Synthesis and Antifungal Screening of 2-{[1-(5-Alkyl/arylalkylpyrazin-2-yl)ethylidene]hydrazono}-1,3-thiazolidin-4-ones †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biology
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Preparation of Thiosemicarbazones 10a–10h
3.1.2. General Procedure for the Cyclization of Thiosemicarbazones 10a–10h to 1,3-Thiazolidin-4-ones 11a–11h
3.2. Biology
Evaluation of In Vitro Antifungal Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Casadevall, A. The Emergence of Invasive Fungal Diseases among Humans. Available online: http://www.healio.com/infectious-disease/vaccine-preventable-diseases/news/print/infectious-disease-news/%7B2c458852-25bc-4818-a020-b3dfd61b9d06%7D/the-emergence-of-invasive-fungal-diseases-among-humans (accessed on 28 September 2016).
- Kontoyiannis, D. Emerging Resistance, Continuous Progress in Antifungal Drug Development. Available online: http://www.healio.com/infectious-disease/antimicrobials/news/print/infectious-disease-news/%7B2ba9bc1e-4639-41e4-81c7-07654f0eb25d%7D/emerging-resistance-continuous-progress-in-antifungal-drug-development (accessed on 28 September 2016).
- Sundriyal, S.; Sharma, R.K.; Jain, R. Current advances in antifungal targets and drug development. Curr. Med. Chem. 2006, 13, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Kollipara, R.; Peranteau, A.J.; Nawas, Z.Y.; Tong, Y.; Woc-Colburn, L.; Yan, A.C.; Lupi, O.; Tyring, S.K. Emerging infectious diseases with cutaneous manifestations fungal, helminthic, protozoan and ectoparasitic infections. J. Am. Acad. Dermatol. 2016, 75, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Bojsen, R.; Regenberg, B.; Folkesson, A. Persistence and drug tolerance in pathogenic yeast. Curr. Genet. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D. Emerging threats in antifungal-resistant fungal pathogens. Front. Med. 2016, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, A.; Kolaczkowski, M. Drug resistance mechanisms and their regulation in non-albicans Candida species. J. Antimicrob. Chemother. 2016, 71, 1438–1450. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Daigle, D.; Carviel, J.L. The role of biofilms in onychomycosis. J. Am. Acad. Dermatol. 2016, 74, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P.; Patterson, T.F. What's new in antifungals: An update on the in vitro activity and in vivo efficacy of new and investigational antifungal agents. Curr. Opin. Infect. Dis. 2015, 28, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Rosa, E.A. Editorial: Antifungal drug discovery: New theories and new therapies. Front. Microbiol. 2016, 7, 728. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Studholme, C. Novel investigational therapies for onychomycosis: An update. Expert Opin. Investig. Drugs 2016, 25, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Brown, F.C. 4-Thiazolidinones. Chem. Rev. 1961, 61, 463–521. [Google Scholar] [CrossRef]
- Singh, S.P.; Parmar, S.S.; Raman, K.; Stenberg, V.I. Chemistry and biological activity of thiazolidinones. Chem. Rev. 1981, 81, 175–203. [Google Scholar] [CrossRef]
- Lesyk, R.B.; Zimenkovsky, B.S. 4-Thiazolidones: Centenarian history, current status and perspectives for modern organic and medicinal chemistry. Curr. Org. Chem. 2004, 8, 1547–1577. [Google Scholar] [CrossRef]
- Hamama, W.S.; Ismail, M.A.; Shaaban, S.; Zoorob, H.H. Progress in the chemistry of 4-thiazolidinones. J. Heterocycl. Chem. 2008, 45, 939–956. [Google Scholar] [CrossRef]
- Verma, A.; Saraf, S.K. 4-Thiazolidinone: A biologically active scaffold. Eur. J. Med. Chem. 2008, 43, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Vaidya, A.; Ravichandran, V.; Kashaw, S.K.; Agrawal, R.K. Recent developments and biological activities of thiazolidinone derivatives: A review. Bioorg. Med. Chem. 2012, 20, 3378–3395. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.C.; Gupta, S.J.; Fatima, G.N.; Sonar, P.K.; Verma, A.; Saraf, S.K. 4-Thiazolidinones: The advances continue. Eur. J. Med. Chem. 2014, 72, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Mashrai, A.; Dar, A.M.; Mir, S.; Shamsuzzaman. Strategies for the synthesis of thiazolidinone heterocycles. Med. Chem. (Los Angeles) 2016, 6, 280–291. [Google Scholar] [CrossRef]
- Opletalova, V.; Kalinowski, D.S.; Vejsova, M.; Kunes, J.; Pour, M.; Jampilek, J.; Buchta, V.; Richardson, D.R. Identification and characterization of thiosemicarbazones with antifungal and antitumor effects: Cellular iron chelation mediating cytotoxic activity. Chem. Res. Toxicol. 2008, 21, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Opletalova, V.; Dolezel, J. Thiosemicarbazones and their antimycobacterial effects. Ceska Slov. Farm. 2013, 62, 78–83. [Google Scholar] [PubMed]
- Vontor, T.; Palat, K.; Oswald, J.; Odlerova, Z. Antituberculotics. XXXII. Functional derivatives of 5-methyl-2-pyrazinecarboxylic acid. Cesk. Farm. 1985, 34, 74–78. [Google Scholar]
- Vontor, T.; Palat, K.; Odlerova, Z. Antituberculotics XLI. Functional derivatives of 5-alkyl-2-pyrazinecarboxylic acid. Cesk. Farm. 1987, 36, 277–280. [Google Scholar]
- Dlabal, K.; Palat, K.; Lycka, A.; Odlerova, Z. Synthesis and 1H and 13C NMR spectra of sulfur derivatives of pyrazine derived from amidation product of 2-chloropyrazine and 6-chloro-2-pyrazinecarbonitrile. Tuberculostatic activity. Collect. Czech. Chem. Commun. 1990, 55, 2493–2501. [Google Scholar] [CrossRef]
- Krinkova, J.; Dolezal, M.; Hartl, J.; Buchta, V.; Pour, M. Synthesis and biological activity of 5-alkyl-6-(alkylsulfanyl)- or 5-alkyl-6-(arylsulfanyl)pyrazine-2-carboxamides and corresponding thioamides. Farmaco 2002, 57, 71–78. [Google Scholar] [CrossRef]
- Dolezal, M.; Zitko, J.; Osicka, Z.; Kunes, J.; Vejsova, M.; Buchta, V.; Dohnal, J.; Jampilek, J.; Kralova, K. Synthesis, antimycobacterial, antifungal and photosynthesis-inhibiting activity of chlorinated N-phenylpyrazine-2-carboxamides. Molecules 2010, 15, 8567–8581. [Google Scholar] [CrossRef] [PubMed]
- Servusova, B.; Eibinova, D.; Dolezal, M.; Kubicek, V.; Paterova, P.; Pesko, M.; Kralova, K. Substituted N-benzylpyrazine-2-carboxamides: Synthesis and biological evaluation. Molecules 2012, 17, 13183–13198. [Google Scholar] [CrossRef] [PubMed]
- Zitko, J.; Servusova, B.; Paterova, P.; Mandikova, J.; Kubicek, V.; Kucera, R.; Hrabcova, V.; Kunes, J.; Soukup, O.; Dolezal, M. Synthesis, Antimycobacterial Activity and In Vitro Cytotoxicity of 5-Chloro-N-phenylpyrazine-2-carboxamides. Molecules 2013, 18, 14807–14825. [Google Scholar] [CrossRef] [PubMed]
- Jandourek, O.; Dolezal, M.; Kunes, J.; Kubicek, V.; Paterova, P.; Pesko, M.; Buchta, V.; Kralova, K.; Zitko, J. New potentially active pyrazinamide derivatives synthesized under microwave conditions. Molecules 2014, 19, 9318–9338. [Google Scholar] [CrossRef] [PubMed]
- Semelkova, L.; Konecna, K.; Paterova, P.; Kubicek, V.; Kunes, J.; Novakova, L.; Marek, J.; Naesens, L.; Pesko, M.; Kralova, K.; et al. Synthesis and Biological Evaluation of N-Alkyl-3-(alkylamino)-pyrazine-2-carboxamides. Molecules 2015, 20, 8687–8711. [Google Scholar] [CrossRef] [PubMed]
- Opletalova, V.; Domonhedo, C. Methods for preparation of acetylpyrazines. Chem. Listy 1999, 93, 15–18. [Google Scholar]
- Ried, W.; Russ, T. Homolytic acylation of methyl 3-amino-2-pyrazinecarboxylates. Synthesis 1991, 581–582. [Google Scholar] [CrossRef]
- Opletalova, V.; Hartl, J.; Patel, A.; Boulton, M. Homolytic acetylation of 2,5-dimethylpyrazine. Collect. Czech. Chem. Commun. 1995, 60, 1551–1554. [Google Scholar] [CrossRef]
- Fontana, F.; Minisci, F.; Nogueira Barbosa, M.C.; Vismara, E. Homolytic acylation of protonated pyridines and pyrazines with α-keto acids: The problem of monoacylation. J. Org. Chem. 1991, 56, 2866–2869. [Google Scholar] [CrossRef]
- Sato, N.; Kadota, H. Studies on pyrazine. 23. Homolytic acylation of 2-amino-3-cyanopyrazine and related compounds with α-keto acids: A synthesis of 5-acyl-3-aminopyrazinecarboxylic acid derivatives. J. Heterocycl. Chem. 1992, 29, 1685–1688. [Google Scholar] [CrossRef]
- Punta, C.; Minisci, F. Minisci reaction: A friedel-crafts type process with opposite reactivity and selectivity. Selective homolytic alkylation, acylation, carboxylation and carbamoylation of heterocyclic aromatic bases. Trends Heterocycl. Chem. 2008, 13, 1–68. [Google Scholar] [CrossRef]
- Opletalova, V.; Hartl, J.; Domonhedo, C.; Patel, A. Homolytic acetylation of 2-pyrazinecarbonitrile. Folia Pharm. Univ. Carol. 1999, 24, 29–32. [Google Scholar]
- Kucerova-Chlupacova, M.; Kunes, J.; Buchta, V.; Vejsova, M.; Opletalova, V. Novel pyrazine analogs of chalcones: Synthesis and evaluation of their antifungal and antimycobacterial activity. Molecules 2015, 20, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
- Kucerova-Chlupacova, M.; Vyskovska-Tyllova, V.; Richterova-Finkova, L.; Kunes, J.; Buchta, V.; Vejsova, M.; Paterova, P.; Semelkova, L.; Jandourek, O.; Opletalova, V. Novel halogenated pyrazine-based chalcones as potential antimicrobial drugs. Molecules 2016, 21, 1421. [Google Scholar] [CrossRef] [PubMed]
- Brown, F.C.; Bradsher, C.K.; Bond, S.M. Mildew-preventing activity of rhodanine derivatives. Some 5-arylidene derivatives. Ind. Eng. Chem. 1953, 45, 1030–1033. [Google Scholar] [CrossRef]
- Bluestone, H. The Use of Cyclic, Nitrogen- and Sulfur-Containing Compounds as Fungicides. German Patent DE1019122B, 7 November 1957. [Google Scholar]
- Junghaehnel, R.; Renckhoff, G.; Thewalt, K. Bacteriostat and Fungistat Compositions Containing N,S-Heterocyclic Compounds. U.S. Patent US3681496A, 1 August 1972. [Google Scholar]
- Legocki, J.; Matysiak, J.; Niewiadomy, A.; Kostecka, M. Synthesis and fungistatic activity of new groups of 2,4-dihydroxythiobenzoyl derivatives against phytopathogenic fungi. J. Agric. Food Chem. 2003, 51, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Kostecka, M. Synthesis of a new group of aliphatic hydrazide derivatives and the correlations between their molecular structure and biological activity. Molecules 2012, 17, 3560–3573. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, K.; Sharma, M.; Singh, P.; Kumar, V.; Shukla, P.K.; Siddiqi, M.I.; Chauhan, P.M.S. Discovery of a new class of dithiocarbamates and rhodanine scaffolds as potent antifungal agents: Synthesis, biology and molecular docking. Med. Chem. Commun. 2012, 3, 1104–1110. [Google Scholar] [CrossRef]
- Zou, Y.; Yu, S.; Li, R.; Zhao, Q.; Li, X.; Wu, M.; Huang, T.; Chai, X.; Hu, H.; Wu, Q. Synthesis, antifungal activities and molecular docking studies of novel 2-(2,4-difluorophenyl)-2-hydroxy-3-(1H-1,2,4-triazol-1-yl)propyl dithiocarbamates. Eur. J. Med. Chem. 2014, 74, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Wiles, D.M.; Suprunchuk, T. Antifungal activity of the thiosemicarbazones of some heterocyclic aldehydes. J. Med. Chem. 1971, 14, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Addor, R.W.; Lamb, G. Thiosemicarbazone Fungicides. U.S. Patent US3824317A, 16 July 1974. [Google Scholar]
- Liberta, A.E.; West, D.X. Antifungal and antitumor activity of heterocyclic thiosemicarbazones and their metal complexes: Current status. Biometals 1992, 5, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Reis, D.C.; Despaigne, A.A.R.; Da Silva, J.G.; Silva, N.F.; Vilela, C.F.; Mendes, I.C.; Takahashi, J.A.; Beraldo, H. Structural studies and investigation on the activity of imidazole-derived thiosemicarbazones and hydrazones against crop-related fungi. Molecules 2013, 18, 12645–12662. [Google Scholar] [CrossRef] [PubMed]
- Degola, F.; Morcia, C.; Bisceglie, F.; Mussi, F.; Tumino, G.; Ghizzoni, R.; Pelosi, G.; Terzi, V.; Buschini, A.; Restivo, F.M.; et al. In vitro evaluation of the activity of thiosemicarbazone derivatives against mycotoxigenic fungi affecting cereals. Int. J. Food Microbiol. 2015, 200, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Altintop, M.D.; Atli, O.; Ilgin, S.; Demirel, R.; Ozdemir, A.; Kaplancikli, Z.A. Synthesis and biological evaluation of new naphthalene substituted thiosemicarbazone derivatives as potent antifungal and anticancer agents. Eur. J. Med. Chem. 2016, 108, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Meher, S.S.; Naik, S.; Behera, R.K.; Nayak, A. Studies on thiazolidinones. Part XI: Synthesis and fungitoxicities of thiazolidinones, thiohydantoins and their derivatives derived from thiosemicarbazones. J. Indian Chem. Soc. 1981, 58, 274–276. [Google Scholar] [CrossRef]
- Naik, H.; Naik, S.K.; Meher, S.S.; Nayak, A. Studies on thiazolidinones. Part XIII: Synthesis and antimicrobial activities of thiazolidinones and their derivatives possessing, oxadiazole and isothiazole moieties. J. Indian Chem. Soc. 1983, 60, 674–678. [Google Scholar]
- Mohan, J.; Chadha, V.K.; Chaudhary, H.S.; Sharma, B.D.; Pujari, H.K.; Mohapatra, L.N. Heterocyclic systems containing bridgehead nitrogen atom. XIII. Antifungal and antibacterial activities of thiazole and thiazolidinone derivatives. Indian J. Exp. Biol. 1972, 10, 37–40. [Google Scholar] [PubMed]
- Kumar, D.; Sharma, R.C. Synthesis and Antimicrobial Activity of Some New 4-Thiazolidinones Derived from Heterocyclic Schiff Bases. J. Indian Chem. Soc. 2002, 79, 284–285. [Google Scholar] [CrossRef]
- Nizami, S.A.; Gurumurthy, M.; Chattarjee, S.J.; Panda, D. Evaluation of antimicrobial potency of some synthesized thiazolidin-4-one substituted 1,2,4-triazoles. J. Adv. Pharm. Res. 2010, 1, 26–35. [Google Scholar]
- Pan, B.; Huang, R.-Z.; Han, S.-Q.; Qu, D.; Zhu, M.-L.; Wei, P.; Ying, H.-J. Design, synthesis, and antibiofilm activity of 2-arylimino-3-aryl-thiazolidine-4-ones. Bioorg. Med. Chem. Lett. 2010, 20, 2461–2464. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Huang, R.; Zheng, L.; Chen, C.; Han, S.; Qu, D.; Zhu, M.; Wei, P. Thiazolidione derivatives as novel antibiofilm agents: Design, synthesis, biological evaluation, and structure-activity relationships. Eur. J. Med. Chem. 2011, 46, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Panzariu, A.-T.; Apotrosoaei, M.; Vasincu, I.M.; Dragan, M.; Constantin, S.; Buron, F.; Routier, S.; Profire, L.; Tuchilus, C. Synthesis and biological evaluation of new 1,3-thiazolidine-4-one derivatives of nitro-l-arginine methyl ester. Chem. Cent. J. 2016, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- De Monte, C.; Carradori, S.; Bizzarri, B.; Bolasco, A.; Caprara, F.; Mollica, A.; Rivanera, D.; Mari, E.; Zicari, A.; Akdemir, A.; et al. Anti-candida activity and cytotoxicity of a large library of new N-substituted-1,3-thiazolidin-4-one derivatives. Eur. J. Med. Chem. 2016, 107, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Secci, D.; Carradori, S.; Bizzarri, B.; Chimenti, P.; De Monte, C.; Mollica, A.; Rivanera, D.; Zicari, A.; Mari, E.; Zengin, G.; et al. Novel 1,3-thiazolidin-4-one derivatives as promising anti-Candida agents endowed with anti-oxidant and chelating properties. Eur. J. Med. Chem. 2016, 117, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.H.; Xu, Y.Y.; Yang, Y.S.; Lin, G.L. A facile synthesis and antimicrobial activity evaluation of sydnonyl-substituted thiazolidine derivatives. Molecules 2015, 20, 6520–6532. [Google Scholar] [CrossRef] [PubMed]
- Hozien, Z.A. Synthesis of some new heterocyclic systems derived from 2-acetylbenzimidazole. J. Chem. Technol. Biotechnol. 1993, 57, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Opletalova, V.; Hartl, J.; Patel, A.; Palat, K., Jr.; Buchta, V. Ring substituted 3-phenyl-1-(2-pyrazinyl)-2-propen-1-ones as potential photosynthesis-inhibiting, antifungal and antimycobacterial agents. Farmaco 2002, 57, 135–144. [Google Scholar] [CrossRef]
- Opletalova, V.; Pour, M.; Kunes, J.; Buchta, V.; Silva, L.; Kralova, K.; Chlupacova, M.; Meltrova, D.; Peterka, M.; Poslednikova, M. Synthesis and biological evaluation of (E)-3-(Nitrophenyl)-1-(pyrazin-2-yl)prop-2-en-1-ones. Collect. Czech. Chem. Commun. 2006, 71, 44–58. [Google Scholar] [CrossRef]
- Paramythiotou, E.; Frantzeskaki, F.; Flevari, A.; Armaganidis, A.; Dimopoulos, G. Invasive fungal infections in the ICU: How to approach, how to treat. Molecules 2014, 19, 1085–1119. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Ananda-Rajah, M.; Andes, D.; Arendrup, M.C.; Bruggemann, R.J.; Chowdhary, A.; Cornely, O.A.; Denning, D.W.; Groll, A.H.; Izumikawa, K.; et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist. Updat. 2015, 21–22, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.; Casadevall, A. Cryptococcal therapies and drug targets: The old, the new and the promising. Cell Microbiol. 2016, 18, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, C.; Su, H.; Zhang, W.; Sheng, C. Strategies in the discovery of novel antifungal scaffolds. Future Med. Chem. 2016, 8, 1435–1454. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.H.; Diaz, J.A.; Lee, S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef]
- Rodriguez-Gutierrez, G.; Carrillo-Casas, E.M.; Arenas, R.; Garcia-Mendez, J.O.; Toussaint, S.; Moreno-Morales, M.E.; Schcolnik-Cabrera, A.A.; Xicohtencatl-Cortes, J.; Hernandez-Castro, R. Mucormycosis in a non-Hodgkin lymphoma patient caused by Syncephalastrum racemosum: Case report and review of literature. Mycopathologia 2015, 180, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Perez-Torrado, R.; Querol, A. Opportunistic strains of Saccharomyces cerevisiae: A potential risk sold in food products. Front. Microbiol. 2016, 6, 1522. [Google Scholar] [CrossRef] [PubMed]
- Dioverti, M.V.; Cawcutt, K.A.; Abidi, M.; Sohail, M.R.; Walker, R.C.; Osmon, D.R. Gastrointestinal mucormycosis in immunocompromised hosts. Mycoses 2015, 58, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, L.; Bednarova, D.; Ruzicka, F.; Chrenkova, V.; Dobias, R.; Mallatova, N.; Buchta, V.; Kocmanova, I.; Olisarova, P.; Stromerova, N.; et al. High frequency of Candida fabianii among clinical isolates biochemically identified as Candida pelliculosa and Candida utilis. Mycoses 2016, 59, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Glockner, A.; Cornely, O.A. Candida glabrata: Unique features and challenges in the clinical management of invasive infections. Mycoses 2015, 58, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Bolotin-Fukuhara, M.; Fairhead, C. Candida glabrata: A deadly companion? Yeast 2014, 31, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Perlin, D.S. Echinocandin resistance: An emerging clinical problem? Curr. Opin. Infect. Dis. 2014, 27, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Nguyen, M.H.; Clancy, C.J. Clinical perspectives on echinocandin resistance among Candida species. Curr. Opin. Infect. Dis. 2015, 28, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Krogh-Madsen, M.; Arendrup, M.C.; Heslet, L.; Knudsen, J.D. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin. Infect. Dis. 2006, 42, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Opletalova, V.; Patel, A.; Boulton, M.; Dundrova, A.; Lacinova, E.; Prevorova, M.; Appeltauerova, M.; Coufalova, M. 5-alkyl-2-pyrazinecarboxamides, 5-alkyl-2-pyrazinecarbonitriles and 5-alkyl-2-acetylpyrazines as synthetic intermediates for antiinflammatory agents. Collect. Czech. Chem. Commun. 1996, 61, 1093–1101. [Google Scholar] [CrossRef]
- Kucerova-Chlupacova, M.; Opletalova, V.; Jampilek, J.; Dolezel, J.; Dohnal, J.; Pour, M.; Kunes, J.; Vorisek, V. New hydrophobicity constants of substituents in pyrazine rings derived from RP-HPLC study. Collect. Czech. Chem. Commun. 2008, 73, 1–18. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 10g, 10h and 11a–11h are available from the authors.

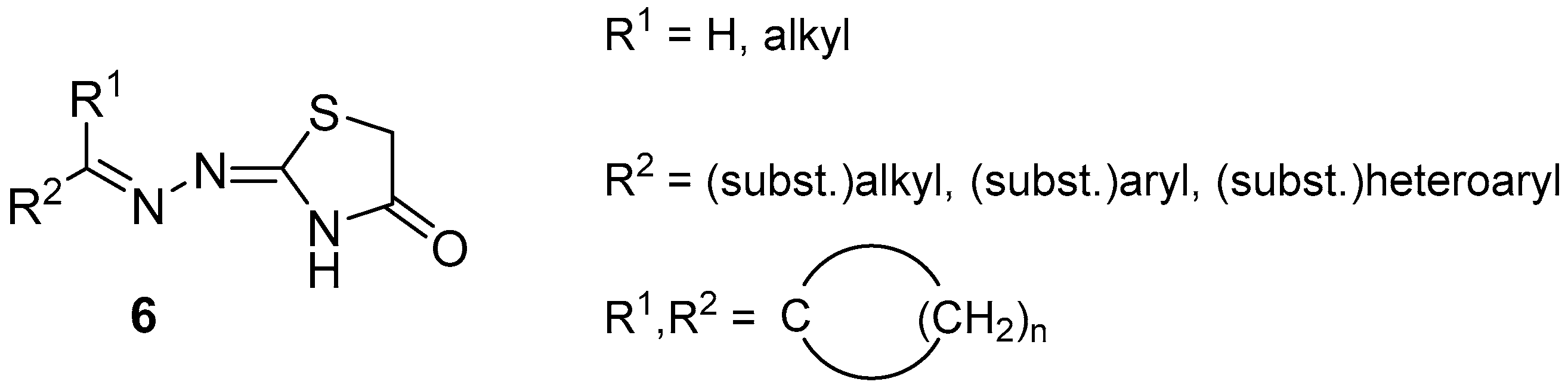
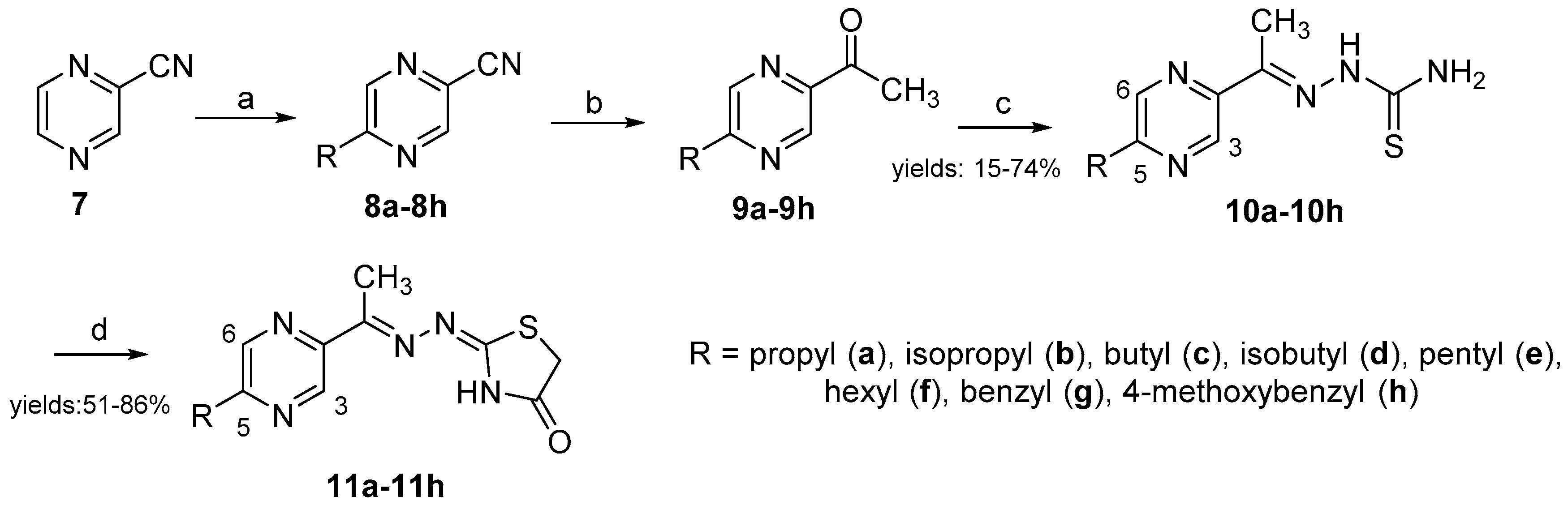
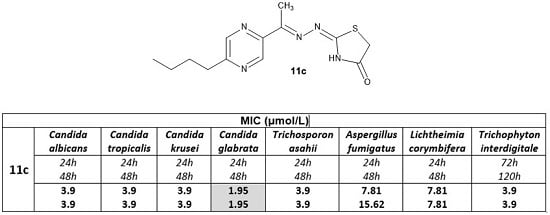
| Compound | MIC (mg/L) 1 | |||||||
| CA | CT | CK | CG | TA | AF | LC | TI | |
| 24 h | 24 h | 24 h | 24 h | 24 h | 24 h | 24 h | 72 h | |
| 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | 48 h | 120 h | |
| 10g | ˃142.69 | ˃142.69 | 8.92 | 4.46 | 4.46 | 17.84 | 35.67 | 8.92 |
| ˃142.69 | ˃142.69 | 8.92 | 4.46 | 4.46 | 17.84 | 35.67 | 8.92 | |
| 10h | ˃157.70 | ˃157.70 | 19.71 | 4.93 | 19.71 | 39.42 | 19.71 | 4.93 |
| ˃157.70 | ˃157.70 | 19.71 | 4.93 | 19.71 | 39.42 | 19.71 | 4.93 | |
| 11a | 2.17 | 2.17 | 2.17 | 1.08 | 2.17 | 4.33 | 4.33 | 2.17 |
| 2.17 | 2.17 | 2.17 | 1.08 | 2.17 | 8.67 | 4.33 | 2.17 | |
| 11b | 1.08 | 2.17 | 2.17 | 1.08 | 2.17 | 17.33 | 8.67 | 2.17 |
| 1.08 | 4.33 | 4.33 | 1.08 | 2.17 | 17.33 | 17.33 | 2.17 | |
| 11c | 1.14 | 1.14 | 1.14 | 0.57 | 1.14 | 2.28 | 2.28 | 1.14 |
| 1.14 | 1.14 | 1.14 | 0.57 | 1.14 | 4.55 | 2.28 | 1.14 | |
| 11d | 4.55 | ˃36.42 | ˃36.42 | 2.28 | 2.28 | ˃36.42 | 9.11 | 9.11 |
| 9.11 | ˃36.42 | ˃36.42 | 2.28 | 2.28 | ˃36.42 | 9.11 | 9.11 | |
| 11e | 2.39 | 2.39 | 2.39 | 2.39 | 2.39 | 2.39 | 2.39 | 2.39 |
| 2.39 | 2.39 | 2.39 | 2.39 | 2.39 | 2.39 | 9.54 | 2.39 | |
| 11f | 9.98 | ˃39.93 | ˃39.93 | 1.25 | 2.49 | 39.93 | 39.93 | ˃39.93 |
| 9.98 | ˃39.93 | ˃39.93 | 1.25 | 2.49 | ˃39.93 | 39.93 | ˃39.93 | |
| 11g | 2.54 | 5.08 | 5.08 | 2.54 | 5.08 | ˃40.67 | ˃40.67 | 10.17 |
| 5.08 | 10.17 | 5.08 | 2.54 | 5.08 | ˃40.67 | ˃40.67 | 20.34 | |
| 11h | 2.78 | 5.55 | 5.55 | 2.78 | 2.78 | 22.21 | 11.11 | 5.55 |
| 2.78 | 11.11 | 5.55 | 2.78 | 2.78 | 44.43 | 22.21 | 5.55 | |
| FLU | 0.07 | ˃153.14 | 38.29 | 12.76 | 76.57 | ˃153.14 | ˃153.14 | 1.99 |
| 0.07 | ˃153.14 | 76.57 | 76.57 | 76.57 | ˃153.14 | ˃153.14 | 31.85 | |
| VOR | 0.002 | 43.67 | 0.23 | 29.2 | 1.14 | 0.17 | 72.66 | 0.03 |
| 0.002 | 87.33 | 0.68 | 87.33 | 5.00 | 0.45 | 87.33 | 0.04 | |
| AmpB | 0.03 | 0.08 | 0.13 | 0.03 | 1.00 | 0.17 | 1.00 | 1.00 |
| 0.06 | 0.10 | 0.17 | 0.08 | 1.66 | 0.21 | 2.00 | 1.00 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opletalova, V.; Dolezel, J.; Kunes, J.; Buchta, V.; Vejsova, M.; Kucerova-Chlupacova, M. Synthesis and Antifungal Screening of 2-{[1-(5-Alkyl/arylalkylpyrazin-2-yl)ethylidene]hydrazono}-1,3-thiazolidin-4-ones. Molecules 2016, 21, 1592. https://doi.org/10.3390/molecules21111592
Opletalova V, Dolezel J, Kunes J, Buchta V, Vejsova M, Kucerova-Chlupacova M. Synthesis and Antifungal Screening of 2-{[1-(5-Alkyl/arylalkylpyrazin-2-yl)ethylidene]hydrazono}-1,3-thiazolidin-4-ones. Molecules. 2016; 21(11):1592. https://doi.org/10.3390/molecules21111592
Chicago/Turabian StyleOpletalova, Veronika, Jan Dolezel, Jiri Kunes, Vladimir Buchta, Marcela Vejsova, and Marta Kucerova-Chlupacova. 2016. "Synthesis and Antifungal Screening of 2-{[1-(5-Alkyl/arylalkylpyrazin-2-yl)ethylidene]hydrazono}-1,3-thiazolidin-4-ones" Molecules 21, no. 11: 1592. https://doi.org/10.3390/molecules21111592
APA StyleOpletalova, V., Dolezel, J., Kunes, J., Buchta, V., Vejsova, M., & Kucerova-Chlupacova, M. (2016). Synthesis and Antifungal Screening of 2-{[1-(5-Alkyl/arylalkylpyrazin-2-yl)ethylidene]hydrazono}-1,3-thiazolidin-4-ones. Molecules, 21(11), 1592. https://doi.org/10.3390/molecules21111592