Emerging Phytochemicals for the Prevention and Treatment of Head and Neck Cancer
Abstract
:1. Introduction
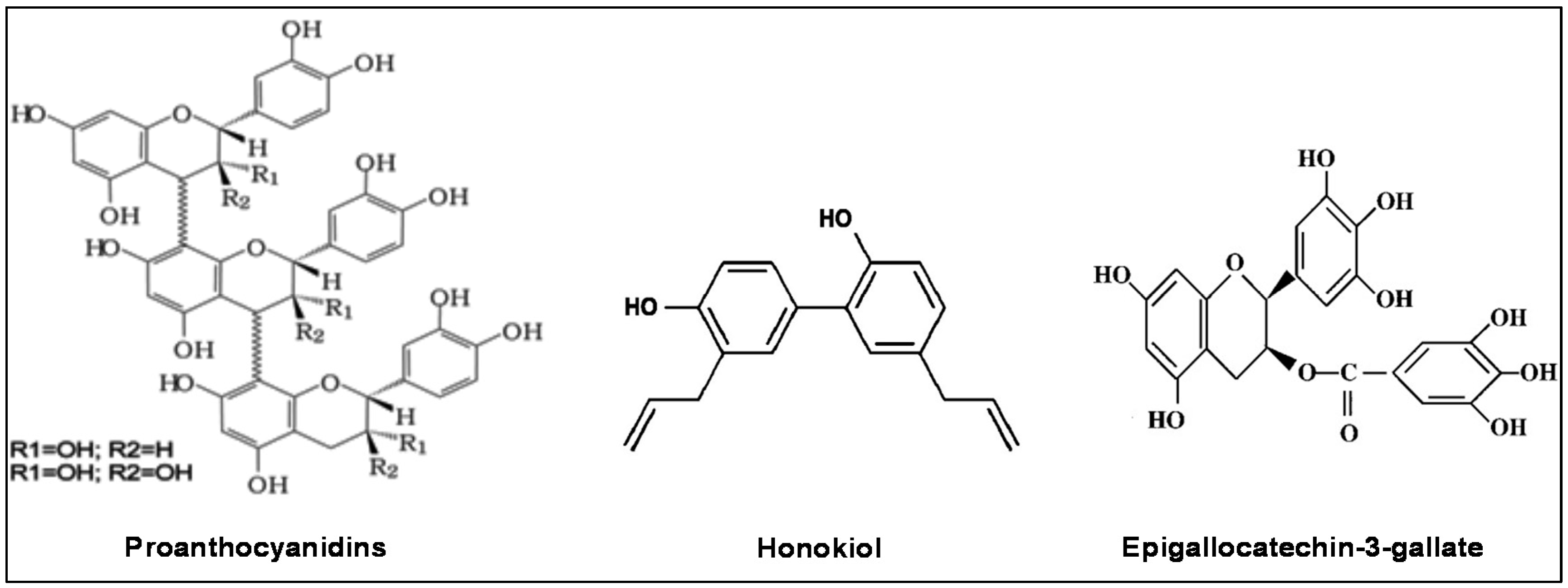
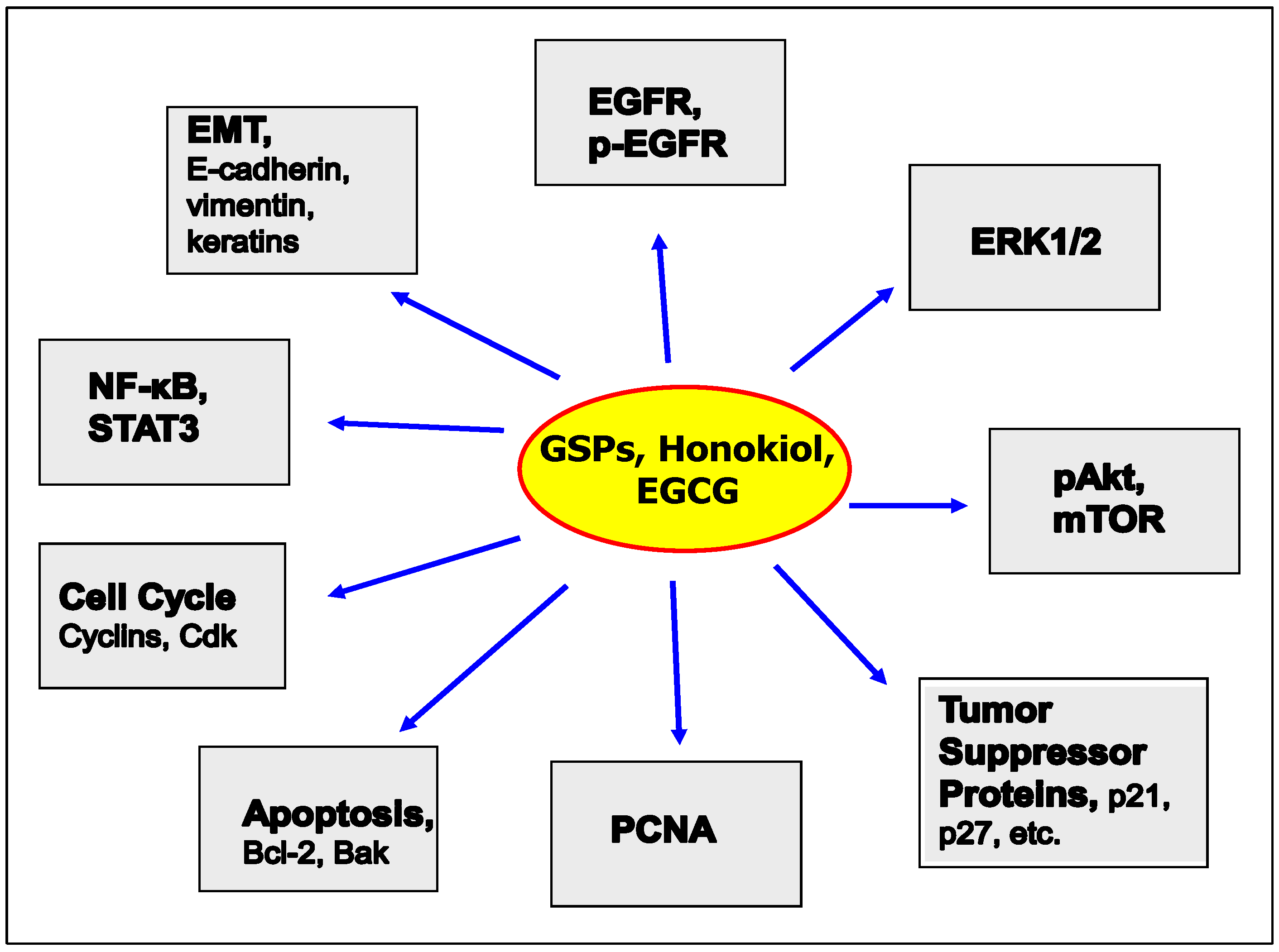
2. Promising Phytochemicals
2.1. Grape Seed Proanthocyanidins (GSPs)
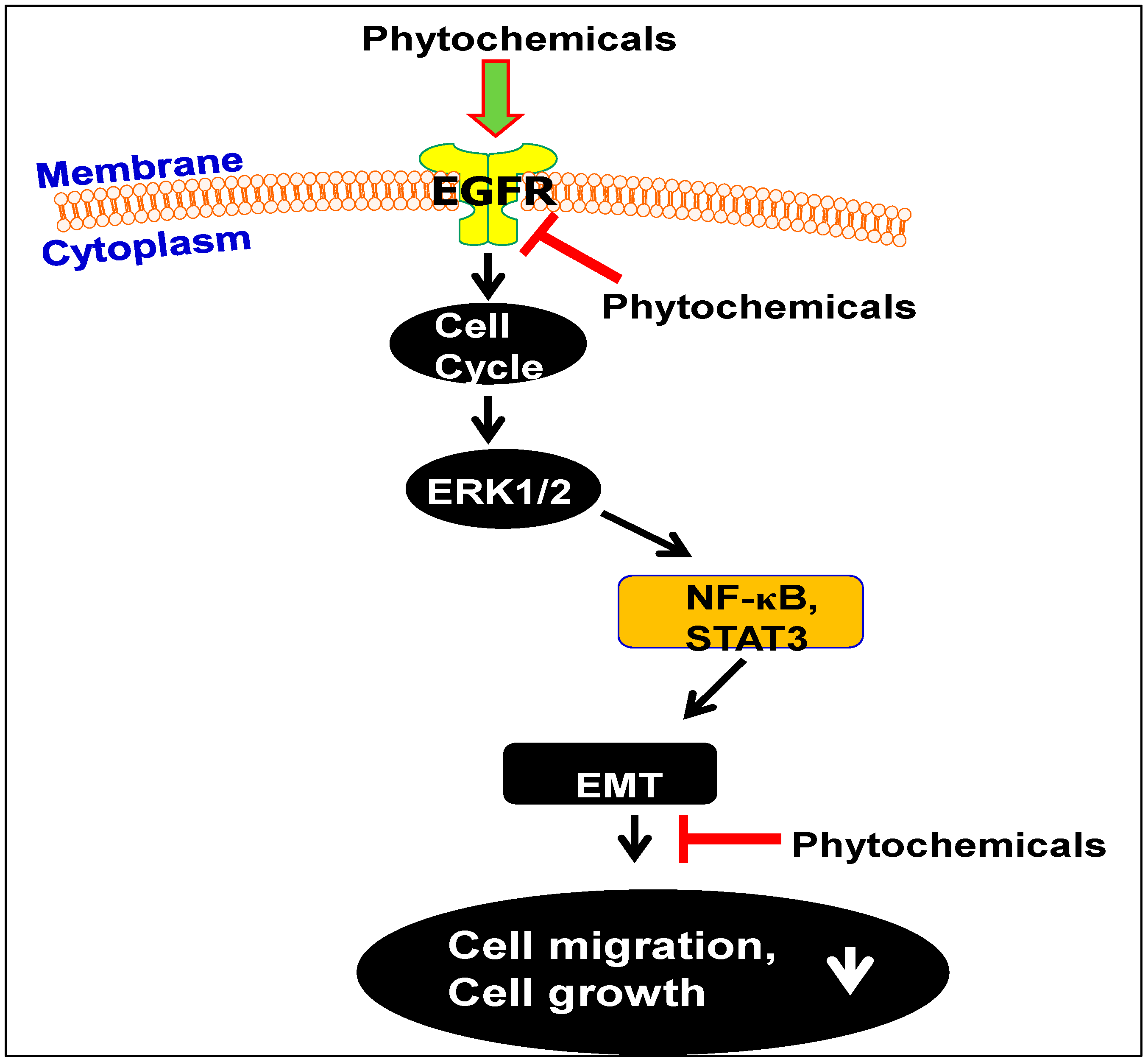
2.1.1. GSPs Inhibit the Growth of HNSCC Cells
2.1.2. GSPs Inhibit the Migration Potential of HNSCC Cells
2.2. Honokiol and HNSCC
2.3. Green Tea Polyphenols and HNSCC
3. Bioavailability and Toxicities of GSPs, GTPs and Honokiol
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hunter, K.D.; Parkinson, E.K.; Harrison, P.R. Profiling early head and neck cancer. Nat. Rev. Cancer 2005, 5, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Arbes, S.J., Jr.; Olshan, A.F.; Caplan, D.J.; Schoenbach, V.J.; Slade, G.D.; Symons, M.J. Factors contributing to the poorer survival of black Americans diagnosed with oral cancer (United States). Cancer Causes Contr. 1999, 10, 513–523. [Google Scholar] [CrossRef]
- Leon, X.; Quer, M.; Orus, C.; del Prado Venegas, M. Can cure be achieved in patients with head and neck carcinomas? The problem of second neoplasm. Expert Rev. Anticancer Ther. 2001, 1, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Casiglia, J.; Woo, S.B. A comprehensive review of oral cancer. Gen. Dent. 2001, 49, 72–82. [Google Scholar] [PubMed]
- Posner, M.R.; Hershock, D.M.; Blajman, C.R.; Mickiewicz, E.; Winquist, E.; Gorbounova, V.; Tjulandin, S.; Shin, D.M.; Cullen, K.; Ervin, T.J.; et al. TAX 324 Study Group. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N. Engl. J. Med. 2007, 357, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Remenar, E.; van Herpen, C.; Gorlia, T.; Mesia, R.; Degardin, M.; Stewart, J.S.; Jelic, S.; Betka, J.; Preiss, J.H. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N. Engl. J. Med. 2007, 357, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Perlmutter, M.A.; Johnson, J.T.; Snyderman, C.H.; Cano, E.R.; Myers, E.N. Functional outcomes after treatment of squamous cell carcinoma of the base of the tongue. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Gellrich, N.C.; Schimming, R.; Schramm, A.; Schmalohr, D.; Bremerich, A.; Kugler, J. Pain, function, and psychologic outcome before, during, and after intraoral tumor resection. J. Oral Maxillofac. Surg. 2002, 60, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, P.; Caruana, S.; Singh, B.; Shaha, A.; Kraus, D.; Harrison, L.; McKiernan, J.; Solan, J.; Polyak, T.; Shah, J.P. Functional status after primary surgical therapy for squamous cell carcinoma of the base of the tongue. Head Neck 2002, 24, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Robertson, M.; Emerton, S.; Phillips, N.; Stevenson-Moore, P. Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck 2001, 23, 389–398. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zeng, Q.; Drenning, S.D.; Melhem, M.F.; Tweardy, D.J.; Huang, L.; Grandis, J.R. Inhibition of human squamous cell carcinoma growth in vivo by epidermal growth factor receptor antisense RNA transcribed from the U6 promoter. J. Natl. Cancer Inst. 1998, 90, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Grandis, J.R.; Melhem, M.F.; Barnes, E.L.; Tweardy, D.J. Quantitative immune-histochemical analysis of transforming growth factor-α and epidermal growth factor receptor in patients with squamous cell carcinoma of the head and neck. Cancer 1996, 78, 1284–1292. [Google Scholar] [CrossRef]
- Grandis, J.R.; Melhem, M.F.; Gooding, W.E.; Day, R.; Holst, V.A.; Wagener, M.M.; Drenning, S.D.; Tweardy, D.J. Levels of TGF-α and EGFR protein in head and neck squamous cell carcinoma and patient survival. J. Natl. Cancer Inst. 1998, 90, 824–832. [Google Scholar] [CrossRef]
- Loeffler-Ragg, J.; Schwentner, I.; Sprinzl, G.M.; Zwierzina, H. EGFR inhibition as a therapy for head and neck squamous cell carcinoma. Expert Opin. Investig. Drugs 2008, 17, 1517–1531. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Suh, N. Chemoprevention: An essential approach to controlling cancer. Nat. Rev. Cancer 2002, 7, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Kikuchi, M. Safety evaluation of proanthocyanidins-rich extract from grape seeds. Food Chem. Toxicol. 2002, 40, 599–607. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in grape seeds-biochemistry and functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Prieur, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry 1994, 36, 781–789. [Google Scholar] [CrossRef]
- Silva, R.C.; Rigaud, J.; Cheynier, V.; Chemina, A. Procyanidin dimers and trimers from grape seeds. Phytochemistry 1991, 30, 1259–1264. [Google Scholar] [CrossRef]
- Mantena, S.K.; Baliga, M.S.; Katiyar, S.K. Grape seed proanthocyanidins induce apoptosis and inhibit metastasis of highly metastatic breast carcinoma cells. Carcinogenesis 2006, 27, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Vayalil, P.K.; Mittal, A.; Katiyar, S.K. Proanthocyanidins from grape seeds inhibit expression of matrix metalloproteinases in human prostate carcinoma cells, which is associated with the inhibition of activation of MAPK and NFκB. Carcinogenesis 2004, 25, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Meeran, S.M.; Katiyar, S.K. Grape seed proanthocyanidins promote apoptosis in human epidermoid carcinoma A431 cells through alterations in Cdki-Cdk-cyclin cascade, loss of mitochondrial membrane potential, caspase-3 activation. Exp. Dermatol. 2007, 16, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Mantena, S.K.; Katiyar, S.K. Grape seed proanthocyanidins inhibit UV radiation-induced oxidative stress and activation of MAPK and NF-κB signaling in human epidermal keratinocytes. Free Radic. Biol. Med. 2006, 40, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Bagchi, M.; Stohs, S.; Ray, S.D.; Sen, C.K.; Preuss, H.G. Cellular protection with proanthocyanidins derived from grape seeds. Ann. N. Y. Acad. Sci. 2002, 957, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, V.; Singh, T.; Katiyar, S.K. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008, 269, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Katiyar, S.K. Polyphenols: Skin photoprotection and inhibition of photocarcinogenesis. Mini Rev. Med. Chem. 2011, 11, 1200–1215. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Meeran, S.M.; Katiyar, N.; Katiyar, S.K. Grape seed proanthocyanidins inhibit the growth of human non-small cell lung cancer xenografts by targeting IGFBP-3, tumor cell proliferation and angiogenic factors. Clin. Cancer Res. 2009, 15, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Elmets, C.A.; Katiyar, S.K. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: Relationship to decreased fat and lipid peroxidation. Carcinogenesis 2003, 24, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Meeran, S.M.; Vaid, M.; Punathil, T.; Katiyar, S.K. Dietary grape seed proanthocyanidins inhibit 12-O-tetradecanoyl phorbol-13-acetate-caused skin tumor promotion in 7,12-dimethylbenz[a]anthracene-initiated mouse skin, which is associated with the inhibition of inflammatory responses. Carcinogenesis 2009, 30, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Sharma, S.D.; Katiyar, S.K. Grape seed proanthocyanidins induce apoptosis by loss of mitochondrial membrane potential of human non-small cell lung cancer cells in vitro and in vivo. PLoS ONE 2011, 6, e27444. [Google Scholar] [CrossRef] [PubMed]
- Raina, K.; Singh, R.P.; Agarwal, R.; Agarwal, C. Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer Res. 2007, 67, 5976–5982. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Katiyar, S.K. Bioactive phytochemical proanthocyanidins inhibit growth of head and neck squamous cell carcinoma cells by targeting multiple signaling molecules. PLoS ONE 2012, 7, e46404. [Google Scholar] [CrossRef] [PubMed]
- Pavletich, N.P. Mechanisms of cyclin-dependent kinase regulation: Structures of CDKS, their cyclin activators, and CIP and INK4 inhibitors. J. Mol. Biol. 1999, 287, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Grana, X.; Reddy, P. Cell cycle control in mammalian cells: Role of cyclins, cyclin-dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CDKIs). Oncogene 1995, 11, 211–219. [Google Scholar] [PubMed]
- Hickman, J.A. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992, 11, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; McDonnell, J.M.; Korsmeyer, S.J. Bcl-2 family members and the mitochondria in apoptosis. Genes Dev. 1999, 13, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Hockenbery, D.; Nunez, G.; Milliman, C.; Schreiber, R.D.; Korsmeyer, S.J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 1990, 348, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.D.; Meeran, S.M.; Katiyar, S.K. Proanthocyanidins inhibit in vitro and in vivo growth of human non-small cell lung cancer cells by inhibiting the prostaglandin E2 and prostaglandin E2 receptors. Mol. Cancer Ther. 2010, 9, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Prasad, R.; Rosenthal, E.; Katiyar, S.K. Grape seed proanthocyanidins inhibit the invasive potential of human HNSCC cells by targeting EGFR and the epithelial-to-mesenchymal transition. PLoS ONE 2012, 7, e31093. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.A.; Azoitei, N.; Baumann, B.; Grünert, S.; Sommer, A.; Pehamberger, H.; Kraut, N.; Beug, H.; Wirth, T. NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Investig. 2004, 114, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Min, C.; Eddy, S.F.; Sherr, D.H.; Sonenshein, G.E. NF-κB and epithelial to mesenchymal transition of cancer. J. Cell Biochem. 2008, 104, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Chua, H.L.; Bhat-Nakshatri, P.; Clare, S.E.; Morimiya, A.; Badve, S.; Nakshatri, H. NF-κB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2. Oncogene 2007, 26, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Maier, H.J.; Wirth, T.; Beug, H. Epithelial-mesenchymal transition in pancreatic carcinoma. Cancers 2010, 2, 2058–2083. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.A.; Beug, H.; Wirth, T. Epithelial-mesenchymal transition: NF-κB takes center stage. Cell Cycle 2004, 3, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Prasad, R.; Rosenthal, E.; Katiyar, S.K. Grape seed proanthocyanidins inhibit the invasive potential of head and neck cutaneous squamous cell carcinoma cells by targeting EGFR expression and epithelial-to-mesenchymal transition. BMC Complement. Altern. Med. 2011, 11, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.R.; Arlotti, J.A.; Marynowski, S.W.; Singh, S.V. Honokiol, a constituent of oriental medicinal herb Magnolia officinalis, inhibits growth of PC-3 xenograft in vivo in association with apoptosis induction. Clin. Cancer Res. 2008, 14, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Cerimele, F.; Ushio-Fukai, M.; Waqas, M.; Campbell, P.M.; Govindarajan, B.; Der, C.J.; Battle, T.; Frank, D.A.; Ye, K.; et al. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J. Biol. Chem. 2003, 278, 35501–35507. [Google Scholar] [CrossRef] [PubMed]
- Munroe, M.E.; Arbiser, J.L.; Bishop, G.A. Honokiol, a natural plant product, inhibits inflammatory signals and alleviates inflammatory arthritis. J. Immunol. 2007, 179, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Prasad, R.; Katiyar, S.K. Inhibition of class I histone deacetylases in non-small cell lung cancer by honokiol leads to suppression of cancer cell growth and induction of cell death in vitro and in vivo. Epigenetics 2013, 8, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Vaid, M.; Sharma, S.D.; Katiyar, S.K. Honokiol, a phytochemical from the Magnolia plant, inhibits photocarcinogenesis by targeting UVB-induced inflammatory mediators and cell cycle regulators: Development of topical formulation. Carcinogenesis 2010, 31, 2004–2011. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Gupta, N.A.; Xu, S.; Prasad, R.; Velu, S.E.; Katiyar, S.K. Honokiol inhibits the growth of head and neck squamous cell carcinoma by targeting epidermal growth factor receptor. Oncotarget 2015, 6, 21268–21282. [Google Scholar] [CrossRef] [PubMed]
- Leeman-Neill, R.J.; Cai, Q.; Joyce, S.C.; Thomas, S.M.; Bhola, N.E.; Neill, D.B.; Arbiser, J.L.; Grandis, J.R. Honokiol inhibits epidermal growth factor receptor signaling and enhances the antitumor effects of epidermal growth factor receptor inhibitors. Clin. Cancer Res. 2011, 16, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Katiyar, S.K. Chemoprevention of photocarcinogenesis by selected dietary botanicals. Photochem. Photobiol. Sci. 2006, 5, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y. Fermentation of tea. In Green Tea, Health Benefits and Applications; Marcel Dekker: New York, NY, USA, 2001; pp. 16–21. [Google Scholar]
- Katiyar, S.K.; Mukhtar, H. Tea in chemoprevention of cancer: Epidemiologic and experimental studies (review). Int. J. Oncol. 1996, 8, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Ahmad, N.; Mukhtar, H. Green tea and skin. Arch. Dermatol. 2000, 136, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Agarwal, R.; Ekker, S.; Wood, G.S.; Mukhtar, H. Protection against 12-O-tetradecanoylphorbol-13-acetate-caused inflammation in SENCAR mouse ear skin by polyphenolic fraction isolated from green tea. Carcinogenesis 1993, 14, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Agarwal, R.; Zaim, M.T.; Mukhtar, H. Protection against N-nitrosodiethylamine and benzo[a]pyrene-induced forestomach and lung tumorigenesis in A/J mice by green tea. Carcinogenesis 1993, 14, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Agarwal, R.; Mukhtar, H. Protection against malignant conversion of chemically induced benign skin papillomas to squamous cell carcinomas in SENCAR mice by a polyphenolic fraction isolated from green tea. Cancer Res. 1993, 53, 5409–5412. [Google Scholar] [PubMed]
- Mantena, S.K.; Meeran, S.M.; Elmets, C.A.; Katiyar, S.K. Orally administered green tea polyphenols prevent ultraviolet radiation-induced skin cancer in mice through activation of cytotoxic T cells and inhibition of angiogenesis in tumors. J. Nutr. 2005, 135, 2871–2877. [Google Scholar] [PubMed]
- Meeran, S.M.; Mantena, S.K.; Elmets, C.A.; Katiyar, S.K. (−)-Epigallocatechin-3-gallate prevents photocarcinogenesis in mice through interleUKin-12-dependent DNA repair. Cancer Res. 2006, 66, 5512–5520. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.; Elmets, C.A.; Katiyar, S.K. Green tea and skin cancer: Photoimmunology, angiogenesis and DNA repair. J. Nutr. Biochem. 2007, 18, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Meeran, S.M.; Akhtar, S.; Katiyar, S.K. Inhibition of UVB-induced skin tumor development by drinking green tea polyphenols is mediated through DNA repair and subsequent inhibition of inflammation. J. Investig. Dermatol. 2009, 129, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Vaid, M.; van Steeg, H.; Meeran, S.M. Green tea polyphenols prevent UV-induced immunosuppression by rapid repair of DNA damage and enhancement of nucleotide excision repair genes. Cancer Prev. Res. 2010, 3, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Katiyar, S.K. Polyphenols from green tea inhibit the growth of melanoma cells through inhibition of class I histone deacetylases and induction of DNA damage. Genes Cancer 2015, 6, 49–61. [Google Scholar] [PubMed]
- Singh, T.; Katiyar, S.K. Green tea catechins reduce invasive potential of human melanoma cells by targeting COX-2, PGE2 receptors and epithelial-to-mesenchymal transition. PLoS ONE 2011, 6, e25224. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Suzui, M.; Weinstein, I.B. Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin. Cancer Res. 2001, 7, 4220–4229. [Google Scholar] [PubMed]
- Masuda, M.; Suzui, M.; Lim, J.T.; Deguchi, A.; Soh, J.W.; Weinstein, I.B. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J. Exp. Ther. Oncol. 2002, 2, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Lee, S.H.; Song, M.H.; Yamaguchi, K.; Yoon, J.H.; Choi, E.C.; Baek, S.J. Growth inhibition and apoptosis by (−)-epicatechin gallate are mediated by cyclin D1 suppression in head and neck squamous carcinoma cells. Eur. J. Cancer 2006, 42, 3260–3266. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Wakasaki, T.; Toh, S.; Shimizu, M.; Adachi, S. Chemoprevention of Head and Neck Cancer by Green Tea Extract: EGCG-The Role of EGFR Signaling and “Lipid Raft”. J. Oncol. 2011, 2011, 540148. [Google Scholar] [CrossRef] [PubMed]
- Vander Broek, R.; Snow, G.E.; Chen, Z.; van Waes, C. Chemoprevention of head and neck squamous cell carcinoma through inhibition of NF-κB signaling. Oral Oncol. 2014, 50, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.U.; Lee, B.S.; Lee, S.H.; Baek, S.J.; Shin, Y.S.; Kim, C.H. Expression of NSAID-activated gene-1 by EGCG in head and neck cancer: Involvement of ATM-dependent p53 expression. J. Nutr. Biochem. 2013, 24, 986–999. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, Z. Clinical trials in chemoprevention of head and neck cancers. Rev. Recent Clin. Trials 2012, 3, 249–254. [Google Scholar] [CrossRef]
- Piao, L.; Mukherjee, S.; Chang, Q.; Xie, X.; Li, H.; Castellanos, M.R.; Banerjee, P.; Iqbal, H.; Ivancic, R.; Wang, X.; et al. TriCurin, a novel formulation of curcumin, epicatechin gallate, and resveratrol, inhibits the tumorigenicity of human papillomavirus-positive head and neck squamous cell carcinoma. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Kalinovsky, T.; Roomi, N.W.; Niedzwiecki, A.; Rath, M. In vitro and in vivo inhibition of human Fanconi anemia head and neck squamous carcinoma by a phytonutrient combination. Int. J. Oncol. 2015, 46, 2261–2266. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Kalinovsky, T.; Roomi, N.W.; Niedzwiecki, A.; Rath, M. In vitro and in vivo inhibition of human Fanconi anemia-associated head and neck squamous cell carcinoma by a novel nutrient mixture. Int. J. Oncol. 2012, 41, 1996–2004. [Google Scholar] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [PubMed]
- Holt, R.R.; Lazarus, S.A.; Sullards, M.C.; Zhu, Q.Y.; Schramm, D.D.; Hammerstone, J.F.; Fraga, C.G.; Schmitz, H.H.; Keen, C.L. Procyanidin dimer B2 [epicatechin-(4β-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am. J. Clin. Nutr. 2002, 76, 798–804. [Google Scholar] [PubMed]
- Sano, A.; Yamakoshi, J.; Tokutake, S.; Tobe, K.; Kubota, Y.; Kikuchi, M. Procyanidin B1 is detected in human serum after intake of proanthocyanidin-rich grape seed extract. Biosci. Biotechnol. Biochem. 2003, 67, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.Y.; Bennett, R.N.; Lazarus, S.A.; Remesy, C.; Scalbert, A.; Williamson, G. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2002, 76, 1106–1110. [Google Scholar] [PubMed]
- Church, R.J.; Gatti, D.M.; Urban, T.J.; Long, N.; Yang, X.; Shi, Q.; Eaddy, J.S.; Mosedale, M.; Ballard, S.; Churchill, G.A.; et al. Sensitivity to hepatotoxicity due to epigallocatechin gallate is affected by genetic background in diversity outbred mice. Food Chem. Toxicol. 2015, 76, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Yu, X.; Guo, Y.; Wang, Y.; Kuang, H.; Wang, X. Honokiol nanosuspensions: Preparation, increased oral bioavailability and dramatically enhanced biodistribution in the cardio-cerebro-vascular system. Colloids Surf. B Biointerfaces 2014, 116, 114–120. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katiyar, S.K. Emerging Phytochemicals for the Prevention and Treatment of Head and Neck Cancer. Molecules 2016, 21, 1610. https://doi.org/10.3390/molecules21121610
Katiyar SK. Emerging Phytochemicals for the Prevention and Treatment of Head and Neck Cancer. Molecules. 2016; 21(12):1610. https://doi.org/10.3390/molecules21121610
Chicago/Turabian StyleKatiyar, Santosh K. 2016. "Emerging Phytochemicals for the Prevention and Treatment of Head and Neck Cancer" Molecules 21, no. 12: 1610. https://doi.org/10.3390/molecules21121610
APA StyleKatiyar, S. K. (2016). Emerging Phytochemicals for the Prevention and Treatment of Head and Neck Cancer. Molecules, 21(12), 1610. https://doi.org/10.3390/molecules21121610




