Induction of Laccase, Lignin Peroxidase and Manganese Peroxidase Activities in White-Rot Fungi Using Copper Complexes
Abstract
:1. Introduction
2. Results and Discussion
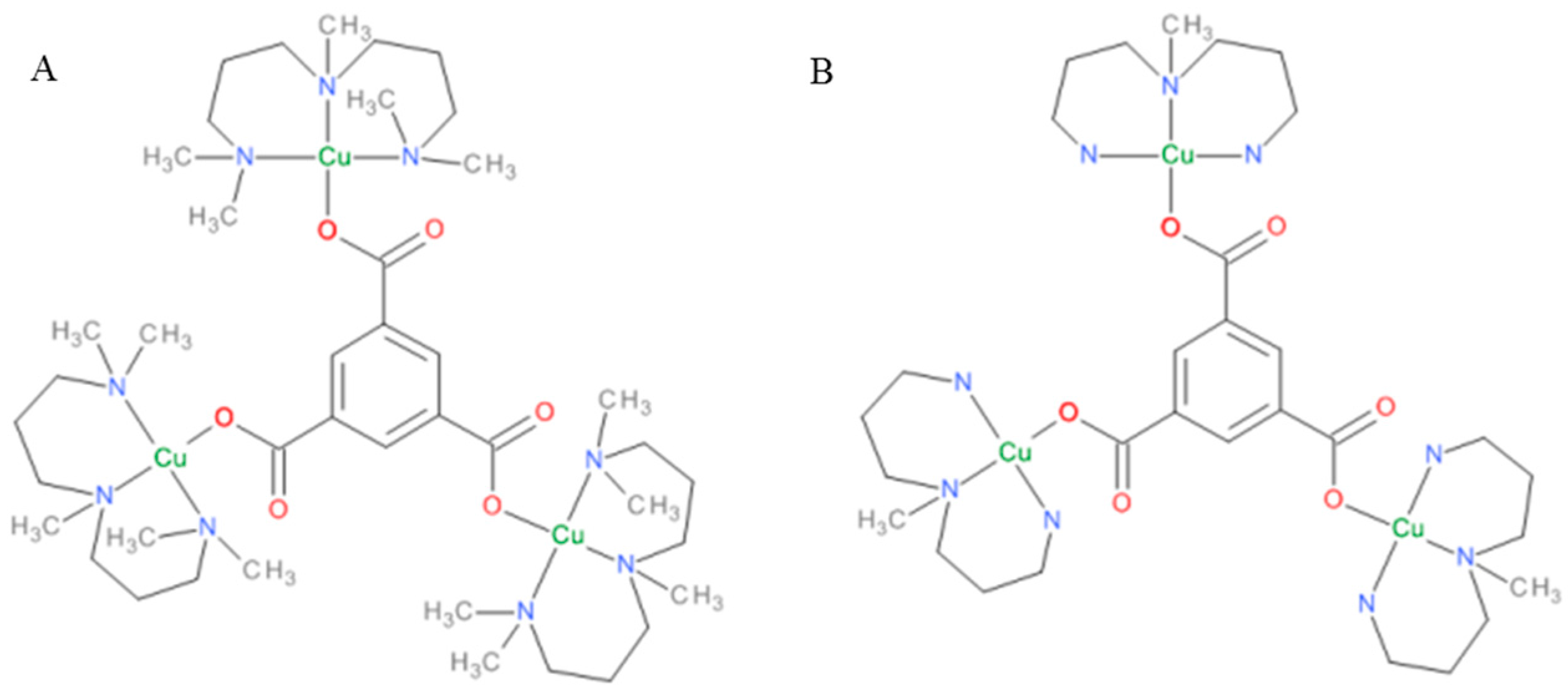
2.1. Synthesis and Characterization of Complexes
2.2. X-ray Structure of (Him)[Cu(im)4(H2O)2](btc)·3H2O (M1)
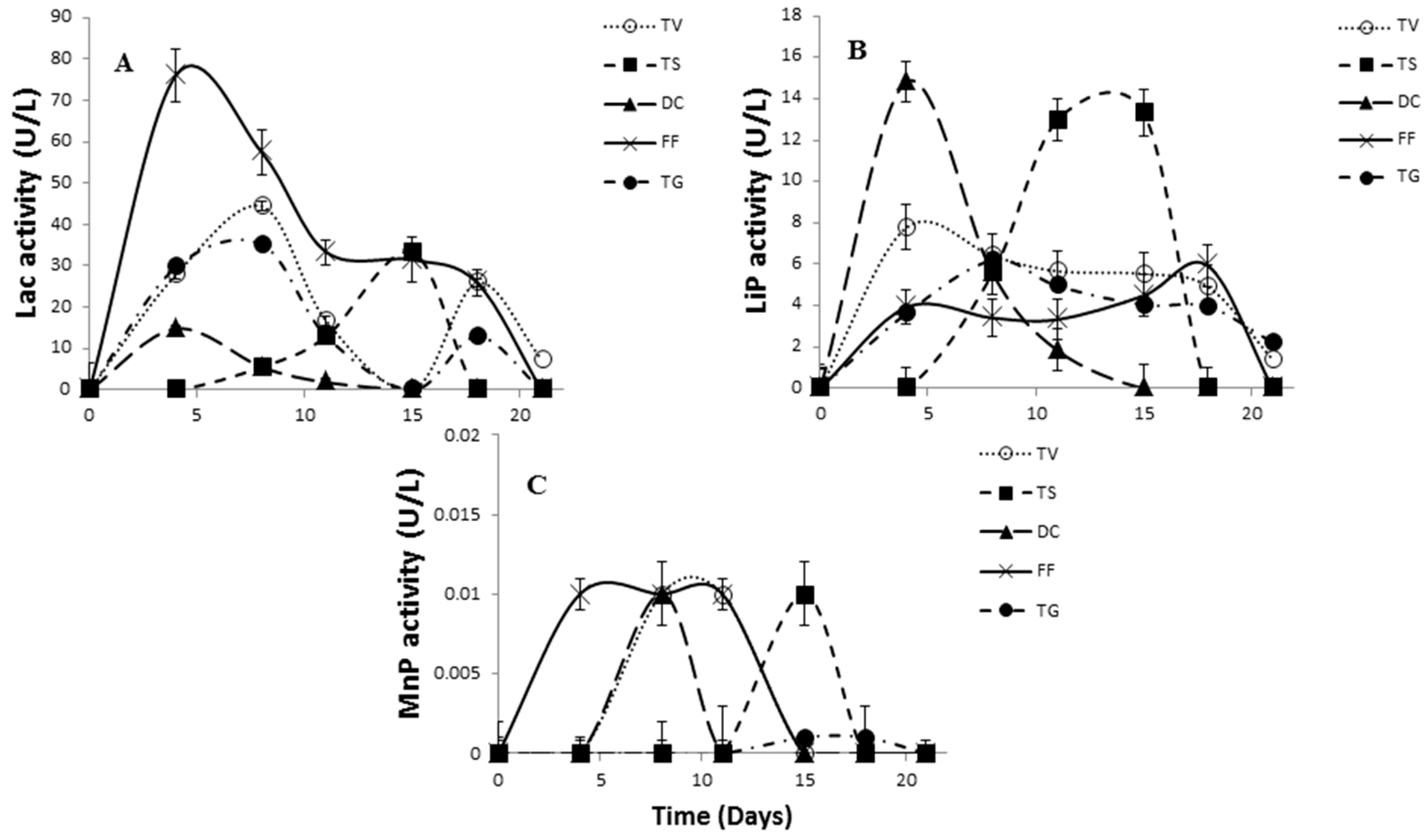
2.3. Comparative Evaluation of White-Rot Fungal Strains
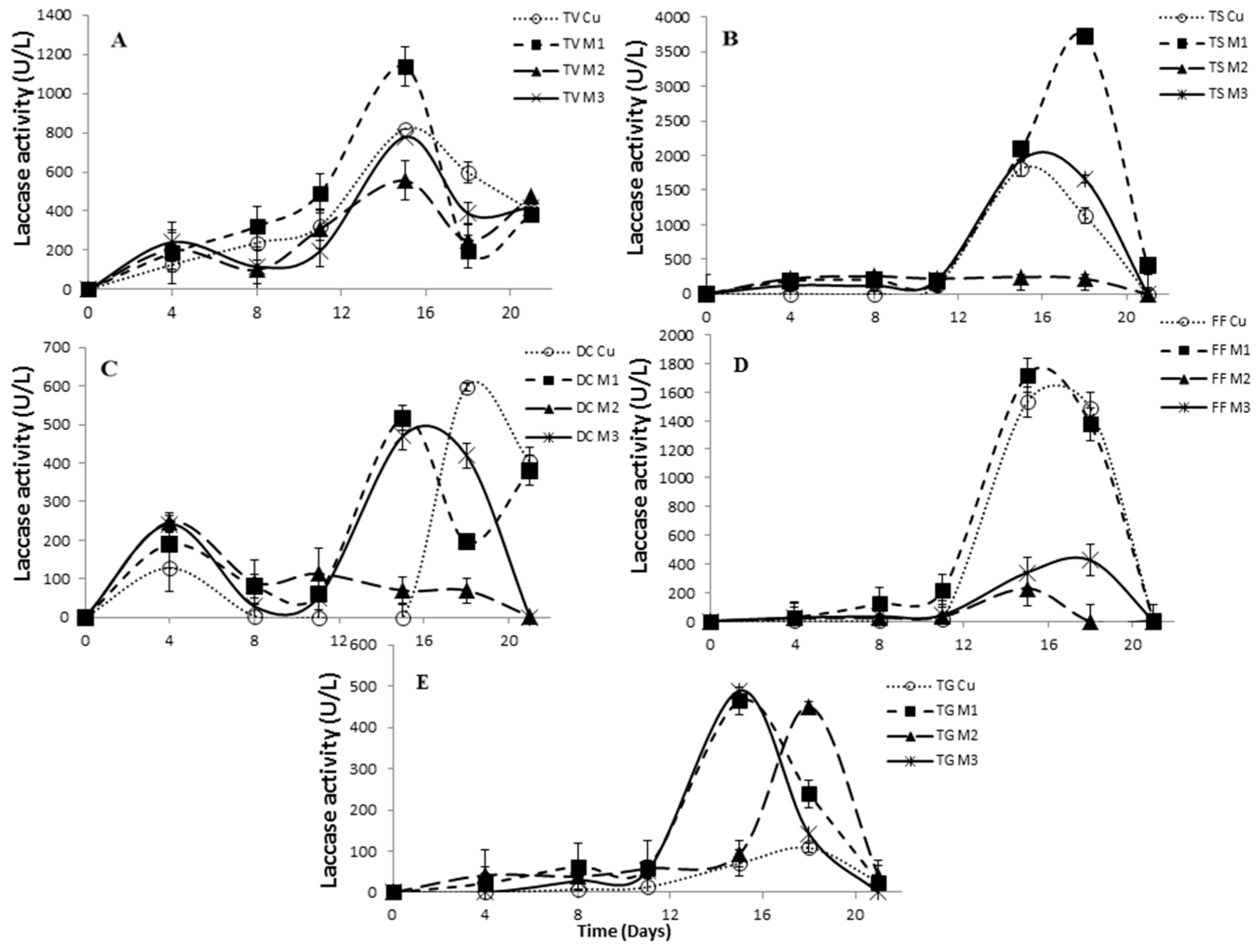
2.4. Effect of Inducers Concentration on Lac, LiP and MnP Activities
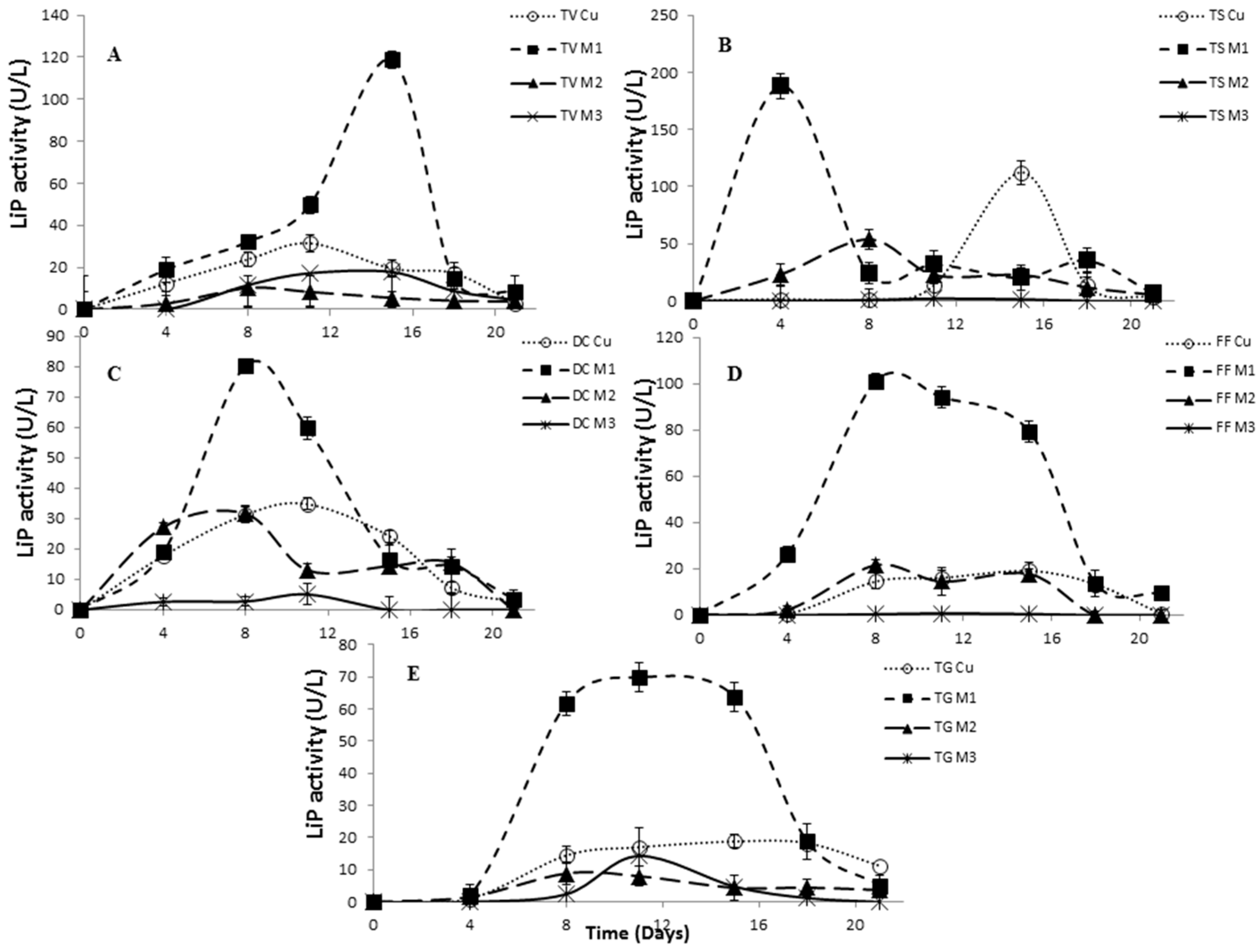
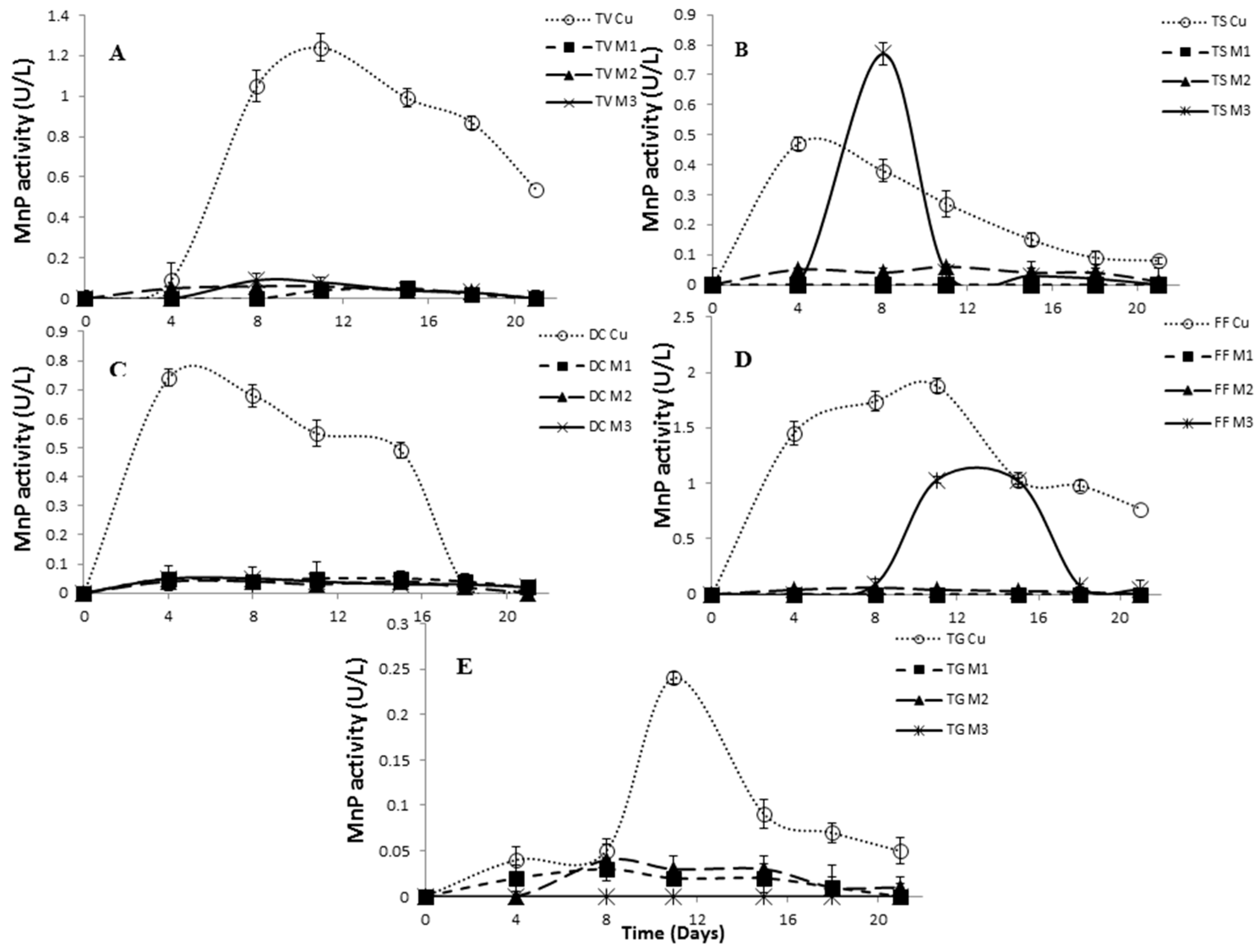
2.5. The Effect of Inducers on Lac, LiP and MnP Activities
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Preparation of the Complexes
3.2.1. Cu3(btc)2·10H2O
3.2.2. (Him)[Cu(im)4(H2O)2](btc)·3H2O: Complex M1
3.2.3. [Cu3(pmdien)3(btc)](ClO4)3·6H2O: Complex M2
3.2.4. [Cu3(mdpta)3(btc)](ClO4)3·4H2O: Complex M3
3.3. X-ray Crystallography
3.4. Microorganisms, Substrates, Inocula
3.5. Enzyme Activity Assay
3.5.1. Laccase Activity
3.5.2. Lignin Peroxidase Activity
3.5.3. Manganese-Dependent Peroxidase Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baldrian, P. Interactions of heavy metals with white-rot fungi. Enzyme Microb. Technol. 2003, 32, 78–91. [Google Scholar] [CrossRef]
- Shah, V.; Dobiasova, P.; Baldrian, P.; Nerud, F.; Kumar, A.; Seal, S. Influence of iron and copper nanoparticle powder on the production of lignocellulose degrading enzymes in the fungus Trametes versicolor. J. Hazard. Mater. 2010, 178, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Thurston, C.F. The structure and function of fungal laccases. Microbiology 1994, 140, 19–26. [Google Scholar] [CrossRef]
- Vasina, D.V.; Pavlov, A.R.; Koroleva, O.V. Extracellular proteins of trametes hirsuta St. 072 induced by copper ions and a lignocellulose substrate. BMC Microbiol. 2016, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell. Mol. Life Sci. 2009, 67, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Sadhasivam, S.; Savitha, S.; Swaminathan, K.; Lin, F.H. Production, purification and characterization of mid-redox potential laccase from a newly isolated trichoderma harzianum Wl1. Process Biochem. 2008, 43, 736–742. [Google Scholar] [CrossRef]
- Collins, P.J.; Dobson, A.D.W. Regulation of laccase gene transcription in Trametes versicolor. Appl. Environ. Microbiol. 1997, 63, 3444–3450. [Google Scholar] [PubMed]
- Galhaup, C.; Wagner, H.; Hinterstoisser, B.; Haltrich, D. Increased production of laccase by the wood-degrading basidiomycete Trametes pubescens. Enzyme Microb. Technol. 2002, 30, 529–536. [Google Scholar] [CrossRef]
- Palmieri, G.; Giardina, P.; Bianco, C.; Fontanella, B.; Sannia, G. Copper induction of laccase isoenzymes in the ligninolytic fungus Pleurotus ostreatus. Appl. Environ. Microbiol. 2000, 66, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Alvares, J.M.; Canessa, P.; Mancilla, R.A.; Polanco, R.; Santibanes, P.A.; Vicuna, R. Expression of genes encoding laccase and manganese-dependent peroxidase in the fungus Cerioporiopsis subvermisporais mediated by an ace1-like copper-fist transcription factor. Fungal Genet. Biol. 2009, 46, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.; Herrmann, V.; Papinutti, V.L. Optimization of lignocellulolytic enzyme production by the white-rot fungus Trametes trogii in solid-state fermentation using response surface methodology. Biochem. Eng. J. 2008, 39, 1207–1214. [Google Scholar] [CrossRef]
- Plackova, M.; Svobodova, K.; Cajthaml, T. Laccase activity profiling and gene expression in PCB-degrading cultures of Trametes versicolor. Int. Biodeterior. Biodegrad. 2012, 71, 22–28. [Google Scholar] [CrossRef]
- Kahraman, S.S.; Gurdal, I.H. Effect of synthetic and natural culture media on laccase production by white rot fungi. Bioresour. Technol. 2002, 82, 215–217. [Google Scholar] [CrossRef]
- Elisashvili, V.; Kachlishvili, E. Physiological regulation of laccase and manganese peroxidase production by white-rot basidiomycetes. J. Biotechnol. 2009, 144, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Hoyos, C.M.; Morales-Alvarez, E.D.; Poutou-Pinales, R.A.; Pedroza-Rodriguez, A.M.; Rodriguez-Vazquez, R.; Delgado-Boada, J.M. Fungal laccases. Fungal Biol. Rev. 2013, 27, 67–82. [Google Scholar] [CrossRef]
- Rao, M.A.; Scelza, R.; Acevedo, F.; Diez, M.C.; Gianfreda, L. Enzymes as useful tools for environmental purposes. Chemosphere 2014, 107, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Rancano, G.; Lorenzo, M.; Molares, N.; Couto, S.R.; Sanroman, M.A. Production of laccase by Trametes versicolor in an airlift fermentor. Process Biochem. 2003, 39, 467–473. [Google Scholar] [CrossRef]
- Fillat, U.; Martin-Sampedro, R.; Macaya-Sanz, D.; Martin, J.A.; Ibarra, D.; Martinez, M.J.; Eugenio, M.E. Screening of eucalyptus wood endophytes for laccase activity. Process Biochem. 2016, 51, 589–598. [Google Scholar] [CrossRef]
- Baldrian, P.; Gabriel, J. Copper and cadmium increase laccase activity in pleurotus ostreatus. FEMS Microbiol. Lett. 2002, 206, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.P.; Khan, M.A.; Houser, R.P. Coordination polymers composed of Copper(II), Trimesic acid, and Imidazole: 3D architecture stabilized by hydrogen bonding. Inorg. Chem. 2001, 40, 6858–6859. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Su, J.; Yang, S.H.; Li, G.B.; Liao, F.H.; Xiong, M.; Lin, J.H. Syntheses, structures and magnetic properties of Mn(II), Co(II) and Ni(II) metal-organic frameworks constructed from 1,3,5-benzenetricarboxylate and formate ligands. Inorg. Chim. Acta 2010, 363, 645–652. [Google Scholar] [CrossRef]
- Mrozinski, J.; Bienko, A.; Kopel, P.; Langer, V. Structure and magnetic properties of a trinuclear Nickel(II) complex with benzenetricarboxylate bridge. Inorg. Chim. Acta 2008, 361, 3723–3729. [Google Scholar] [CrossRef]
- Brown, K.; Zolezzi, S.; Aguirre, P.; Venegas-Yazigi, D.; Paredes-Garcia, V.; Baggio, R.; Novak, M.A.; Spodine, E. Cu(H2Btec)(Bipy) (infinity): A novel metal organic framework (MOF) as heterogeneous catalyst for the oxidation of olefins. Dalton Trans. 2009, 2009, 1422–1427. [Google Scholar] [CrossRef]
- Wang, X.L.; Le, M.; Lin, H.Y.; Luan, J.; Liu, G.C.; Liu, D.N. Aminopyridine derivatives controlled the assembly and various properties of Cu-btc metal-organic frameworks. Dalton Trans. 2015, 44, 14008–14018. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.Y.; Shi, L.; Huang, H.B.; Yu, Q.; Ye, Z.Z.; Peng, X.S. Mesoporous separation membranes of {Cu(BTC-H2)2·(H2O)·3H2O} nanobelts synthesized by ultrasonication at room temperature. Crystengcomm 2013, 15, 265–270. [Google Scholar] [CrossRef]
- Hosseinabadi, F.; Ghadermazi, M.; Taran, M.; Derikvand, Z. Synthesis, crystal structure, spectroscopic, thermal analyses and biological properties of novel F-block coordination polymers containing 2,2′-thiodiacetic acid and piperazine. Inorg. Chim. Acta 2016, 443, 186–197. [Google Scholar] [CrossRef]
- Kopel, P.; Kamenicek, J.; Petricek, V.; Kurecka, A.; Kalinska, B.; Mrozinski, J. Syntheses and study on Nickel and Copper complexes with 1,3,5-benzenetricarboxylic acid. Crystal and molecular structure of Cu3(Mdpta)3(Btc)·(ClO4)3·4H2O. Polyhedron 2007, 26, 535–542. [Google Scholar] [CrossRef]
- Kopel, P.; Travnicek, Z.; Marek, J.; Mrozinski, J. Syntheses and study on Nickel(II) complexes with thiodiglycolic acid and nitrogen-donor ligands. X-ray structures of Ni(Bpy)(Tdga)(H2O)·4H2O and (En)Ni(Mu-Tdga)2Ni(en)·4H2O·(TdgaH2)= thiodiglycolic acid). Polyhedron 2004, 23, 1573–1578. [Google Scholar] [CrossRef]
- Vrsanska, M.; Palovcikova, D.; Voberkova, S. Monitoring of Laccase Production by Fungal Isolates from Czech Forest. In Proceedings of International PhD Students Conference, Brno, Czech Republic, 11 November 2015; Polak, O., Cerkal, C., Belcredi, N.B., Eds.; Mendel University in Brno: Brno, Czech Republic; pp. 103–107.
- Couto, S.R.; Gundin, M.; Lorenzo, M.; Sanroman, M.N. Screening of supports and inducers for laccase production by Trametes versicolor in semi-solid-state conditions. Process Biochem. 2002, 38, 249–255. [Google Scholar] [CrossRef]
- Gasser, C.A.; Ammann, E.M.; Schaffer, A.; Shahgaldian, P.; Corvini, P.F.X. Production of superparamagnetic nanobiocatalysts for green chemistry applications. Appl. Microbiol. Biotechnol. 2016, 100, 7281–7296. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hu, J.H.; Guo, C.H.; Liu, C.-Z. Enhanced laccase production by Trametes versicolor using corn steep liquor as both nitrogen source and inducer. Bioresour. Technol. 2014, 166, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Songulashvili, G.; Elisashvili, V.; Wasser, S.P.; Nevo, E.; Hadar, Y. Basidiomycetes laccase and manganese peroxidase activity in submerged fermentation of food industry wastes. Enzyme Microb. Technol. 2007, 41, 57–61. [Google Scholar] [CrossRef]
- Rodrigues, M.A.M.; Pinto, P.; Bezerra, R.M.F.; Dias, A.A.; Guedes, C.V.M.; Cardoso, V.M.G.; Cone, J.W.; Ferreira, L.M.M.; Colaco, J.; Sequeira, C.A. Effect of enzyme extracts isolated from white-rot fungi on chemical composition and in vitro digestibility of wheat straw. Anim. Feed Sci. Technol. 2008, 141, 326–338. [Google Scholar] [CrossRef]
- Jarvinen, J.; Taskila, S.; Isomaki, R.; Ojamo, H. Screening of white-rot fungi manganese peroxidases: A comparison between the specific activities of the enzyme from different native producers. AMB Express 2012, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Arantes, V.; Milagres, A.M.F. The synergistic action of ligninolytic enzymes (mnp and laccase) and Fe3+ reducing activity from white-rot fungi for degradation of azure b. Enzyme Microb. Technol. 2007, 42, 17–22. [Google Scholar] [CrossRef]
- Hughes, M.N.; Poole, R.K. Metal speciation and microbial growth the hard (and soft) facts. J. Gen. Microbiol. 1991, 137, 725–734. [Google Scholar] [CrossRef]
- Makela, M.R.; Lundell, T.; Hatakka, A.; Hilden, K. Effect of copper, nutrient nitrogen, and wood-supplement on the production of lignin-modifying enzymes by the white-rot fungus phlebia radiata. Fungal Biol. 2013, 117, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Halaburgi, V.M.; Sharma, S.; Sinha, M.; Singh, T.P.; Karegoudar, T.B. Purification and characterization of a thermostable laccase from the ascomycetes cladosporium cladosporioides and its applications. Process Biochem. 2011, 46, 1146–1152. [Google Scholar] [CrossRef]
- Sklenar, J.; Niku-Paavola, M.L.; Santos, S.; Man, P.; Kruus, K.; Novotny, C. Isolation and characterization of novel pi 4.8 mnp isoenzyme from white-rot fungus irpex lacteus. Enzyme Microb. Technol. 2010, 46, 550–556. [Google Scholar] [CrossRef]
- Gettemy, J.M.; Ma, B.; Alic, R.; Gold, M.H. Reverse transcription pcr analysis of the regulation of the manganese peroxidase gene family. Appl. Environ. Microbiol. 1998, 64, 569–574. [Google Scholar] [PubMed]
- Loera, C.O.; Perez, M.C.P.; Barbosa, I.R.; Ricardo, J.; Villasenor, F.O. Laccases. In Advances in Agricultural and Food Biotechnology; Ramón, G.G., Irineo, T.-P., Eds.; Research Signpost: Trivandrum, Kerala, India, 2006; p. 347. [Google Scholar]
- Kopel, P.; Travnicek, Z.; Kvitek, L.; Biler, M.; Pavlicek, M.; Sindelar, Z.; Marek, J. Coordination compounds of nickel with trithiocyanuric acid. Part IV. Structure of ni(pmdien)(ttch) (pmdien = N,N,N′,N′,N′′-pentamethyldiethylenetriamine, TtcH3 = trithiocyanuric acid). Transit. Met. Chem. 2001, 26, 282–286. [Google Scholar] [CrossRef]
- Kopel, P.; Mrozinski, J.; Dolezal, K.; Langer, V.; Boca, R.; Bienko, A.; Pochaba, A. Ferromagnetic properties of a trinuclear Nickel(II) complex with a trithiocyanurate bridge. Eur. J. Inorg. Chem. 2009, 2009, 5475–5482. [Google Scholar] [CrossRef]
- Bienko, A.; Kopel, P.; Kizek, R.; Kruszynski, R.; Bienko, D.; Titis, J.; Boca, R. Synthesis, crystal structure and magnetic properties of trithiocyanurate or thiodiacetate polynuclear Ni(II) and Co(II) complexes. Inorg. Chim. Acta 2014, 416, 147–156. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of shelx. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the (Him)[Cu(im)4(H2O)2](btc)·3H2O, where im = imidazole are available from the authors.
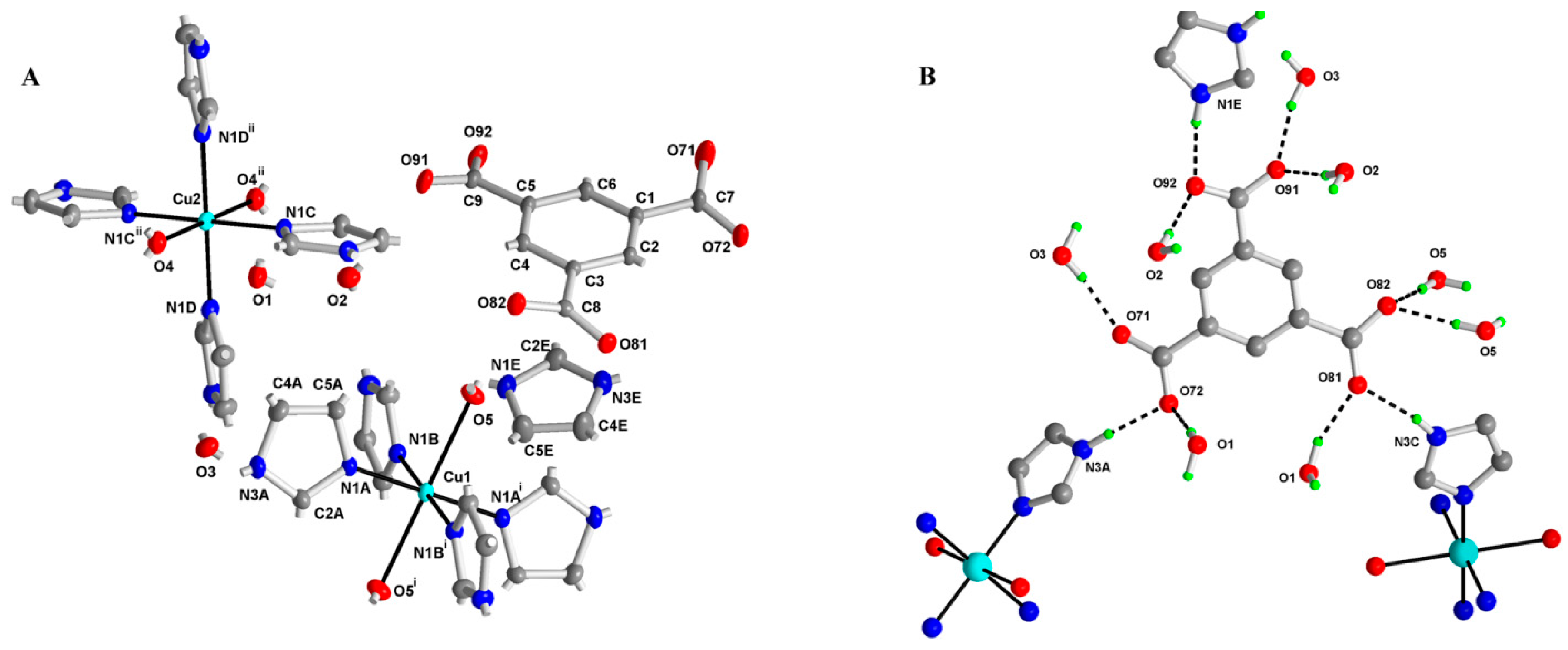
| Cu(1)-N(1B) | 2.0099 (15) | Cu(2)-N(1D) | 2.0156 (15) |
| Cu(1)-N(1B) i | 2.0099 (15) | Cu(2)-N(1D) ii | 2.0156 (15) |
| Cu(1)-N(1A) i | 2.0219 (14) | Cu(2)-N(1C) ii | 2.0219 (14) |
| Cu(1)-N(1A) | 2.0220 (14) | Cu(2)-N(1C) | 2.0220 (14) |
| N(1B)-Cu(1)-N(1B) i | 180.0 | N(1D)-Cu(2)-N(1D) ii | 179.999 (2) |
| N(1B)-Cu(1)-N(1A) i | 90.23 (6) | N(1D)-Cu(2)-N(1C) ii | 90.65 (6) |
| N(1B)i-Cu(1)-N(1A) i | 89.77 (6) | N(1D) ii-Cu(2)-N(1C) ii | 89.35 (6) |
| N(1B)-Cu(1)-N(1A) | 89.77 (6) | N(1D)-Cu(2)-N(1C) | 89.35 (6) |
| N(1B)i-Cu(1)-N(1A) | 90.23 (6) | N(1D) ii-Cu(2)-N(1C) | 90.65 (6) |
| N(1A)i-Cu(1)-N(1A) | 180.0 | N(1C) ii-Cu(2)-N(1C) | 180.0 |
| D-H...A | d(D-H) | d(H...A) | d(D...A) | <(DHA) |
|---|---|---|---|---|
| O(1)-H(11)...O(72) iii | 0.80 (3) | 1.91 (3) | 2.712 (2) | 174 (3) |
| O(1)-H(12)...O(81) iv | 0.86 (3) | 1.90 (3) | 2.7145 (19) | 159 (3) |
| O(2)-H(21)...O(91) i | 0.80 (3) | 1.95 (3) | 2.734 (2) | 169 (3) |
| O(2)-H(22)...O(92) v | 0.83 (3) | 1.90 (3) | 2.726 (2) | 170 (3) |
| O(3)-H(31)...O(91) vi | 0.87 (3) | 1.84 (3) | 2.699 (2) | 167 (2) |
| O(3)-H(32)...O(71) v | 0.78 (3) | 1.85 (3) | 2.616 (2) | 170 (3) |
| O(4)-H(41)...O(2) ii | 0.80 (3) | 2.04 (3) | 2.825 (2) | 166 (2) |
| O(4)-H(42)...O(3) vii | 0.86 (3) | 1.92 (3) | 2.771 (2) | 173 (2) |
| O(5)-H(51)...O(82) i | 0.80 (2) | 1.95 (3) | 2.7472 (19) | 177 (2) |
| O(5)-H(52)...O(82) iv | 0.79 (3) | 2.04 (3) | 2.826 (2) | 174 (2) |
| N(3A)-H(3A)...O(72) viii | 0.88 | 1.86 | 2.7312 (19) | 170.3 |
| N(3B)-H(3B)...O(2) i | 0.88 | 1.99 | 2.853 (2) | 168.2 |
| N(3C)-H(3C)...O(81) iv | 0.88 | 1.96 | 2.813 (2) | 162.6 |
| N(3D)-H(3D)...O(3) i | 0.88 | 1.95 | 2.792 (2) | 159.9 |
| N(1E)-H(1E)...O(92) v | 0.88 | 1.9 | 2.773 (2) | 172.2 |
| N(3E)-H(3E)...O(1) ix | 0.88 | 1.81 | 2.684 (2) | 174.1 |
| 0.1 mM | Lac (U/L) | LiP (U/L) | MnP (U/L) | 0.3 mM | Lac (U/L) | LiP (U/L) | MnP (U/L) | 0.5 mM | Lac (U/L) | LiP (U/L) | MnP (U/L) | 1.0 mM | Lac (U/L) | LiP (U/L) | MnP (U/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TV | TV | TV | TV | ||||||||||||
| Cu | 583.30 | 9.44 | 0.54 | Cu | 708.30 | 37.40 | 0.15 | Cu | 819.40 | 31.50 | 1.20 | Cu | 1291.70 | 1.81 | 0.09 |
| M1 | 833.30 | 333.30 | 0.02 | M1 | 777.80 | 43.90 | 0.14 | M1 | 1138.90 | 118.80 | 0.05 | M1 | 533.30 | 1.76 | 0.06 |
| M2 | 1194.40 | 50.20 | 0.05 | M2 | 1472.20 | 26.70 | 0.05 | M2 | 555.60 | 11.60 | 0.06 | M2 | 344.60 | 0.50 | 0.48 |
| M3 | 1305.60 | 135.40 | 0.06 | M3 | 1416.70 | 25.30 | 0.03 | M3 | 777.80 | 17.80 | 0.09 | M3 | 777.80 | 11.70 | 0.05 |
| TS | TS | TS | TS | ||||||||||||
| Cu | 987.50 | 17.50 | 0.05 | Cu | 444.40 | 17.80 | 0.09 | Cu | 1813.50 | 112.50 | 0.47 | Cu | 2108.30 | 1.90 | 0.08 |
| M1 | 186.10 | 0 | 0 | M1 | 175.00 | 15.30 | 0.02 | M1 | 3725.20 | 187.80 | 0 | M1 | 1234.70 | 1.60 | 0 |
| M2 | 152.80 | 52.80 | 0.03 | M2 | 206.90 | 15.60 | 0.05 | M2 | 253.90 | 53.90 | 0.06 | M2 | 11.70 | 0.31 | 0.31 |
| M3 | 627.80 | 44.40 | 0.04 | M3 | 306.80 | 26.10 | 0 | M3 | 1941.70 | 2.04 | 0.77 | M3 | 5.10 | 0.55 | 0.05 |
| DC | DC | DC | DC | ||||||||||||
| Cu | 569.40 | 69.40 | 0.25 | Cu | 569.40 | 16.30 | 0.24 | Cu | 597.20 | 34.80 | 0.74 | Cu | 750.00 | 0.98 | 0.38 |
| M1 | 272.20 | 42.20 | 0.01 | M1 | 500.60 | 15.60 | 0 | M1 | 516.70 | 80.30 | 0.05 | M1 | 413.90 | 1.70 | 0 |
| M2 | 83.30 | 16.40 | 0 | M2 | 142.30 | 8.90 | 0.05 | M2 | 241.20 | 31.60 | 0.04 | M2 | 175.40 | 0.65 | 0.27 |
| M3 | 84.20 | 21.40 | 0.05 | M3 | 469.40 | 460.10 | 0 | M3 | 434.70 | 5.10 | 0.05 | M3 | 409.40 | 0.33 | 0 |
| FF | FF | FF | FF | ||||||||||||
| Cu | 1022.20 | 13.10 | 0.24 | Cu | 1133.30 | 14.40 | 0.05 | Cu | 1529.20 | 17.70 | 0.05 | Cu | 1551.40 | 1.50 | 0.69 |
| M1 | 433.30 | 54.30 | 0.03 | M1 | 1980.60 | 18.10 | 0.06 | M1 | 1719.40 | 101.20 | 0.06 | M1 | 715.30 | 2.00 | 0.07 |
| M2 | 227.80 | 13.30 | 0.07 | M2 | 311.10 | 31.40 | 0.05 | M2 | 227.80 | 21.60 | 0.06 | M2 | 325.00 | 1.78 | 1.70 |
| M3 | 286.10 | 27.40 | 0.08 | M3 | 513.90 | 19.90 | 0.04 | M3 | 425.00 | 0.74 | 1.03 | M3 | 425.00 | 0.40 | 0.08 |
| TG | TG | TG | TG | ||||||||||||
| Cu | 100.00 | 16.70 | 0.02 | Cu | 106.90 | 18.50 | 0.04 | Cu | 108.30 | 18.80 | 0.24 | Cu | 156.90 | 0.30 | 0.25 |
| M1 | 80.60 | 70.40 | 0.01 | M1 | 91.70 | 23.70 | 0 | M1 | 463.90 | 69.80 | 0.03 | M1 | 136.10 | 0.60 | 0 |
| M2 | 350.00 | 38.90 | 0 | M2 | 169.40 | 31.20 | 0.05 | M2 | 450.40 | 8.00 | 0.04 | M2 | 54.70 | 1.65 | 0.05 |
| M3 | 1.67 | 41.80 | 0.03 | M3 | 380.60 | 19.40 | 0 | M3 | 488.90 | 14.30 | 0 | M3 | 51.40 | 0.74 | 0.04 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrsanska, M.; Voberkova, S.; Langer, V.; Palovcikova, D.; Moulick, A.; Adam, V.; Kopel, P. Induction of Laccase, Lignin Peroxidase and Manganese Peroxidase Activities in White-Rot Fungi Using Copper Complexes. Molecules 2016, 21, 1553. https://doi.org/10.3390/molecules21111553
Vrsanska M, Voberkova S, Langer V, Palovcikova D, Moulick A, Adam V, Kopel P. Induction of Laccase, Lignin Peroxidase and Manganese Peroxidase Activities in White-Rot Fungi Using Copper Complexes. Molecules. 2016; 21(11):1553. https://doi.org/10.3390/molecules21111553
Chicago/Turabian StyleVrsanska, Martina, Stanislava Voberkova, Vratislav Langer, Dagmar Palovcikova, Amitava Moulick, Vojtech Adam, and Pavel Kopel. 2016. "Induction of Laccase, Lignin Peroxidase and Manganese Peroxidase Activities in White-Rot Fungi Using Copper Complexes" Molecules 21, no. 11: 1553. https://doi.org/10.3390/molecules21111553
APA StyleVrsanska, M., Voberkova, S., Langer, V., Palovcikova, D., Moulick, A., Adam, V., & Kopel, P. (2016). Induction of Laccase, Lignin Peroxidase and Manganese Peroxidase Activities in White-Rot Fungi Using Copper Complexes. Molecules, 21(11), 1553. https://doi.org/10.3390/molecules21111553









