A New Two-Photon Ratiometric Fluorescent Probe for Detecting Alkaline Phosphatase in Living Cells
Abstract
:1. Introduction
2. Results and Discussion
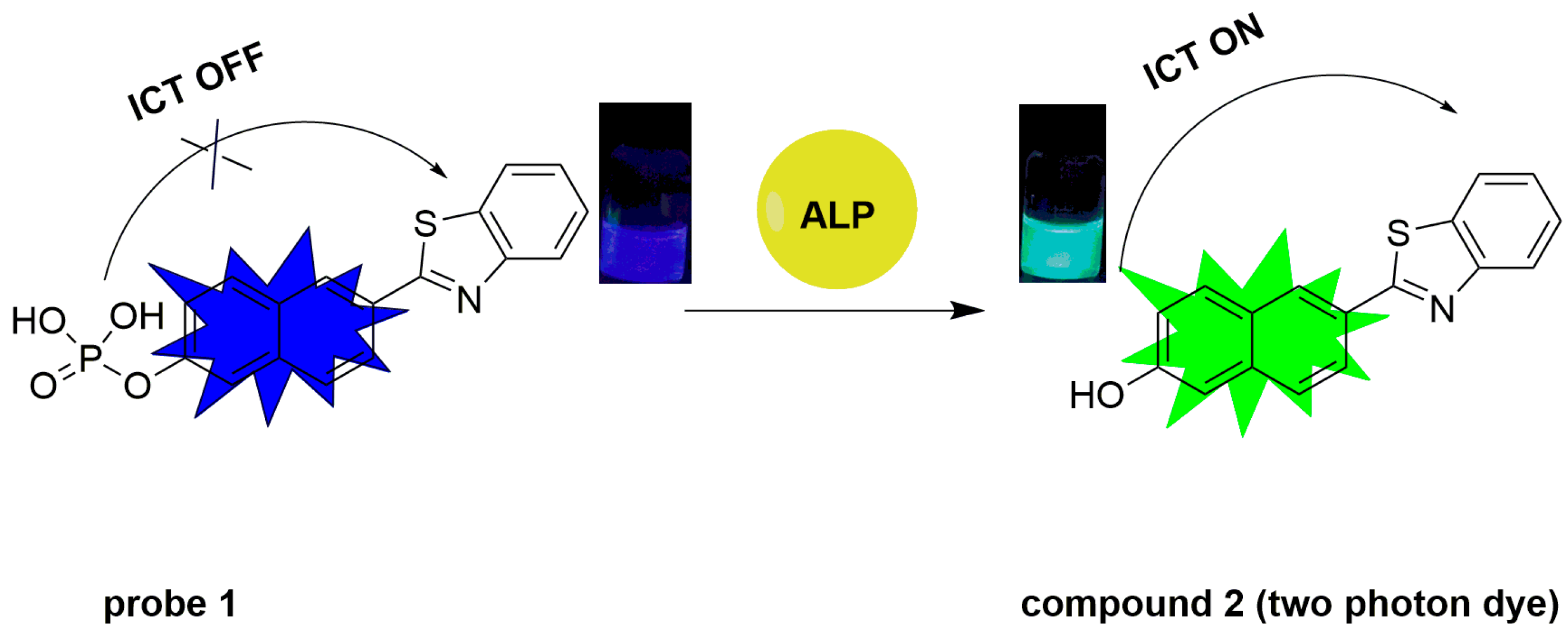
2.1. Design and Response Principle of Probe 1
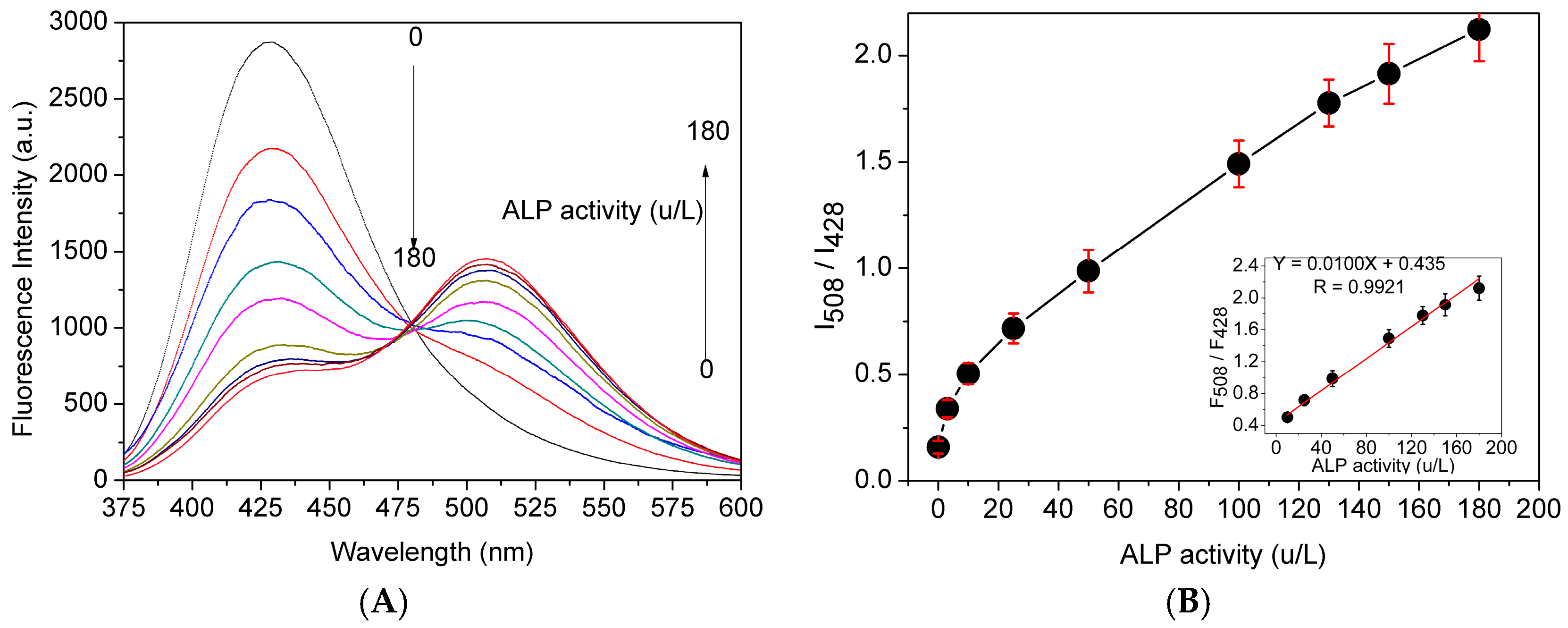
2.2. Analytical Spectral Properties of Probe 1
2.3. Effect of pH
2.4. Selectivity Study
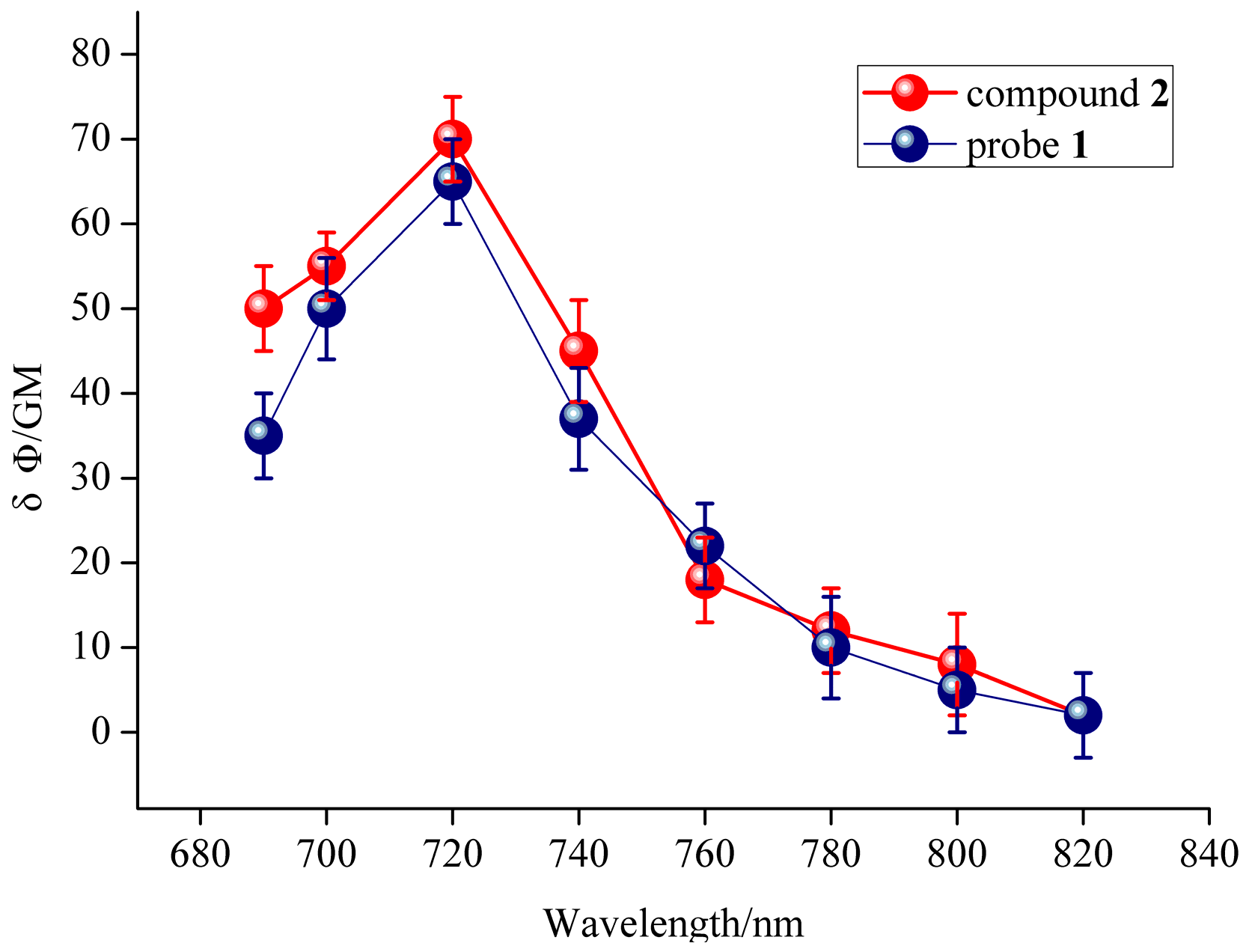
2.5. TP Fluorescence Propertiess
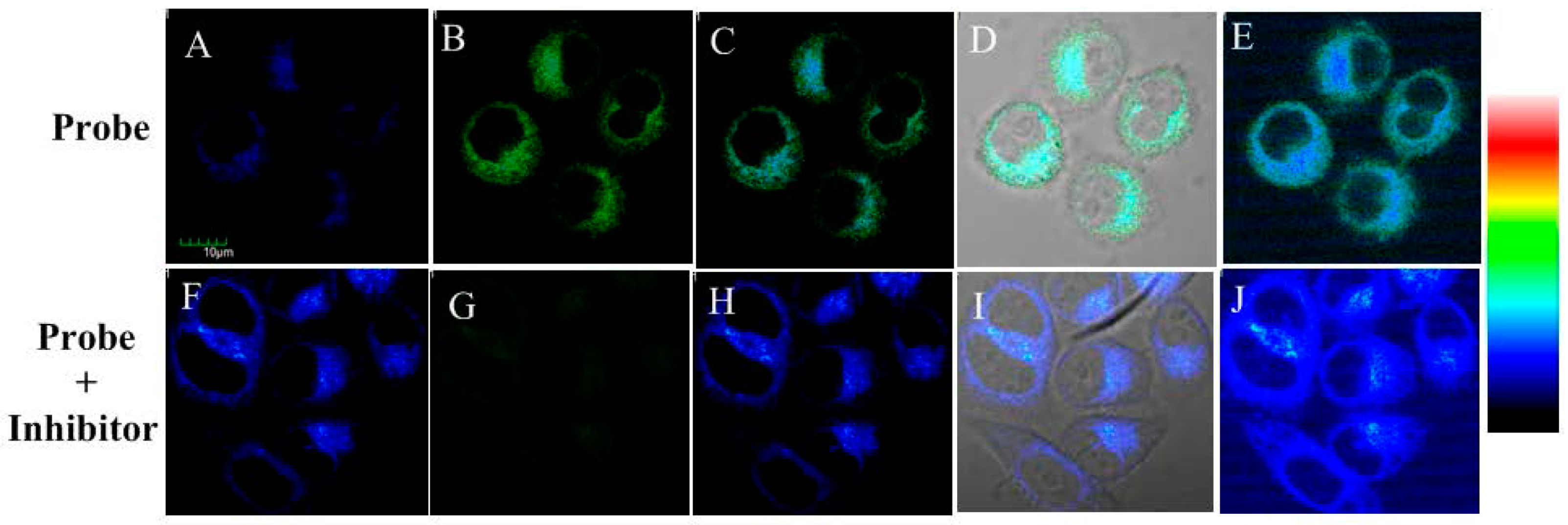
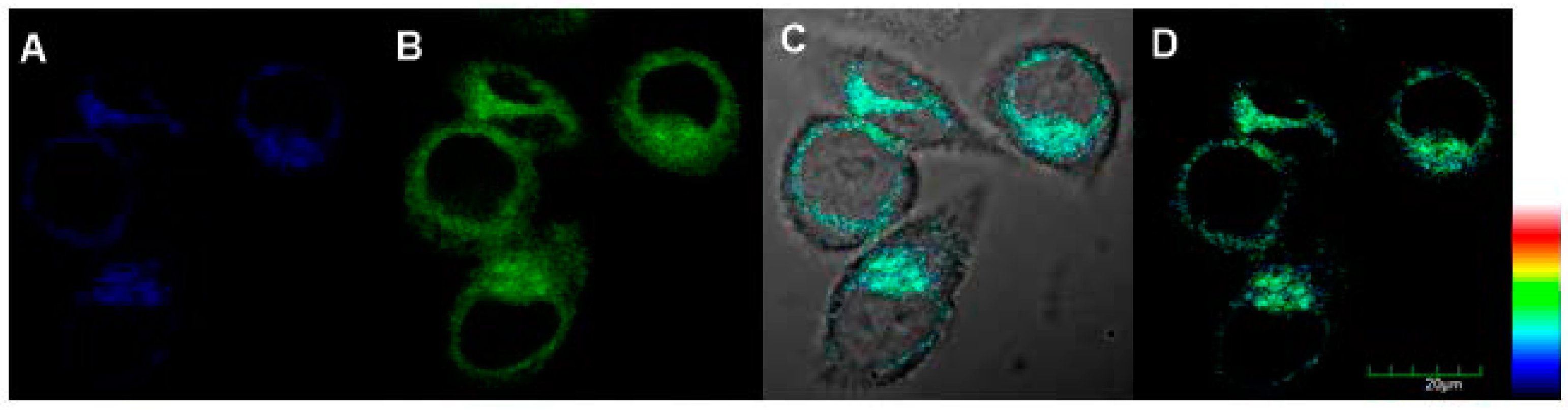
2.6. Imaging of Endogenous ALP in Living Cells
3. Experimental Section
3.1. Materials and Apparatus
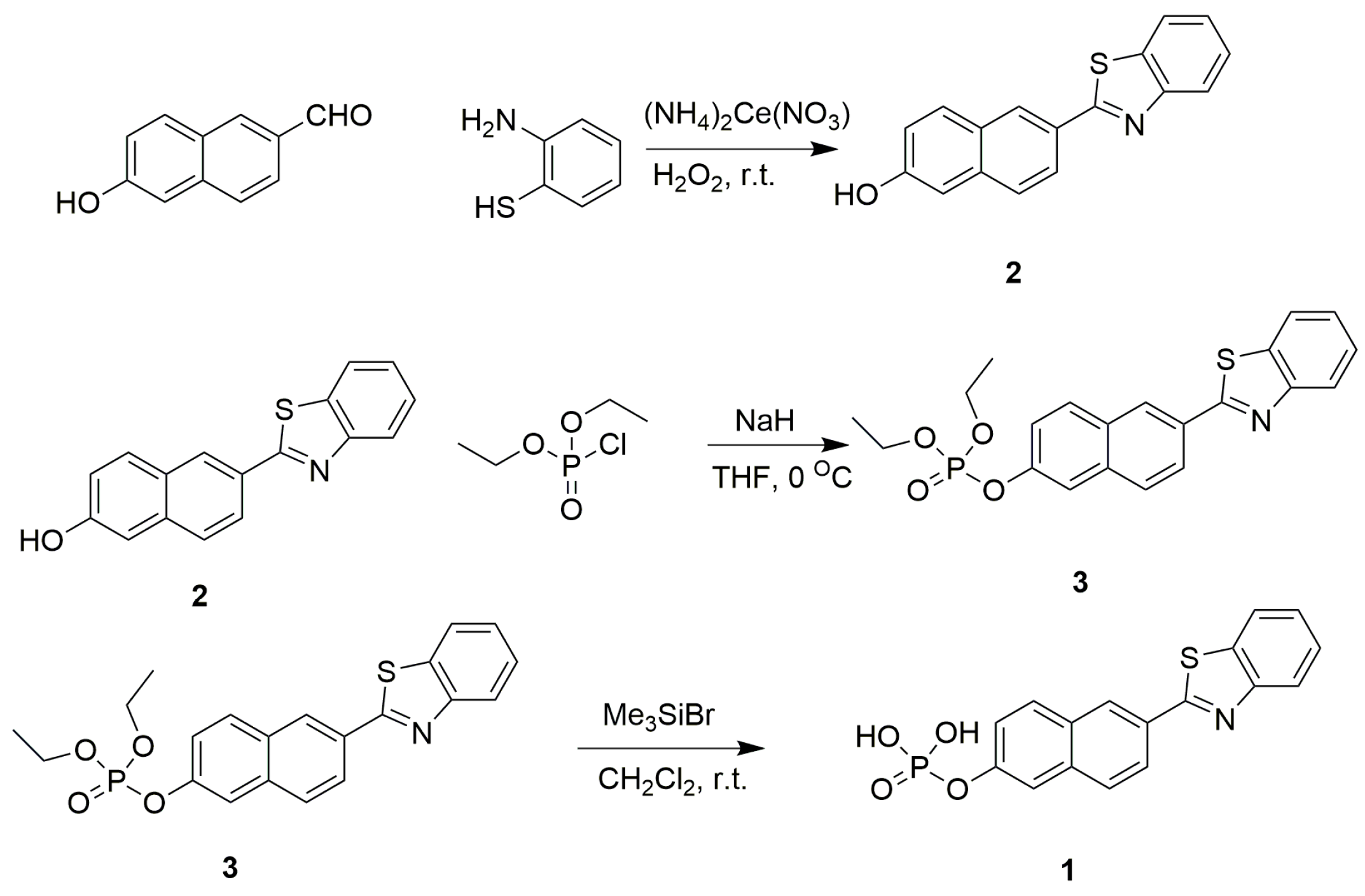
3.2. Synthesis
3.3. Optical Property
3.4. Cell Viability and Imaging
3.5. The Fluorescent Quantum Yields and Two-Photon Excited Fluorescence Measurement
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| D–π–A | donor–π–acceptor |
| ICT | intramolecular charge transfer |
| TPM | two-photon microscopy |
| I | Intensity |
| Tris | tris(hydroxymethyl)aminomethane |
| DMSO | dimethyl sulfoxide |
| Ex | excitation |
| BSA | bovine serum albumin |
| AChE | acetyl cholinesterase |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide |
| Em | emission |
| UV | ultraviolet light |
| LC-MS | liquid chromatography-mass spectrometry |
| CCD | charge coupled device |
| Fs | femtosecond |
References
- Syakalima, M.; Takiguchi, M.; Yasuda, J.; Hashimoto, A. A study of liver and corticosteroid-induced alkaline phosphatase isoenzymes activities associated with glucocorticoid hepatopathy in the dog. Jpn. J. Vet. Res. 1998, 46, 119–121. [Google Scholar]
- Coleman, J.E. Structure and mechanism of alkaline phosphatase. Annu. Rev. Biophys. Biomol. Struct. 1992, 21, 441–483. [Google Scholar] [CrossRef]
- Pike, A.F.; Kramer, N.I.; Blaauboer, B.J.; Seinen, W.; Brands, R. A novel hypothesis for an alkaline phosphatase ‘rescue’ mechanism in the hepatic acute phase immune response. Biochim. Biophys. Acta. 2013, 1832, 2044–2056. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.G.; Hong, Y.N.; Kwok, R.T.K.; Lam, J.W.Y.; Liu, B.; Tang, B.Z. A dual-mode fluorescence “turn-on” biosensor based on an aggregation-induced emission luminogen. J. Mater. Chem. B 2014, 2, 1717–1723. [Google Scholar] [CrossRef]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, X.; Zheng, Z.; Lu, X.; Lin, M.; Pan, C.; Liu, J. A novel ATP7B gene mutation in a liver failure patient with normal ceruloplasmin and low serum alkaline phosphatase. Gene 2014, 538, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Kampanatkosol, R.; Thomson, T.; Habeeb, O.; Glynn, L.; DeChristopher, P.J.; Yong, S.; Jeske, W.; Maheshwari, A.; Muraskas, J. The relationship between reticulated platelets, intestinal alkaline phosphatase, and necrotizing enterocolitis. J. Pediatr. Surg. 2014, 49, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Lacivita, E.; Leopoldo, M.; Berardi, F.; Colabufo, N.A.; Perrone, R. Activatable fluorescent probes: A new concept in optical molecular imaging. Curr. Med. Chem. 2012, 19, 4731–4741. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, S.U.; Prasai, B.; McCarley, R.L. Detection and cellular imaging of human cancer enzyme using a turn-on, wavelength-shiftable, self-immolative profluorophore. J. Am. Chem. Soc. 2014, 136, 7575–7578. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Liu, T.; Zhang, X.B.; Huan, S.Y.; Wu, C.C.; Fu, T.; Tan, W.H. Multicolor fluorescent biosensor for multiplexed detection of DNA. Anal. Chem. 2014, 86, 5009–5016. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schanze, K.S. Conjugated polyelectrolyte-based real-time fluorescence assay for alkaline phosphatase with pyrophosphate as substrate. Anal. Chem. 2008, 80, 8605–8612. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Tang, Y.; Feng, F.; He, F.; Wang, S.J. Water-soluble conjugated polymers for continuous and sensitive fluorescence assays for phosphatase and peptidase. J. Mater. Chem. 2007, 17, 4147–4152. [Google Scholar] [CrossRef]
- Zheng, F.Y.; Guo, S.H.; Zeng, F.; Li, J.; Wu, S.Z. Ratiometric fluorescent probe for alkaline phosphatase based on betaine-modified polyethylenimine via excimer/monomer conversion. Anal. Chem. 2014, 86, 9873–9879. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.; Finder, T.; Gill, R.; Willner, I. Probing protein kinase (CK2) and alkaline phosphatase with CdSe/ZnS quantum dots. Nano Lett. 2010, 10, 2192–2196. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Xu, J.P.; Li, D.; Pang, S.P.; Fang, Y.; Song, Z.G.; Ji, J. Fluorescence detection of alkaline phosphatase activity with β-cyclodextrin-modified quantum dots. Chem. Commun. 2010, 46, 7166–7168. [Google Scholar] [CrossRef] [PubMed]
- Li, G.L.; Fu, H.L.; Chen, X.J.; Gong, P.W.; Chen, G.; Xia, L.; Wang, H.; You, J.M.; Wu, Y.N. Facile and sensitive fluorescence sensing of alkaline phosphatase activity with photoluminescent carbon dots Based on inner filter effect. Anal. Chem. 2016, 88, 2720–2726. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.H.; Wang, G.F.; Jiang, H.; Chen, L.; Zhang, X.J. One-step ultrasonic synthesis of graphene quantum dots with high quantum yield and their application in sensing alkaline phosphatase. Chem. Commun. 2015, 51, 948–951. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.P.; Chen, J.; Li, W.Y.; Wang, F.Y.; Zhou, H.P.; Li, Y.X.; Yu, C. Nucleic acid-regulated perylene brobe-induced gold nanoparticle aggregation: A new strategy for colorimetric sensing of alkaline phosphatase activity and inhibitor screening. ACS Appl. Mater. Interfaces 2014, 6, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Miao, Q.Q.; Hai, Z.J.; Yuan, Y.; Liang, G.L. Enzymatic hydrogelation-induced fluorescence turn-off for sensing alkaline phosphatase in vitro and in living cells. Anal. Chem. 2015, 87, 6475–6478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Xu, C.L.; Liu, J.; Li, X.H.; Guo, L.; Li, X.M. An enzyme-activatable probe with a self-immolative linker for rapid and sensitive alkaline phosphatase detection and cell imaging through a cascade reaction. Chem. Commun. 2015, 51, 7031–7034. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.X.; Wu, J.S.; Liu, W.M.; Zhang, G.Y.; Wang, P.F. A ratiometric fluorescent probe for quantification of alkaline phosphatase in living cells. RSC Adv. 2016, 6, 32046–32051. [Google Scholar] [CrossRef]
- Kuhn, B.; Denk, W.; Bruno, R.M. In vivo two-photon voltage-sensitive dye imaging reveals top-down control of cortical layers 1 and 2 during wakefulness. Proc. Natl. Acad. Sci. USA 2008, 105, 7588–7593. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.H.; Han, J.H.; Heo, C.H.; Kim, H.M.; Liu, Z.H.; Cho, B.R. Dual-color imaging of magnesium/calcium ion activities with two-photon fluorescent probes. Anal. Chem. 2012, 84, 8110–8113. [Google Scholar] [CrossRef] [PubMed]
- Masanta, G.; Lim, C.S.; Kim, H.J.; Han, J.H.; Kim, H.M.; Cho, B.R. A mitochondrial-targeted two-photon probe for zinc ion. J. Am. Chem. Soc. 2011, 133, 5698–5700. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Lu, D.Q.; Wang, Q.Q.; Hu, S.Q.; Wang, H.F.; Sun, H.Y.; Zhang, X.B. A high-resolution mitochonghdria-targeting ratiometric fluorescent probe for detection of the endogenous hypochlorous acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 166, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Hu, S.Q.; Wang, H.F.; Sun, H.Y.; Zhang, X.B. A novel ratiometric two-photon fluorescent probe for imaging of Pd2+ ions in living cells and tissues. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2016, 166, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Cho, B.R. Two-photon probes for intracellular free metal ions, acidic vesicles, and lipid rafts in live tissues. Acc. Chem. Res. 2009, 42, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.W.; Ding, C.Q.; Tian, Y. A two-photon ratiometric fluorescence probe for cupric ions in live cells and tissues. Sci. Rep. 2013, 3, 2933. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ryu, H.G.; Ahn, K.H. Recent development of two-photon fluorescent probes for bioimaging. Org. Biomol. Chem. 2014, 12, 4550–4566. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.R.; Kang, D.E.; Kim, H.M.; Cho, B.R. Recent development of two-photon fluorescent probes for bioimaging. Inorg. Chem. 2014, 53, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; Masanta, G.; Kim, H.J.; Han, J.H.; Kim, H.M.; Cho, B.R. Ratiometric detection of mitochondrial thiols with a two-photon fluorescent probe. J. Am. Chem. Soc. 2011, 133, 11132–11135. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.K.; Heo, C.H.; Choi, D.J.; Sen, D.; Joe, E.H.; Cho, B.R.; Kim, H.M. A ratiometric two-photon fluorescent probe reveals reduction in mitochondrial H2S production in Parkinson’s disease gene knockout astrocytes. J. Am. Chem. Soc. 2013, 135, 9915–9923. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.J.; Xu, J.H.; Zhang, X.B.; Zhou, L.Y.; Liu, H.W.; Zhang, J. A naphthalene-based two-photon fluorescent probe for selective and sensitive detection of thiophenols. Sens. Actuators B Chem. 2016, 229, 434–440. [Google Scholar] [CrossRef]
- Xia, X.T.; Zeng, F.; Zhang, P.S.; Lyu, J.; Huang, Y.; Wu, S.Z. An ICT-based ratiometric fluorescent probe for hydrazine detection and its application in living cells and in vivo. Sens. Actuators B Chem. 2016, 227, 411–418. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, D.Q.; Wang, Q.; Hu, S.; Wang, H.; Sun, H.; Zhang, X. An efficient two-photon ratiometric fluorescent probe platform for dual-channel imaging of lysosomes in living cells and tissues. Sens. Actuators B Chem. 2017, 238, 274–280. [Google Scholar] [CrossRef]
- Hou, X.F.; Yu, Q.X.; Zeng, F.; Ye, J.H.; Wu, S.Z. A ratiometric fluorescent probe for in vivo tracking of alkaline phosphatase level variation resulting from drug-induced organ damage. J. Mater. Chem. B 2015, 3, 1042–1048. [Google Scholar] [CrossRef]
- Bahrami, K.; Khodaei, M.M.; Naali, F. Mild and highly efficient method for the synthesis of 2-arylbenzimidazoles and 2-arylbenzothiazoles. J. Org. Chem. 2008, 73, 6835–6837. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.H.; Zeng, F.; Yu, C.M.; Wu, S.Z. A fluorescent probe for alkaline phosphatase via excited state intramolecular proton transfer. Sens. Actuators B Chem. 2015, 220, 720–726. [Google Scholar] [CrossRef]
- Zhou, X.H.; Jiang, Y.R.; Zhao, X.J.; Guo, D. A naphthalene-based two-photon fluorescent probe for selective and sensitive detection of endogenous hypochlorous acid. Talanta 2016, 160, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1, 2 and 3 are available from the authors.


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Jiang, Y.; Zhao, X.; Zhu, Y. A New Two-Photon Ratiometric Fluorescent Probe for Detecting Alkaline Phosphatase in Living Cells. Molecules 2016, 21, 1619. https://doi.org/10.3390/molecules21121619
Zhou X, Jiang Y, Zhao X, Zhu Y. A New Two-Photon Ratiometric Fluorescent Probe for Detecting Alkaline Phosphatase in Living Cells. Molecules. 2016; 21(12):1619. https://doi.org/10.3390/molecules21121619
Chicago/Turabian StyleZhou, Xiaohong, Yuren Jiang, Xiongjie Zhao, and Yao Zhu. 2016. "A New Two-Photon Ratiometric Fluorescent Probe for Detecting Alkaline Phosphatase in Living Cells" Molecules 21, no. 12: 1619. https://doi.org/10.3390/molecules21121619
APA StyleZhou, X., Jiang, Y., Zhao, X., & Zhu, Y. (2016). A New Two-Photon Ratiometric Fluorescent Probe for Detecting Alkaline Phosphatase in Living Cells. Molecules, 21(12), 1619. https://doi.org/10.3390/molecules21121619




