Temperature-Triggered Switchable Helix-Helix Inversion of Poly(phenylacetylene) Bearing l-Valine Ethyl Ester Pendants and Its Chiral Recognition Ability
Abstract
:1. Introduction
2. Results and Discussion
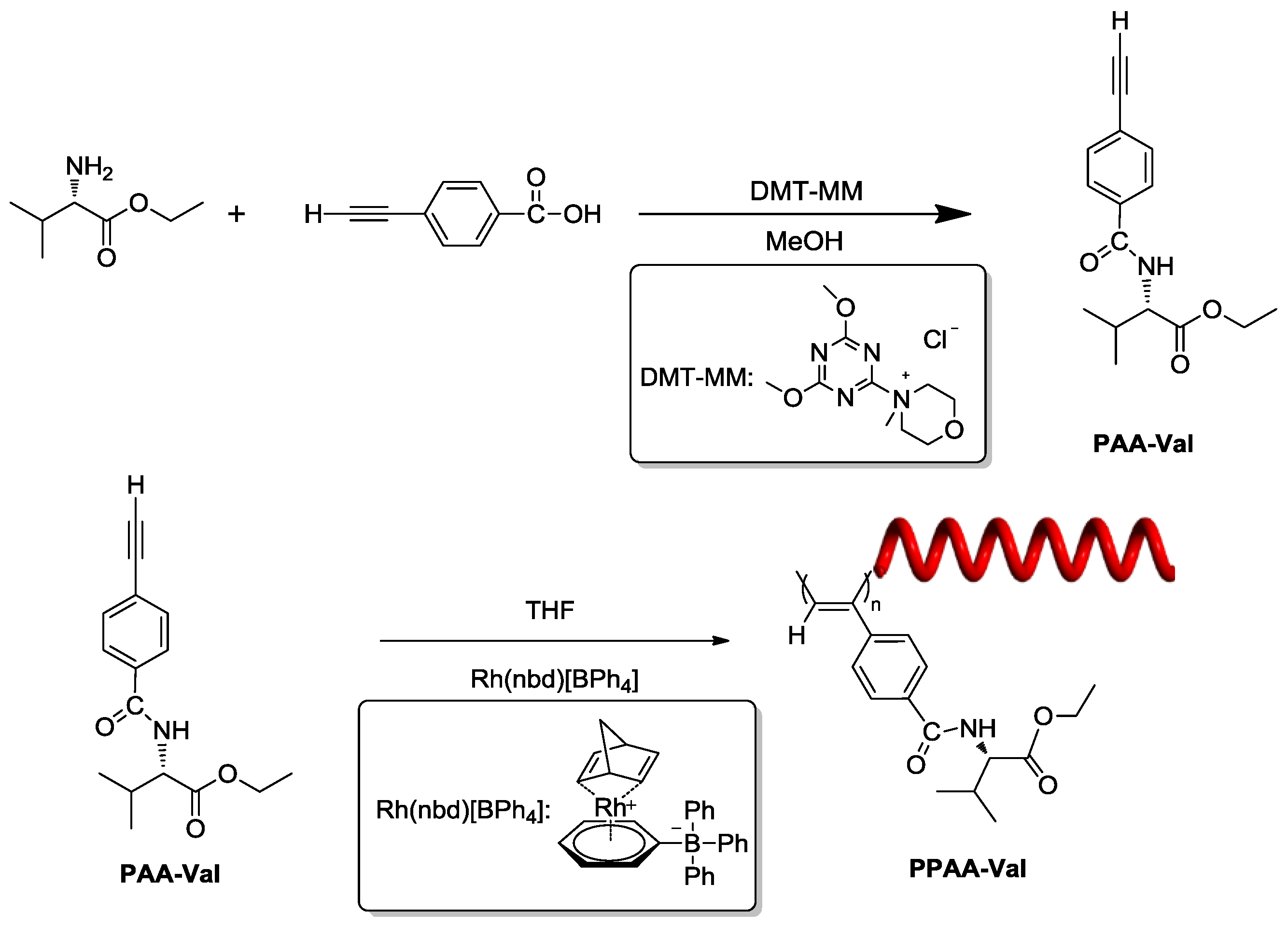
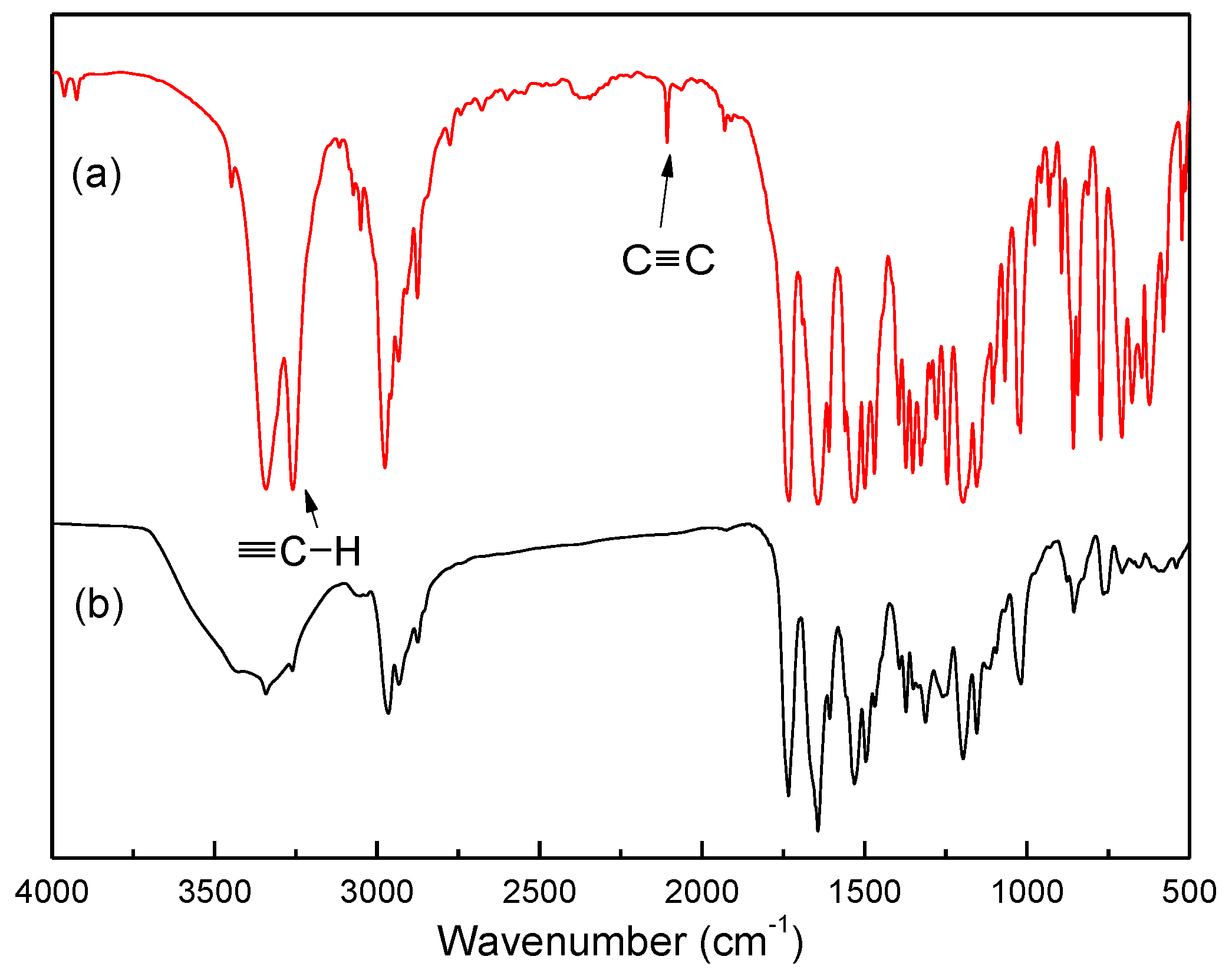
2.1. Synthesis and Polymerization of PAA-Val
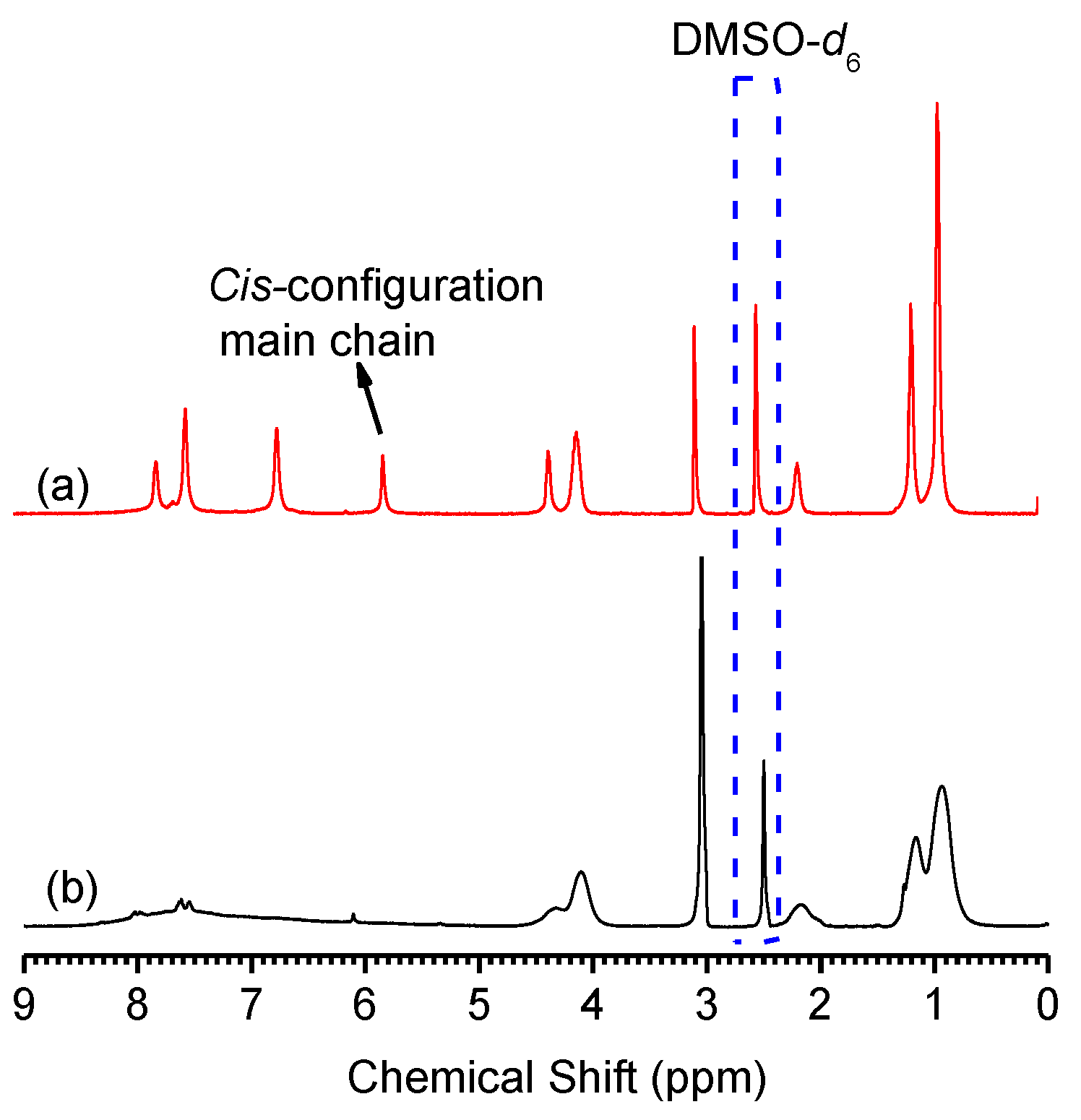
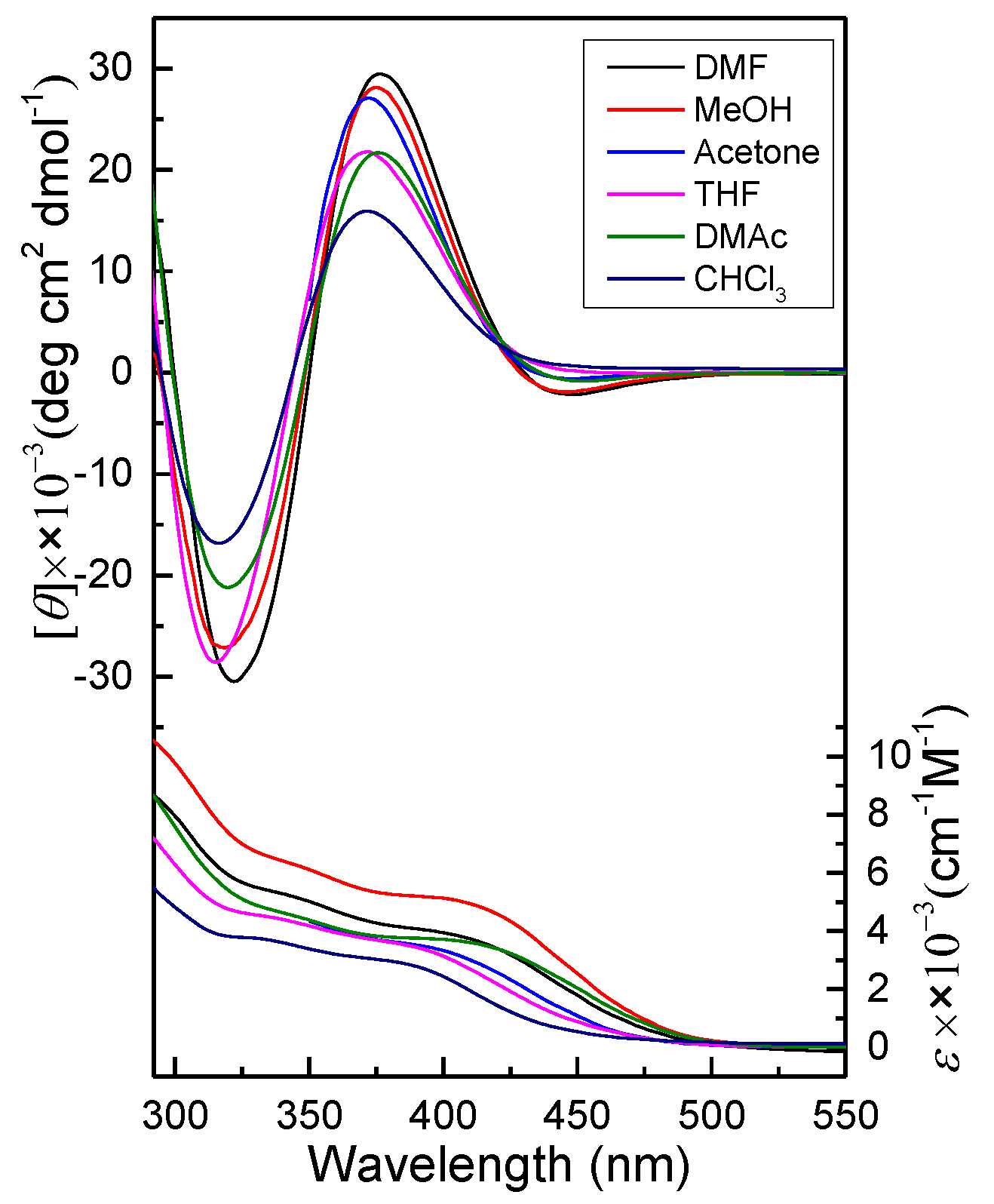
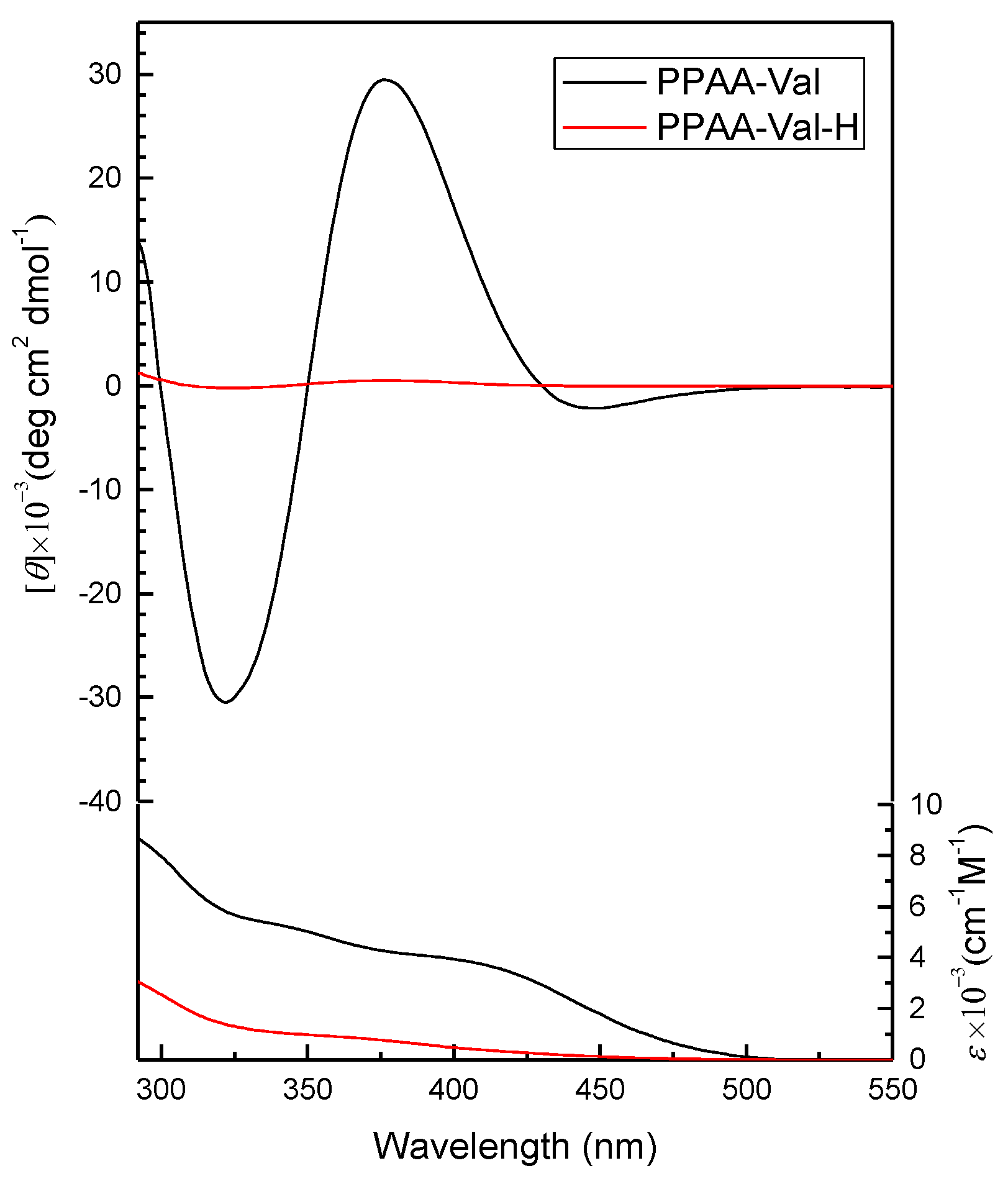
2.2. Secondary Structure of PPAA-Val
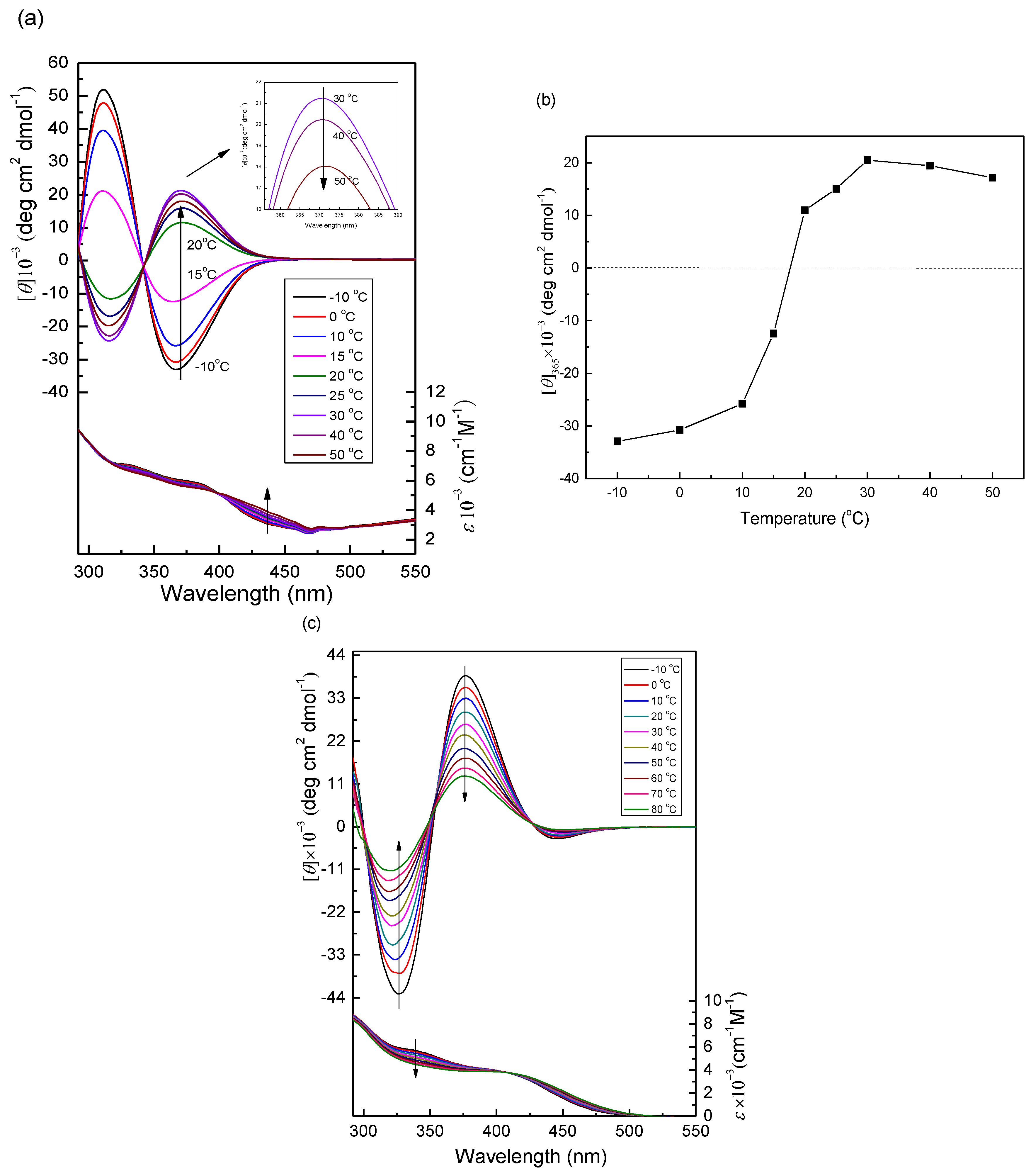
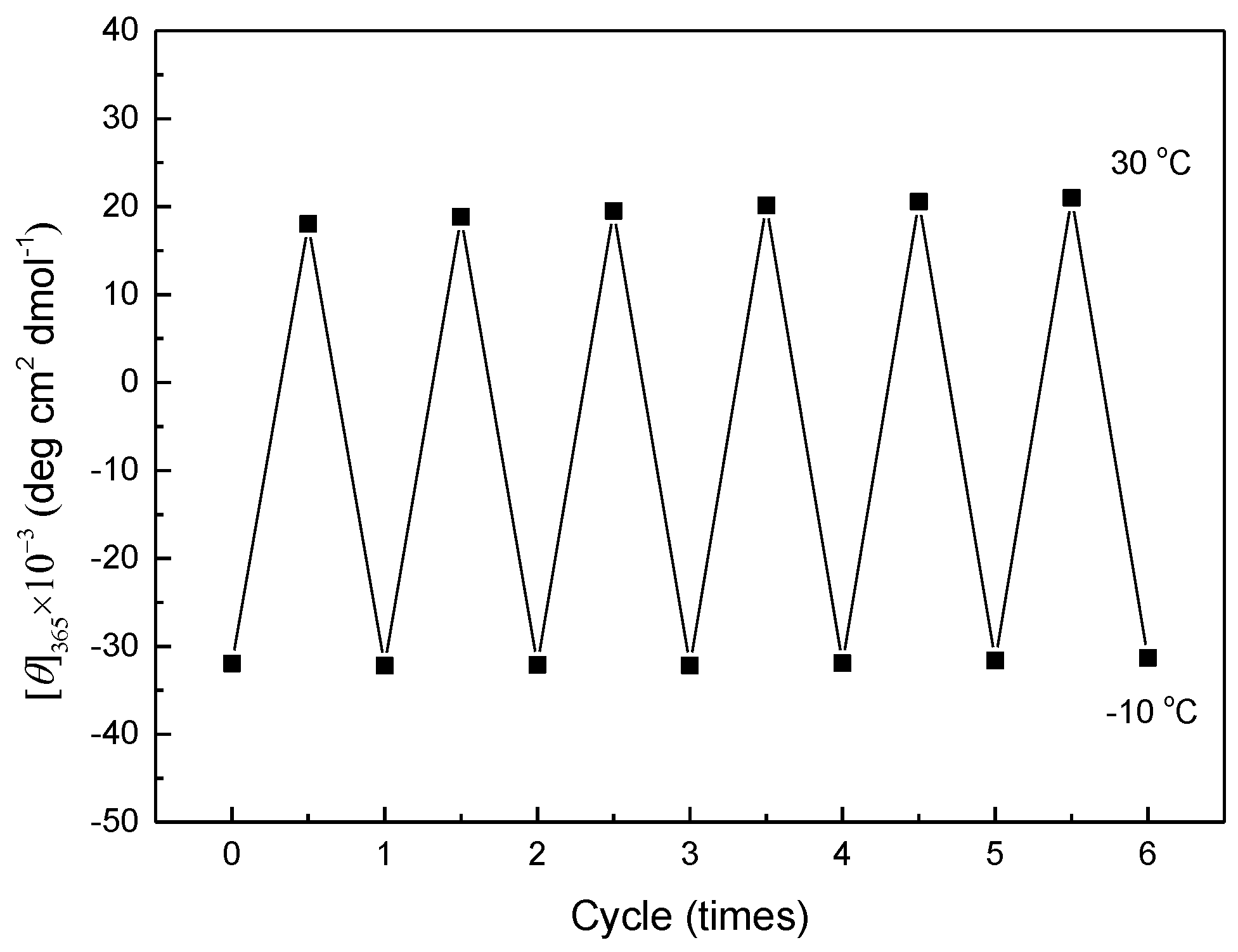
2.3. Temperature-Triggered Helix-Helix Inversion of PPAA-Val
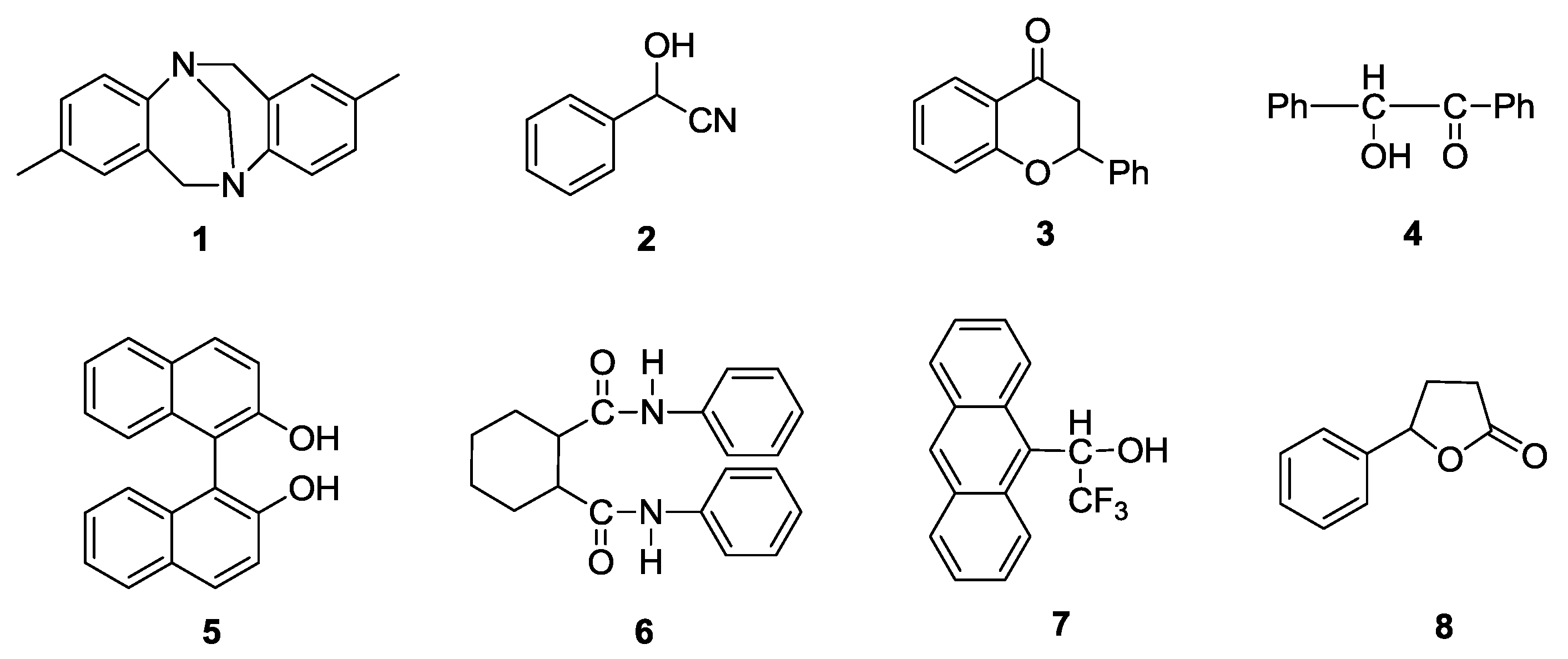
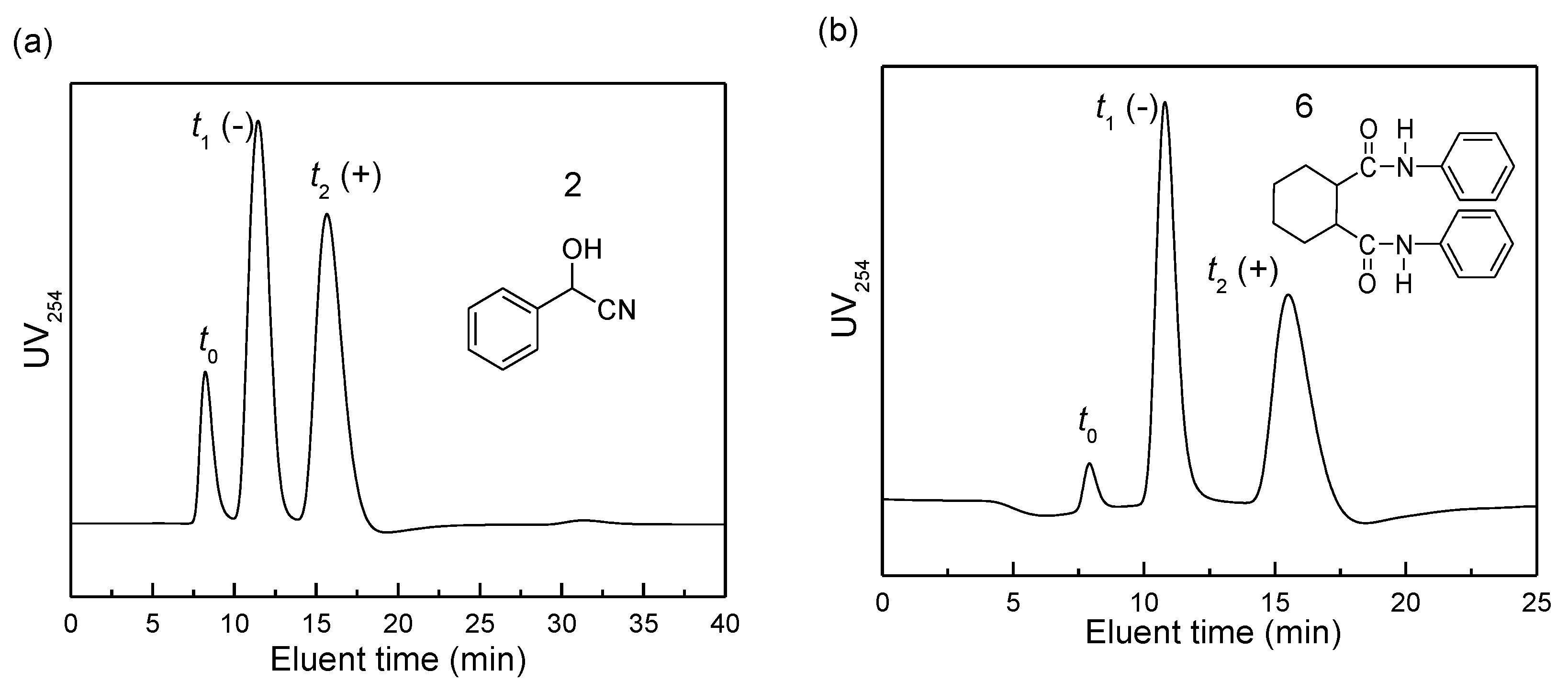
2.4. Chiral Recognition Ability of PPAA-Val
2.5. Effects of One-Handed Helical cis-Polyene Main Chain of PPAA-Val on its Chiral Recognition Ability
3. Materials and Methods
3.1. Materials
3.2. Instruments
3.3. Synthesis of l-Valine Ethyl Ester
3.4. Synthesis of N-(4-Ethynylbenzoyl)-l-Valine Ethyl Ester (PAA-Val)
3.5. Polymerization
3.6. Heat Treatment of PPAA-Val
3.7. Preparation of Chiral Stationary Phases (CSPs)
3.8. HPLC Measurement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tabei, J.; Nomura, R.; Sanda, F.; Masuda, T. Design of helical poly(N-propargylamides) that switch the helix sense with thermal stimuli. Macromolecules 2004, 37, 1175–1179. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Bai, J.; Zhang, J.; Liu, A.; Wan, X. Chiroptical and thermotropic properties of helical styrenic polymers: Effect of achiral group. Macromolecules 2014, 47, 1553–1562. [Google Scholar] [CrossRef]
- Green, M.M.; Peterson, N.C.; Sato, T.; Teramoto, A.; Cook, R.; Lifson, S. A helical polymer with a cooperative response to chiral information. Science 1995, 268, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Wakasone, S.; Shimomura, K.; Ikai, T.; Kanoh, S. Helical polymer brushes with a preferred-handed helix-sense triggered by a terminal optically active group in the pendant. Chem. Commun. 2012, 48, 3342–3344. [Google Scholar] [CrossRef] [PubMed]
- Engelkamp, H.; Middelbeek, S.; Nolte, R.J.M. Self-assembly of disk-shaped molecules to coiled-coil aggregates with tunable helicity. Science 1999, 284, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.; Koepf, M.; Kitto, H.J.; Nolte, R.J.M.; Rowan, A.E. Helical poly(isocyanides): Past, present and future. Polym. Chem. 2011, 2, 33–47. [Google Scholar] [CrossRef]
- Kajitani, T.; Okoshi, K.; Sakurai, S.; Kumaki, J.; Yashima, E. Helix-sense controlled polymerization of a single phenyl isocyanide enantiomer leading to diastereomeric helical polyisocyanides with opposite helix-sense and cholesteric liquid crystals with opposite twist-sense. J. Am. Chem. Soc. 2006, 128, 708–709. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Suzuki, K.; Ohta, K.; Hatada, K.; Yuki, H. Optically active poly(triphenylmethyl methacrylate) with one-handed helical conformation. J. Am. Chem. Soc. 1979, 101, 4763–4765. [Google Scholar] [CrossRef]
- Nakano, T.; Okamoto, Y. Synthetic helical polymers: Conformation and function. Chem. Rev. 2001, 101, 4013–4038. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Kamiya, N.; Yashima, E. Poly(phenylacetylene)s bearing a peptide pendant: Helical conformational changes of the polymer backbone stimulated by the pendant conformational change. Chem. Eur. J. 2004, 10, 4000–4010. [Google Scholar] [CrossRef] [PubMed]
- Yashima, E.; Maeda, K.; Sato, O. Switching of a macromolecular helicity for visual distinction of molecular recognition events. J. Am. Chem. Soc. 2001, 123, 8159–8160. [Google Scholar] [CrossRef] [PubMed]
- Tabei, J.; Nomura, R.; Masuda, T. Synthesis and structure of poly(N-propargylbenzamides) bearing chiral ester groups. Macromolecules 2003, 36, 573–577. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K.; Nishimura, T. Detection and amplification of chirality by helical polymers. Chem. Eur. J. 2004, 10, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Yashima, E.; Maeda, K.; Iida, H.; Furusho, Y.; Nagai, K. Helical polymers: Synthesis, structures, and functions. Chem. Rev. 2009, 109, 6102–6211. [Google Scholar] [CrossRef] [PubMed]
- Freire, F.; Quiñoá, E.; Riguera, R. Supramolecular Assemblies from Poly(phenylacetylene)s. Chem. Rev. 2015, 116, 1242–1271. [Google Scholar] [CrossRef] [PubMed]
- Teraguchi, M.; Mottate, K.; Kim, S.Y.; Aoki, T.; Kaneko, T.; Hadano, S.; Masuda, T. Synthesis of chiral helical poly(hydroxyl-containing phenylacetylene) membranes by in-situ depinanylsilylation and their enantioselective permeabilities. Macromolecules 2005, 38, 6367–6373. [Google Scholar] [CrossRef]
- Yashima, E.; Matsushima, T.; Nimura, T.; Okamoto, Y. Enantioseparation on optically active streoregular polyphenylacetylene derivatives as chiral stationary phases for HPLC. Korea Polym. J. 1996, 4, 139–146. [Google Scholar]
- Naito, Y.; Tang, Z.; Iida, H.; Miyabe, T.; Yashima, E. Enantioseparation on helical poly(phenylacetylene)s bearing cinchona alkaloid pendants as chiral stationary phases for HPLC. Chem. Lett. 2012, 41, 809–811. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, F.; Li, Y.; Shen, X.; Xu, X.; Sakai, R.; Satoh, T.; Kakuchi, T.; Okamoto, Y. Influence of stereoregularity and linkage groups on chiral recognition of poly(phenylacetylene) derivatives bearing l-leucine ethyl ester pendants as chiral stationary phases for HPLC. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2271–2278. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Geng, Q.; Yang, T.; Liu, L.; Sakai, R.; Satoh, T.; Kakuchi, T.; Okamoto, Y. Synthesis of helical poly(phenylacetylene)s with amide linkage bearing l-phenylalanine and l-phenylglycine ethyl ester pendants and their applications as chiral stationary phases for HPLC. Macromolecules 2013, 46, 8406–8415. [Google Scholar] [CrossRef]
- Kakuchi, R.; Nagata, S.; Tago, Y.; Sakai, R.; Otsuka, I.; Satoh, T.; Kakuchi, T. Efficient anion recognition property of three dimensionally clustered amide groups organized on a poly(phenylacetylene) backbone. Macromolecules 2009, 42, 1476–1481. [Google Scholar] [CrossRef]
- Li, B.S.; Cheuk, K.K.L.; Ling, L.; Chen, J.; Xiao, X.; Bai, C.; Tang, B.Z. Synthesis and hierarchical structures of amphiphilic polyphenylacetylenes carrying l-valine pendants. Macromolecules 2003, 36, 77–85. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Liu, F.; Shen, X.; Liu, L.; Sakai, R.; Satoh, T.; Kakuchi, T.; Okamoto, Y. Chiral recognition of helical poly(phenylacetylene) derivatives bearing l-amino acid ethyl ester pendants. Acta Polym. Sin. 2013, 6, 811–816. [Google Scholar]
- Masuda, T.; Tang, B.; Tanaka, A.; Higashimura, T. Mechanical properties of substituted polyacetylenes. Macromolecules 1986, 19, 1459–1464. [Google Scholar] [CrossRef]
- Seki, H.; Tang, B.; Tanaka, A.; Masuda, T. Tensile and dynamic viscoelastic properties of various new substituted polyacetylenes. Polymer 1994, 35, 3456–3462. [Google Scholar] [CrossRef]
- Yang, W.; Tabata, M.; Kobayashi, S.; Yokota, K.; Shimizu, A. Synthesis of ultra-high-molecular-weight aromatic polyacetylenes with [Rh(norbornadiene)Cl]2-triethylamine and solvent-induced crystallization of the obtained amorphous polyacetylenes. Polym. J. 1991, 23, 1135–1138. [Google Scholar] [CrossRef]
- Tabata, M.; Yang, W.; Yokota, K. 1H-NMR and UV studies of Rh complexes as a stereoregular polymerization catalysts for phenylacetylenes: Effects of ligands and solvents on its catalyst activity. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 1113–1120. [Google Scholar] [CrossRef]
- Matsunami, S.; Watanabe, T.; Kamimura, H.; Kakuchi, T.; Ishii, F. Thermal isomerization of cis-poly(phenylacetylene). Scission of polymer main-chain and formation of 1,3,5-triphenylbenzene as a pyrolysis product. Polymer 1996, 37, 4853–4855. [Google Scholar] [CrossRef]
- Matsunami, S.; Kakuchi, T.; Ishii, F. Deuterium Nuclear Quadrupole Coupling and cis-trans Isomerization in Poly(phenylacetylene-d1). Macromolecules 1997, 30, 1074–1078. [Google Scholar] [CrossRef]
- Kakuchi, T.; Matsunami, S.; Kamimura, H.; Ishii, F.; Uesaka, T.; Yokota, K. Crowned polyacetylene. I. Synthesis and characterization of poly(4′-ethynylbenzo-15-crown-5). J. Polym. Sci. Part A Polym. Chem. 1995, 33, 1431–1436. [Google Scholar] [CrossRef]
- Tang, B.; Kotera, N. Synthesis of optically active polyacetylene containing an asymmetric silicon by using organotransition-metal complexes as catalysts. Macromolecules 1989, 22, 4388–4390. [Google Scholar] [CrossRef]
- Akagi, K.; Guo, S.; Mori, T.; Goh, M.; Piao, G.; Kyotani, M. Synthesis of helical polyacetylene in chiral nematic liquid crystals using crown ether type binaphthyl derivatives as chiral dopants. J. Am. Chem. Soc. 2005, 127, 14647–14654. [Google Scholar] [CrossRef] [PubMed]
- Sakai, R.; Barasa, E.B.; Sakai, N.; Sato, S.; Satoh, T.; Kakuchi, T. Colorimetric detection of anions in aqueous solution using poly(phenylacetylene) with sulfonamide receptors activated by electron withdrawing group. Macromolecules 2012, 45, 8221–8227. [Google Scholar] [CrossRef]
- Gao, G.; Sanda, F.; Masuda, T. Copolymerization of chiral amino acid-based acetylenes and helical conformation of the copolymers. Macromolecules 2003, 36, 3938–3943. [Google Scholar] [CrossRef]
- Cheuk, K.K.L.; Lam, J.W.Y.; Chen, J.; Lai, L.; Tang, B. Amino acid-containing polyacetylenes: Synthesis, hydrogen bonding, chirality transcription, and chain helicity of amphiphilic poly(phenylacetylene)s carrying l-leucine pendants. Macromolecules 2003, 36, 5947–5959. [Google Scholar] [CrossRef]
- Zhao, H.; Sanda, F.; Masuda, T. Transformation of helical sense of poly(N-propargylamides) controlled by competition between structurally different enantiomeric amino acids. Macromolecules 2004, 37, 8888–8892. [Google Scholar] [CrossRef]
- Ginsburg, E.J.; Gorman, C.B.; Grubbs, R.H. Modern Acetylene Chemistry; Stang, P.J., Diederich, F., Eds.; VCH: New York, NY, USA, 1995; Chapter 10; pp. 353–383. [Google Scholar]
- Mrevlishvili, G.M.; Metreveli, N.O.; Razmadze, G.Z.; Mdzinarashvili, T.D.; Kakabadze, G.R.; Khvedelidze, M.M. Partial heat capacity change-Fundamental characteristic of the process of thermal denaturation of biological macromolecules (proteins and nucleic acids). Thermochim. Acta 1998, 308, 41–48. [Google Scholar] [CrossRef]
- Tabei, J.; Shiotsuki, M.; Sanda, F.; Masuda, T. Effect of chiral substituents on the secondary structure of poly(N-alkynylamides). Macromolecules 2005, 38, 5860–5867. [Google Scholar] [CrossRef]
- Simionescu, C.I.; Percec, V.J. Polypentadeuterophenylacetylene isomers. Polym. Sci. Polym. Lett. Ed. 1979, 17, 421–429. [Google Scholar] [CrossRef]
- Sanford, T.J.; Allendoerfer, R.D.; Kang, E.T.; Ehrlich, P. High-resolution NMR spectra and phase equilibria in poly(phenylacetylene)-diluent mixtures. J. Polym. Sci. Polym. Phys. Ed. 1980, 18, 2277–2286. [Google Scholar] [CrossRef]
- Ishii, F.; Matsunami, S.; Shibata, M.; Kakuchi, T. UV-vis absorption study of cis- and trans-poly(phenylacetylene)s based on CNDO/S calculation. Polym. J. 1999, 31, 84–88. [Google Scholar] [CrossRef]
- Yashima, E.; Matsushima, T.; Okamoto, Y. Chirality assignment of amines and amino alcohols based on circular dichroism induced by helix formation of a stereoregular poly((4-carboxyphenyl) acetylene) through acid-base complexation. J. Am. Chem. Soc. 1997, 119, 6345–6359. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Itou, M.; Miyatake, T.; Ikariya, T.; Noyori, R. Polymerization of monosubstituted acetylenes with a zwitterionic rhodium (I) complex, Rh+(2,5-norbornadiene)[η6-C6H5)B-(C6H5)3]. Macromolecules 1995, 28, 6662–6666. [Google Scholar] [CrossRef]
- Kakuchi, R.; Nagata, S.; Sakai, R.; Otsuka, I.; Nakade, H.; Satoh, T.; Kakuchi, T. Size-Specific, Colorimetric Detection of Counteranions by Using Helical Poly(phenylacetylene) Conjugated to l-Leucine Groups through Urea Acceptors. Chem. Eur. J. 2008, 14, 10259–10266. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Kawashima, M.; Hatada, K. Chromatographic resolution: XI. Controlled chiral recognition of cellulose triphenylcarbamate derivatives supported on silica gel. J. Chromatogr. A 1986, 363, 173–186. [Google Scholar] [CrossRef]
- Okamoto, Y.; Aburatani, R.; Fukumoto, T.; Hatada, K. Useful chiral stationary phases for HPLC. Amylose tris(3,5-dimethylphenylcarbamate) and tris(3,5-dichlorophenylcarbamate) supported on silica gel. Chem. Lett. 1987, 16, 1857–1860. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
| Compound | []/° a | |||||
|---|---|---|---|---|---|---|
| CHCl3 | MeOH | DMF | DMAc | THF | Acetone | |
| PAA-Val | +57.6 | +10.3 | +40.6 | +40.0 | +25.3 | +39.3 |
| PPAA-Val | +433 | +334 | +515 | +453 | +557 | +457 |
| Sample | Condition | Wavenumber (cm−1) | |
|---|---|---|---|
| νC=O (Amide) | νC=O (Ester) | ||
| PAA-Val | CHCl3 | 1662 | 1735 |
| solid | 1644 | 1734 | |
| PPAA-Val | CHCl3 | 1648 | 1735 |
| solid | 1644 | 1734 | |
| Racemates | PPAA-Val | PPAA-Val-H | ||||||
|---|---|---|---|---|---|---|---|---|
| Coating solvent: DMF | Coating Colvent: THF | Coating Solvent: CHCl3 | Coating Solvent: DMF | |||||
| k1′ | α | k1′ | α | k1′ | α | k1′ | α | |
| 1 | 0.19 | 1.00 | 0.05 | 1.00 | 0.13 | 1.00 | 0.11 | 1.00 |
| 2 | 0.44 (−) | 2.18 | 0.13 (−) | 2.15 | 0.24 | 1.00 | 1.14 | 1.00 |
| 3 | 0.65 | 1.00 | 0.18 | 1.00 | 0.33 | 1.00 | 0.36 | 1.00 |
| 4 | 1.67 | 1.00 | 0.58 | 1.00 | 0.84 | 1.00 | 0.99 | 1.00 |
| 5 | 13.54 (+) | ~1 | 13.12 (+) | ~1 | 4.33 | 1.00 | 5.80 | 1.00 |
| 6 | 0.36 (−) | 2.60 | 0.12 (−) | 2.25 | 0.29 (−) | 2.01 | 0.92 | 1.00 |
| 7 | 4.83 | 1.00 | 1.58 | 1.00 | 2.18 | 1.00 | 1.99 | 1.00 |
| 8 | 2.26 | 1.00 | 0.67 | 1.00 | 1.12 | 1.00 | 1.19 | 1.00 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhang, C.; Qiu, Y.; Liu, L.; Yang, T.; Dong, H.; Satoh, T.; Okamoto, Y. Temperature-Triggered Switchable Helix-Helix Inversion of Poly(phenylacetylene) Bearing l-Valine Ethyl Ester Pendants and Its Chiral Recognition Ability. Molecules 2016, 21, 1583. https://doi.org/10.3390/molecules21111583
Zhou Y, Zhang C, Qiu Y, Liu L, Yang T, Dong H, Satoh T, Okamoto Y. Temperature-Triggered Switchable Helix-Helix Inversion of Poly(phenylacetylene) Bearing l-Valine Ethyl Ester Pendants and Its Chiral Recognition Ability. Molecules. 2016; 21(11):1583. https://doi.org/10.3390/molecules21111583
Chicago/Turabian StyleZhou, Yanli, Chunhong Zhang, Yuan Qiu, Lijia Liu, Taotao Yang, Hongxing Dong, Toshifumi Satoh, and Yoshio Okamoto. 2016. "Temperature-Triggered Switchable Helix-Helix Inversion of Poly(phenylacetylene) Bearing l-Valine Ethyl Ester Pendants and Its Chiral Recognition Ability" Molecules 21, no. 11: 1583. https://doi.org/10.3390/molecules21111583
APA StyleZhou, Y., Zhang, C., Qiu, Y., Liu, L., Yang, T., Dong, H., Satoh, T., & Okamoto, Y. (2016). Temperature-Triggered Switchable Helix-Helix Inversion of Poly(phenylacetylene) Bearing l-Valine Ethyl Ester Pendants and Its Chiral Recognition Ability. Molecules, 21(11), 1583. https://doi.org/10.3390/molecules21111583







