and Sugar Analysis
The new compound 2 (2 mg) was added to a solution of concentrated HCl (0.5 mL), and refluxed for 3 h, by adding H2O (1.5 mL)/dioxane (3 mL). After dilution with H2O, the reaction mixture was subjected to extraction twice with ethyl acetate (EtOAc). The H2O layers of compound 2 were then neutralized with NaHCO3 and concentrated to dryness under reduced pressure. The residue was re-dissolved in H2O for TLC analysis.
3.5. Biological Activity
3.5.1. Anti-Tumor Assay
Compounds 1–37 were evaluated for their anti-tumor activity against three human cancer cell lines, the lung adenocarcinoma (A549), liver cancer (HepG2), and gastric carcinoma (MGC-803) cell lines, and one murine foregastric carcinoma (MFC) cell line, which were obtained from Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s modified eagle medium supplemented with 10% fetal bovine serum (both from Hyclone, South Logan, UT, USA), in 5% CO2 at 37 °C. The anti-tumor assay was performed according to the MTT method [58], with some modifications. In brief, A549, HepG2, MGC-803, and MFC cells were seeded into 96-well plates (5 × 103 cells/well) for 20–24 h under the above conditions, treated with 50 µg/mL of each tested compound, and further incubated for 24 h under the same conditions. After this period, 20 µL of MTT stock solution (5 mg/mL in PBS) were added to each well and samples were incubated for another 4 h, under the same conditions. Supernatants were then removed, and 150 µL of dimethyl sulfoxide were added to each well. After 10 min, absorbance was determined on a Multiskan MK 3 Automated Microplate Reader (Thermo Fisher Scientific, Waltham, MA, USA) at 490 nm, using paclitaxel as the positive control. The percentage inhibition was calculated according to:
where AA is the inhibition percentage, Ao is the absorbance of the blank sample, and Ai is the absorbance of the test sample.
3.5.2. ABTS Radical-Scavenging Assay
Samples (extracts and polyphenols from X. sorbifolia husks) ability to scavenge the ABTS radical cation was measured following a previously reported method [59], with some modifications, and using L-ascorbic acid as the positive control. Assays were performed in 96-well plates and absorbance was measured at 734 nm. The radical-scavenging activity of each sample was expressed as the percentage inhibition of the ABTS radical and determined according to Equation (1).
3.6. Statistical Analysis
All experiments were carried out in three replicates to ensure reproducibility. Sample concentrations providing 50% scavenging capability (SC50) were obtained by fitting dose-response data to a four-parametric logistic nonlinear regression model, using GraphPad Prism 5.0 software (GraphPad, La Jolla, CA, USA).
4. Conclusions
In conclusion, quercetin and myricetin polyphenols were the main aglycons in X. sorbifolia husks, and this might be of great chemotaxonomic importance within the genus Xanthoceras and the family Sapindaceae. Pharmacological studies showed that, although compounds 1–37 did not show strong cytotoxicity against the four tumor cell lines (A549, HepG2, MGC-803, and MFC), compared to paclitaxel and under the conditions described in the present research, compounds 3, 4, 7, 8, 10, 18–20, 25, 26, 29, 30, 32, and 35 showed stronger radical-scavenging activity than ascorbic acid in the ABTS assay. This was the first report on the anti-tumor and radical-scavenging activities of the polyphenols isolated from X. sorbifolia husks. The results obtained in the present study contribute important baseline information on the biological activity of X. sorbifolia husks, which might contribute for their pharmacological application.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/12/1694/s1.
Acknowledgments
The work was financially supported by the National Nature Science Foundation of China (No. 21405164, 21575150, and 81673325), the CAS Pioneer Hundred Talents Program), the scientific research project of Central Asia Drug Discovery and Development Centre of Chinese Academy of Sciences (No. CAM201404), the CAS Pioneer Hundred Talents Program, and Postdoctoral Research Projects in Gansu Province.
Author Contributions
Y.-P.S., J.-L.Y. and W.H. conceived and designed the experiments; C.-Y.Y. and K.J. performed the experiments; Y.L., W.H., and C.-Y.Y. analyzed the data; K.J. and W.H. contributed reagents/materials/analysis tools; C.-Y.Y. and Y.L. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Y.Y.; Xiang, Z.; Cui, H.; Xiao, H.; Kang, T.G.; Dou, D.Q.; Kuang, H.X. Two new oleanane-type saponins from the husks of Xanthoceras sorbifolia Bunge. Nat. Prod. Res. 2013, 27, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Wang, X.B.; Wei, X.C.; Wang, M.M.; Chen, L.X.; Cao, S.J.; Kang, N.; Qiu, F. Triterpenoid saponins from Xanthoceras sorbifolia Bunge. and their inhibitory activity on human cancer cell lines. Bioorg. Med. Chem. Lett. 2012, 22, 5232–5238. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, S.C.; Dou, D.Q. Determination of the content of total saponins in the husks of Xanthoceras sorbifolia Bunge. J. Liaoning Univ. TCM 2009, 11, 165–166. [Google Scholar]
- Wan, G.S.; Ren, Y.H.; Gao, H.Y.; Bai, S.; Xi, R.G.; Wang, X.B. Isolation and identification of chemical constituents from the husks of Xanthoceras sorbifolium Bunge. J. Shenyang Pharm. Univ. 2015, 32, 18–21. [Google Scholar]
- Li, Z.L.; Li, X.; Li, L.H.; Li, N.; Yu, M.; Meng, D.L. Two new triterpenes from the husks of Xanthoceras sorbifolia. Planta Med. 2005, 71, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Li, X.; Li, L.; Li, W. Studies on the chemical constituents of the husks of Xanthoceras sorbifolia Bunge. J. Shenyang Pharm. Univ. 2005, 22, 271–272. [Google Scholar]
- Ji, X.F.; Chi, T.Y.; Xu, Q.; He, X.L.; Zhou, X.Y.; Zhang, R.; Zou, L.B. Xanthoceraside ameliorates mitochondrial dysfunction contributing to the improvement of learning and memory impairment in mice with intracerebroventricular injection of a beta 1–42. Evid. Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Zou, L.B.; Liu, P.; Xu, Q.; Zhang, Y.F.; Yu, Y.; Zou, L.; Chi, T.Y.; Ji, X.F. Xanthoceraside induces apoptosis in melanoma cells through the activation of caspases and the suppression of the IGF-1R/Raf/MEK/ERK signaling pathway. J. Med. Food 2014, 17, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Gen, J.; Zhou, Q.C. Inhibitory effect of flavonoids consituents from Xanthoceras Sorbifolia nutshell on tyrosinase. J. Chin. Cereals Oils Assoc. 2013, 28, 96–100. [Google Scholar]
- Xu, J.K.; Zhang, W.; Li, Y.J.; Ji, X.F.; Chi, T.Y.; Zou, L.B. Effects of xanthoceraside on focal cerebral ischemia reperfusion injury in rats and the preliminary mechanism study. J. Shenyang Pharm. Univ. 2014, 31, 793–798. [Google Scholar]
- Wu, W.J. Study on the Separation Process and Antioxidant Activity of the Total Sorbifolia Sheel Saponin; Beijing Forestry University: Beijing, China, 2010. [Google Scholar]
- Gen, J.; Zhang, H.M.; Zhou, Q.C. Inhibitory effect of Xanthoceras sorbifolia nutshell saponin on pancreatic lipase. Mod. Food Sci. Technol. 2014, 30, 89–92. [Google Scholar]
- Katalinic, M.; Rusak, G.; Barovic, J.D.; Sinko, G.; Jelic, D.; Antolovic, R.; Kovarik, Z. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur. J. Med. Chem. 2010, 45, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Keawsa-ard, S.; Natakankitkul, S.; Liawruangrath, S.; Teerawutgulrag, A.; Trisuwan, K.; Charoenying, P.; Pyne, S.G.; Liawruangrath, B. Anticancer and antibacterial activities of the isolated compounds from Solanum spirale Roxb. leaves. Chiang Mai J. Sci. 2012, 39, 445–454. [Google Scholar]
- Lei, J.C.; Yang, C.X.; Yang, Y.; Zhang, W.; Yu, J.Q. Antioxidant and antitumour activities of extracts from Patrinia villosa and its active constituents. J. Funct. Foods 2015, 16, 289–294. [Google Scholar] [CrossRef]
- Zhao, S.M.; Chou, G.X.; Wang, Z.T. Chemical constituents from roots and rhizomes of Physochlaina infundibularis. Chin. Tradit. Herb. Drugs 2013, 44, 938–941. [Google Scholar]
- Zhang, W.K.; WANG, S.B.; Fu, C.Y.; Li, P.; Xu, J.K. Flavonoids from Humulus lupulus. China J. Chin. Meter. Med. 2013, 38, 1539–1542. [Google Scholar]
- Lotti, C.; Piccinelli, A.L.; Arevalo, C.; Ruiz, I.; De Castro, G.M.M.; De Sa, L.F.R.; Tessis, A.C.; Ferreira-Pereira, A.; Rastrelli, L. Constituents of Honduran propolis with inhibitory effects on Saccharomyces cerevisiae multidrug resistance protein pdr5p. J. Agric. Food. Chem. 2012, 60, 10540–10545. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.J.; Zhao, Q.C.; Yang, L.; Shang, Z.P.; Du, Z.Q.; Yan, M. Chemical constituents in roots of Paeonia lactiflora. Chin. Tradit. Herb. Drugs 2010, 41, 1245–1248. [Google Scholar]
- Kayano, S.; Kikuzaki, H.; Fukutsuka, N.; Mitani, T.; Nakatani, N. Antioxidant activity of prune (Prunus domestica L.) constituents and a new synergist. J. Agric. Food Chem. 2002, 50, 3708–3712. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Li, J.; Wang, N.L.; Yao, X.S. Flavonoids and a new polyacetylene from Bidens parviflora Willd. Molecules 2008, 13, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Bellakhdar, J.; Passannanti, S.; Paternostro, M.P.; Piozzi, F. Constituents of Origanum-Compactum. Planta Med. 1988, 94. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, H.; Saito, T.; Ishikawa, H.; Date, H.; Kataoka, S.; Tamura, Y.; Mizutani, K. Antiperoxidative components in Thymus vulgaris. Planta Med. 1996, 62, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Sakushima, A.; Coskun, M.; Hisada, S.; Nishibe, S. Flavonoids from Rhamnus-Pallasii. Phytochemistry 1983, 22, 1677–1678. [Google Scholar] [CrossRef]
- Manthey, J.A.; Guthrie, N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agric. Food Chem. 2002, 50, 5837–5843. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.H.; Dai, Y.; Gao, H.; Ye, W.C.; Yao, X.S. Study on chemical constituents of Sarcandra glaber. Chin. Tradit. Herb. Drugs 2010, 41, 29–32. [Google Scholar]
- Zhang, S.X.; Tani, T.; Yamaji, S.; Ma, C.M.; Wang, M.C.; Cai, S.Q.; Zhao, Y.Y. Glycosyl flavonoids from the roots and rhizomes of Asarum longerhizomatosum. J. Asian Nat. Prod. Res. 2003, 5, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Lee, E.J.; Wu, T.S. Antityrosinase principles and constituents of the petals of Crocus sativus. J. Nat. Prod. 2004, 67, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Lee, S.S. Dibenzocycloheptanoids from the leaves of Cinnamomum subavenium. J. Nat. Prod. 2012, 75, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Cui, C.B. One new and nine known flavonoids from Choerospondias axillaries and their in vitro antitumor, anti-hypoxia and antibacterial activities. Molecules 2014, 19, 21363–21377. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.F.; Zhou, B.; Huang, J.M.; Gao, X.M.; Shu, L.D.; Yang, G.Y.; Che, C.T. Antiviral phenolic compounds from Arundina gramnifolia. J. Nat. Prod. 2013, 76, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.H.; Yang, S.J.; Liang, N.; Hu, D.Y.; Jin, L.H.; Xue, W.; Yang, S. Chemical constituents of Caesalpinia decapetala (Roth) Alston. Molecules 2013, 18, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, J.; Wang, F. A new flavanone glucoside from the flowers of Carthamus tinctorius and assignment of absolute configuration. Chem. Nat. Compd. 2014, 50, 427–429. [Google Scholar] [CrossRef]
- Gao, H.; Nishida, J.; Saito, S.; Kawabata, J. Inhibitory effects of 5,6,7-trihydroxyflavones on tyrosinase. Molecules 2007, 12, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Y.; Huang, M.Y.; Wang, F.; Liu, Q.Y.; Zhang, L.X.; Zhang, Y.H. Chemical constituents of Ajania nematoloba. Chem. Nat. Compd. 2015, 51, 143–145. [Google Scholar] [CrossRef]
- Funari, C.S.; Gullo, F.P.; Napolitano, A.; Carneiro, R.L.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M.; Piacente, S.; Pizza, C.; Silva, D.H.S. Chemical and antifungal investigations of six Lippia species (Verbenaceae) from Brazil. Food Chem. 2012, 135, 2086–2094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kurita, M.; Shinozaki, T.; Ukiya, M.; Yasukawa, K.; Shimizu, N.; Tokuda, H.; Masters, E.T.; Akihisa, M.; Akihisa, T. Triterpene glycosides and other polar constituents of shea (Vitellaria paradoxa) kernels and their bioactivities. Phytochemistry 2014, 108, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.Y.; Zhang, C.H. Studies on the chemical constituents of Xanthoceras sorbifolia. J. Chin. Med. Mater. 2009, 32, 702–704. [Google Scholar]
- Jung, M.; Choi, J.; Chae, H.S.; Cho, J.Y.; Kim, Y.D.; Htwe, K.M.; Lee, W.S.; Chin, Y.W.; Kim, J.; Yoon, K.D. Flavonoids from Symplocos racemosa. Molecules 2015, 20, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, L.; Saturnino, P.; Schettino, O.; Dini, A. Studies on the constituents of Chenopodium Pallidicaule (Canihua) seeds-isolation and characterization of 2 new flavonol glycosides. J. Agric. Food Chem. 1995, 43, 2020–2024. [Google Scholar] [CrossRef]
- Tai, Z.G.; Zhang, F.M.; Cai, L.; Shi, J.; Cao, Q.E.; Ding, Z.T. Flavonol glycosides of Pseudodrynaria coronans and their antioxidant activity. Chem. Nat. Compd. 2012, 48, 221–224. [Google Scholar] [CrossRef]
- Aderogba, M.A.; Ndhlala, A.R.; Rengasamy, K.R.R.; Van Staden, J. Antimicrobial and selected in vitro enzyme inhibitory effects of leaf extracts, flavonols and indole alkaloids isolated from Croton menyharthii. Molecules 2013, 18, 12633–12644. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Lou, G.D.; Ma, Z.J.; Liu, X.M. Chemical constituents with antioxidant activities from litchi (Litchi chinensis Sonn.) seeds. Food Chem. 2011, 126, 1081–1087. [Google Scholar] [CrossRef]
- Lou, H.X.; Yamazaki, Y.; Sasaki, T.; Uchida, M.; Tanaka, H.; Oka, S. A-type proanthocyanidins from peanut skins. Phytochemistry 1999, 51, 297–308. [Google Scholar] [CrossRef]
- Hashimoto, F.; Nonaka, G.; Nishioka, I. Tannins and related-compounds Structures of novel fermentation products, theogallinin, theaflavonin and desgalloyl theaflavonin from black tea, and Changes of tea leaf polyphenols during fermentation. Chem. Pharm. Bull. 1992, 40, 1383–1389. [Google Scholar] [CrossRef]
- Peng, X.; Yu, D.Y.; Feng, B.M.; Wang, Y.Q.; Shi, L.Y. A new acylated flavonoid glycoside from the flowers of Camellia nitidissima and its effect on the induction of apoptosis in human lymphoma U937 cells. J. Asian Nat. Prod. Res. 2012, 14, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Panyadee, A.; Sahakitpichan, P.; Ruchirawat, S.; Kanchanapoom, T. 5-methyl ether flavone glucosides from the leaves of Bruguiera gymnorrhiza. Phytochem. Lett. 2015, 11, 215–219. [Google Scholar] [CrossRef]
- Liao, C.R.; Kuo, Y.H.; Ho, Y.L.; Wang, C.Y.; Yang, C.S.; Lin, C.W.; Chang, Y.S. Studies on cytotoxic constituents from the leaves of Elaeagnus oldhamii Maxim. in non-small cell lung cancer A549 Cells. Molecules 2014, 19, 9515–9534. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.R.; Wu, D.; Jiang, Y.M.; Prasad, K.N.; Lin, S.; Jiang, G.X.; He, J.R.; Zhao, M.M.; Luo, W.; Yang, B. Identification of flavonoids in litchi (Litchi chinensis Sonn.) leaf and evaluation of anticancer activities. J. Funct. Foods 2014, 6, 555–563. [Google Scholar] [CrossRef]
- Chang, R.J.; Wang, C.H.; Zeng, Q.; Guan, B.; Zhang, W.D.; Jin, H.Z. Chemical constituents of the stems of Celastrus rugosus. Arch. Pharm. Res. 2013, 36, 1291–1301. [Google Scholar]
- Ashour, M.L.; El-Readi, M.Z.; Tahrani, A.; Eid, S.Y.; Wink, M. A novel cytotoxic aryltetraline lactone from Bupleurum marginatum (Apiaceae). Phytochem. Lett. 2012, 5, 387–392. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Ind. Crop. Prod. 2015, 70, 238–244. [Google Scholar] [CrossRef]
- Huang, G.J.; Wang, B.S.; Lin, W.C.; Huang, S.S.; Lee, C.Y.; Yen, M.T.; Huang, M.H. Antioxidant and anti-inflammatory properties of Longan (Dimocarpus longan Lour.) pericarp. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Huang, Z.H.; Qin, P.Y.; Yao, Y.; Meng, X.J.; Zou, J.Q.; Zhu, K.; Ren, G.X. Chemical characterization of a procyanidin-rich extract from Sorghum Bran and Its effect on oxidative stress and tumor inhibition in vivo. J. Agric. Food Chem. 2011, 59, 8609–8615. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.C.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A.M. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [PubMed]
- Yang, C.Y.; Li, F.; Du, B.W.; Chen, B.; Wang, F.; Wang, M.K. Isolation and characterization of new phenolic compounds with estrogen biosynthesis-inhibiting and antioxidation activities from Broussonetia papyrifera leaves. PLoS ONE 2014, 9, e94198. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Liu, Z.Q.; Zou, K.; Wang, J.Z.; Zhou, Y.Q.; Liu, X.Q. Preliminary experimental study on the anti-tumor effect of Tupistra chinensis Bak. in vitro. Lishizhen Med. Mater. Med. Res. 2009, 20, 2390–2392. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).