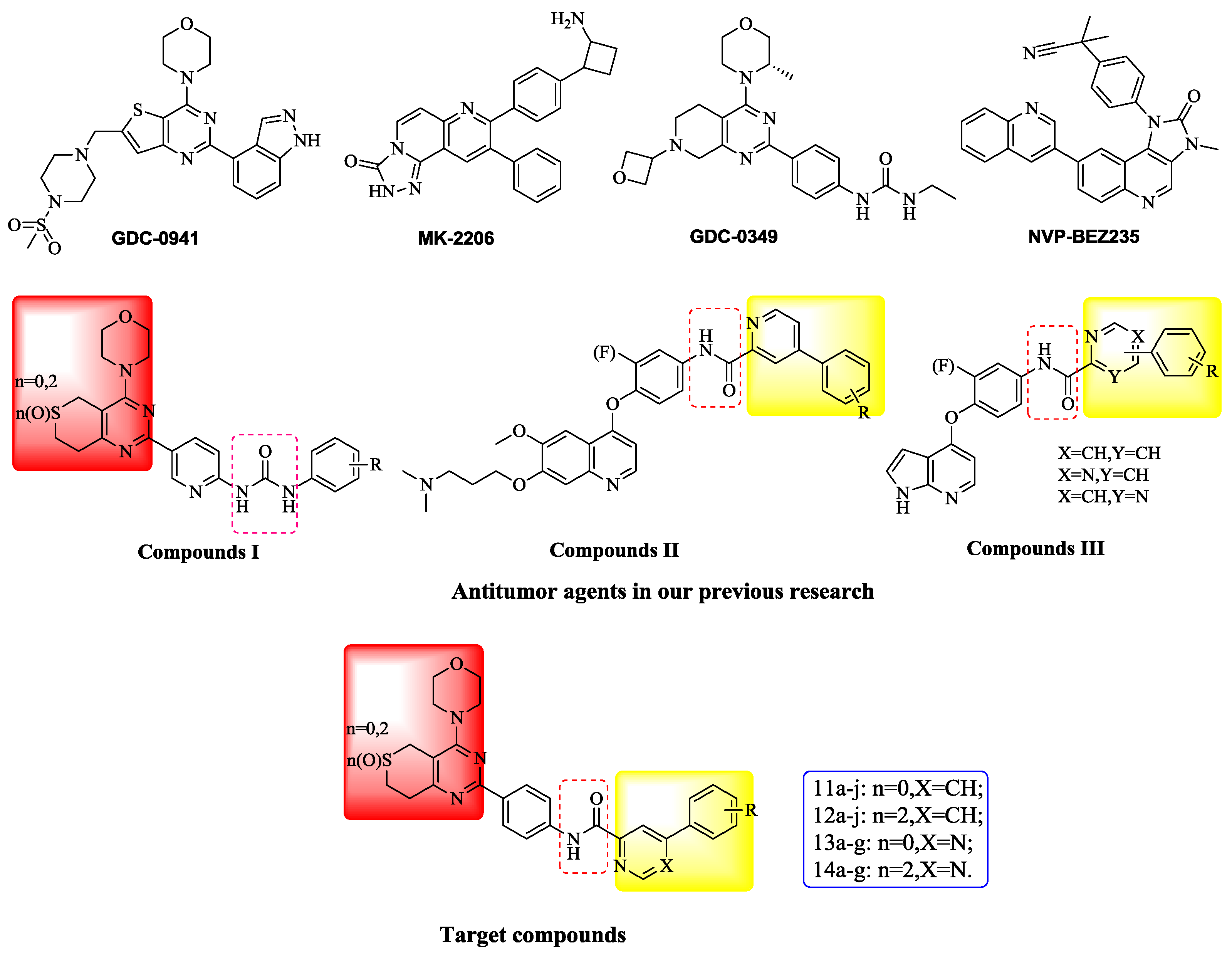
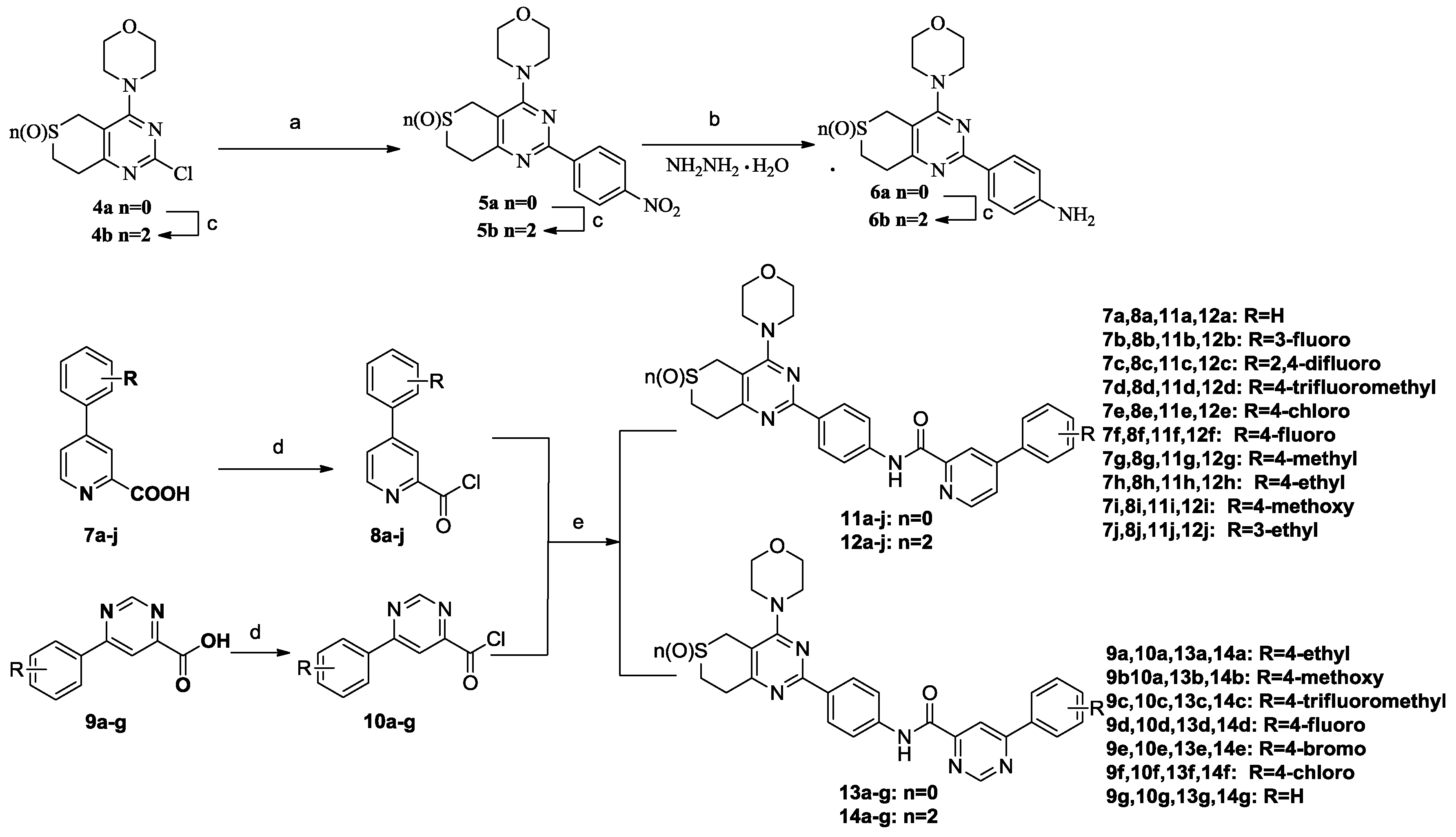
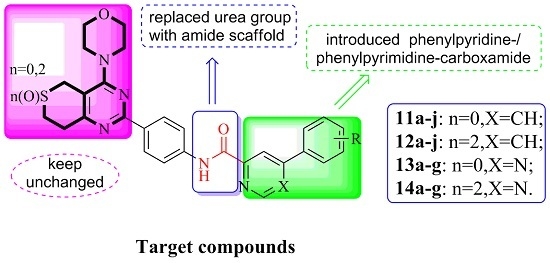
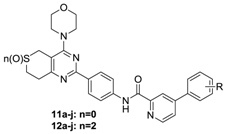
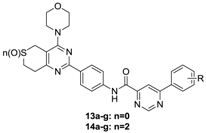
3.2.3. General Procedure for the Preparation of Target Compounds 11a–j, 12a–j, 13a–g and 14a–g
A solution of phenylpyrimidine chloride (0.01 mol, 8a–j and 10a–g) in dichloromethane (10 mL) was added dropwise to a solution of 6a or 6b (0.075 mol) and N,N-diisopropylethylamine (0.03 mol) in dichloromethane (10 mL) in an ice bath. Upon completion of the addition, the reaction mixture was removed from the ice bath, held at room temperature for 15 min and monitored by thin-layer chromatography (TLC). The mixture was washed with 10% K2CO3 (50 mL × 3) followed by brine (50 mL × 1), and the organic phase was separated, dried over anhydrous sodium sulfate, and evaporated to yield the target compounds 11a–11j, 12a–12j, 13a–13g and 14a–14g, which were recrystallized from isopropanol.
N-(4-(4-Morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-phenylpyridine carboxamide (11a). A pale yellow solid; Yield: 66.8%; m.p.: 244.1–242.8 °C; ESI-MS [M + H] m/z: 510.2; 1H-NMR (CDCl3) δ 10.55 (s, 1H), 8.75 (d, J = 5.1 Hz, 1H), 8.71 (d, J = 8.3 Hz, 2H), 8.65 (s, 1H), 8.04 (d, J = 8.2 Hz, 2H), 7.77 (d, J = 3.8 Hz, 1H), 7.60 (d, J = 7.7 Hz, 1H), 7.52 (s, 1H), 7.52–7.48 (m, 1H), 7.47 (s, 1H), 7.20 (d, J = 2.1 Hz, 1H), 3.95 (s, 4H), 3.89 (s, 6H), 3.64 (s, 2H), 3.04 (s, 2H).
N-(4-(4-Morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(3-fluorophenyl)pyridine amide (11b). A pale yellow solid; Yield: 65.3%; m.p.: 236.5–238.4 °C; ESI-MS [M + H] m/z: 528.1; 1H-NMR (CDCl3) δ 11.02 (s, 1H), 8.95 (s, 1H), 8.82 (d, J = 5.4 Hz, 1H), 8.63 (d, J = 8.7 Hz, 2H), 8.08 (d, J = 8.7 Hz, 2H), 7.91 (d, J = 2.1 Hz, 2H), 7.90 (s, 1H), 7.56 (s, 1H), 7.54 (d, J = 1.8 Hz, 1H), 3.95 (s, 4H), 3.87 (s, 4H), 3.84 (s, 2H), 3.65 (s, 2H), 3.05 (d, J = 6.0 Hz, 2H).
N-(4-(4-Morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(2,4-difluorophenyl) picolinamide (11c). A pale yellow solid; Yield: 66.6%; m.p.: 235.0–236.7 °C; ESI-MS [M + H] m/z: 546.1; 1H-NMR (CDCl3) δ 10.42 (s, 1H), 8.73 (d, J = 3.1 Hz, 2H), 8.72 (s, 1H), 8.49 (s, 1H), 8.03 (d, J = 8.5 Hz, 2H), 7.74–7.71 (m, 1H), 7.61 (td, J = 8.7, 6.3 Hz, 1H), 7.07 (td, J = 8.1, 2.3 Hz, 1H), 7.00 (ddd, J = 11.0, 8.7, 2.5 Hz, 1H), 3.94 (s, 4H), 3.89 (s, 6H), 3.62 (s, 2H), 3.04 (s, 2H).
N-(4-(4-Morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(4-trifluoromethylphenyl) picolinamide (11d). A yellow solid; Yield: 64.9%; m.p.: 229.3–231.7 °C; ESI-MS [M + H] m/z: 578.2; 1H-NMR (DMSO-d6) δ 10.86 (s, 1H), 8.87 (s, 1H), 8.47 (s, 1H), 8.34 (d, J = 8.3 Hz, 2H), 8.13 (d, J = 7.5 Hz, 2H), 8.07 (s, 2H), 8.05 (s, 1H), 7.93 (d, J = 7.9 Hz, 2H), 3.76 (s, 4H), 3.71 (s, 2H), 3.39 (s, 4H), 3.07 (s, 2H), 3.00 (s, 2H).
N-(4-(4-Morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(4-chlorophenyl)pyridine amide (11e). A pale yellow solid; Yield: 65.1%; m.p.: 235.3–236.2 °C; ESI-MS [M + H] m/z: 545.2; 1H-NMR (CDCl3) δ 12.09 (s, 1H), 9.81 (s, 1H), 9.14 (s, 1H), 8.30 (s, 3H), 8.23 (s, 2H), 8.10 (s, 2H), 7.56 (s, 2H), 3.95 (s, 4H), 3.88 (s, 4H), 3.75 (s, 2H), 3.68 (s, 2H), 3.08 (s, 2H).
N-(4-(4-Morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(4-fluorophenyl)pyridine amide (11f). A yellow solid; Yield: 63.7%; m.p.: 248.1–249.6 °C; ESI-MS [M + H] m/z: 528.2; 1H-NMR (DMSO-d6) δ 10.84 (s, 1H), 8.82 (s, 1H), 8.43 (s, 1H), 8.35 (d, J = 8.3 Hz, 2H), 8.07 (d, J = 8.4 Hz, 2H), 8.01 (s, 3H), 7.43 (d, J = 8.8 Hz, 2H), 3.78 (s, 4H), 3.73 (s, 2H), 3.40 (s, 4H), 3.09 (s, 2H), 3.02 (s, 2H).
N-(4-(4-Morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(4-methylphenyl) picolinamide (11g). A pale yellow solid; Yield: 64.1%; m.p.: 275.0–276.8 °C; ESI-MS [M + H] m/z: 524.2; 1H-NMR (DMSO-d6) δ 10.84 (s, 1H), 8.79 (s, 1H), 8.42 (s, 1H), 8.35 (d, J = 8.0 Hz, 2H), 8.07 (d, J = 8.7 Hz, 2H), 8.02–7.98 (m, 1H), 7.83 (d, J = 7.3 Hz, 2H), 7.40 (d, J = 7.9 Hz, 2H), 3.78 (s, 4H), 3.73 (s, 2H), 3.41 (s, 4H), 3.09 (s, 2H), 3.02 (s, 2H), 2.41 (s, 3H).
N-(4-(4-Morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(4-ethylphenyl) picolinamide (11h). A pale yellow solid; Yield: 67.3%; m.p.: 229.8–232.1 °C; ESI-MS [M + H] m/z: 538.2; 1H-NMR (DMSO-d6) δ 10.82 (s, 1H), 8.79 (s, 1H), 8.41 (s, 1H), 8.33 (d, J = 8.4 Hz, 2H), 8.05 (d, J = 9.1 Hz, 2H), 7.99 (s, 1H), 7.83 (d, J = 8.0 Hz, 2H), 7.41 (d, J = 7.8 Hz, 2H), 3.76 (s, 4H), 3.71 (s, 2H), 3.37 (s, 4H), 3.08 (s, 2H), 3.00 (s, 2H), 2.69 (d, J = 7.5 Hz, 2H), 1.22 (s, 3H).
N-(4-(4-Morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(4methoxyphenyl) pyridine-carboxamide (11i). A pale yellow solid; Yield: 63.7%; m.p.: 233.6–235.1 °C; ESI-MS [M + H] m/z: 540.2; 1H-NMR (DMSO-d6) δ 10.84 (s, 1H), 8.76 (d, J = 5.2 Hz, 1H), 8.40 (d, J = 1.3 Hz, 1H), 8.36 (s, 1H), 8.34 (s, 1H), 8.08 (s, 1H), 8.06 (s, 1H), 7.98 (dd, J = 5.2, 1.9 Hz, 1H), 7.92–7.89 (m, 1H), 7.89 (d, J = 2.1 Hz, 1H), 7.14 (d, J = 5.4 Hz, 1H), 7.13 (s, 1H), 3.85 (s, 3H), 3.80–3.74 (m, 4H), 3.73 (s, 2H), 3.40 (s, 4H), 3.09 (t, J = 6.4 Hz, 2H), 3.01 (t, J = 6.3 Hz, 2H).
N-(4-(4-Morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(3-methylphenyl) pyridine carboxamide (11j). A white solid; Yield: 65.6%; m.p.: >300.0 °C; ESI-MS [M + H] m/z: 542.1; 1H-NMR (DMSO-d6) δ 10.83 (s, 1H), 8.80 (s, 1H), 8.41 (s, 1H), 8.34 (d, J = 8.1 Hz, 2H), 8.05 (d, J = 8.7 Hz, 2H), 8.00 (s, 1H), 7.72 (s, 1H), 7.67 (s, 1H), 7.46 (s, 1H), 7.35 (s, 1H), 3.76 (s, 4H), 3.71 (s, 2H), 3.39 (s, 4H), 3.07 (s, 2H), 3.00 (s, 2H), 2.43 (s, 3H).
N-(4-(4-Morpholino-7,8-dihydro-6,6-dioxide-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-phenylpyridine Amide (12a). A white solid; Yield: 61.9%; m.p.: 229.0–233.8 °C; ESI-MS [M + H] m/z: 524.1; 1H-NMR (DMSO-d6) δ 10.87 (s, 1H), 8.82 (s, 1H), 8.42 (s, 1H), 8.35 (d, J = 8.6 Hz, 2H), 8.08 (d, J = 8.4 Hz, 2H), 8.02 (s, 1H), 7.90 (d, J = 7.8 Hz, 2H), 7.57 (d, J = 7.4 Hz, 3H), 4.35 (s, 2H), 3.76 (s, 4H), 3.56 (s, 2H), 3.39 (s, 6H).
N-(4-(4-Morpholino-7,8-dihydro-6,6-dioxide-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(3-fluoro-phenyl) pyridinecarboxamide (12b). A white solid; Yield: 62.3%; m.p.: 281.5–283.8 °C; ESI-MS [M + H] m/z: 560.1; 1H-NMR (DMSO-d6) δ 10.90 (s, 1H), 8.84 (d, J = 5.1 Hz, 1H), 8.45 (d, J = 1.2 Hz, 1H), 8.36 (d, J = 8.8 Hz, 2H), 8.09 (d, J = 8.9 Hz, 2H), 8.07 (dd, J = 5.1, 1.9 Hz, 1H), 7.84–7.76 (m, 2H), 7.66–7.60 (m, 1H), 7.42– 7.35 (m, 1H), 4.37 (s, 2H), 3.77 (d, J = 4.5 Hz, 4H), 3.58 (t, J = 6.4 Hz, 2H), 3.40 (t, J = 5.1 Hz, 6H).
N-(4-(4-Morpholino-7,8-dihydro-6,6-dioxide-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(2,4-difluorophenyl) picolinamide (12c). A pale yellow solid; Yield: 63.0%; m.p.: 269.0–272.6 °C; ESI-MS [M + H] m/z: 578.1; 1H-NMR (DMSO-d6) δ 10.91 (s, 1H), 8.86 (d, J = 5.0 Hz, 1H), 8.36 (d, J = 8.7 Hz, 2H), 8.32 (s, 1H), 8.09 (d, J = 8.8 Hz, 2H), 7.90 (d, J = 5.1 Hz, 1H), 7.87–7.81 (m, 1H), 7.55–7.47 (m, 1H), 7.32 (t, J = 8.3 Hz, 1H), 4.37 (s, 2H), 3.78 (s, 4H), 3.57 (d, J = 6.6 Hz, 2H), 3.40 (t, J = 5.2 Hz, 6H).
N-(4-(4-Morpholino-7,8-dihydro-6,6-dioxide-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(4-trifluoro-methylphenyl) pyridinecarboxamide (12d). A white solid; Yield: 62.4%; m.p.: >300.0 °C; ESI-MS [M + H] m/z: 610.1; 1H-NMR (DMSO-d6) δ 10.92 (s, 1H), 8.88 (d, J = 5.1 Hz, 1H), 8.49 (d, J = 1.2 Hz, 1H), 8.35 (s, 2H), 8.15 (d, J = 8.1 Hz, 2H), 8.11 (s, 3H), 7.94 (d, J = 8.3 Hz, 2H), 4.37 (s, 2H), 3.81–3.73 (m, 4H), 3.58 (t, J = 6.6 Hz, 2H), 3.40 (d, J = 4.5 Hz, 6H).
N-(4-(4-Morpholino-7,8-dihydro-6,6-dioxide-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(4-chloro-phenyl) pyridinecarboxamide (12e). A pale yellow solid; Yield: 62.1%; m.p.: 266.8–268.8 °C; ESI-MS [M + H] m/z: 577.1; 1H-NMR (DMSO-d6) δ 10.90 (s, 1H), 8.83 (d, J = 5.1 Hz, 1H), 8.43 (d, J = 1.3 Hz, 1H), 8.36 (d, J = 8.8 Hz, 2H), 8.09 (d, J = 8.9 Hz, 2H), 8.04 (dd, J = 5.2, 1.9 Hz, 1H), 7.98–7.94 (m, 2H), 7.66–7.62 (m, 2H), 4.37 (s, 2H), 3.80–3.76 (m, 4H), 3.58 (t, J = 6.5 Hz, 2H), 3.40 (s, 6H).
N-(4-(4-Morpholino-7,8-dihydro-6,6-dioxide-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(4-fluoro-phenyl) pyridincarboxamide (12f). A white solid; Yield: 63.3%; m.p.: >300.0 °C; ESI-MS [M + H] m/z: 560.2; 1H-NMR (DMSO-d6) δ 10.89 (s, 1H), 8.82 (d, J = 5.1 Hz, 1H), 8.42 (d, J = 1.4 Hz, 1H), 8.36 (d, J = 8.8 Hz, 2H), 8.09 (d, J = 8.9 Hz, 2H), 8.04–7.96 (m, 3H), 7.42 (t, J = 8.8 Hz, 2H), 4.37 (s, 2H), 3.77 (d, J = 4.8 Hz, 4H), 3.57 (t, J = 6.5 Hz, 2H), 3.40 (t, J = 5.2 Hz, 6H).
N-(4-(4-Morpholino-7,8-dihydro-6,6-dioxide-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(4-methylphenyl) pyridinecarboxamide (12g). A pale yellow solid; Yield: 64.1%; m.p.: 290.2–293.1 °C; ESI-MS [M + H] m/z: 556.2; 1H-NMR (CDCl3) δ 10.88 (s, 1H), 8.78 (d, J = 5.1 Hz, 1H), 8.40 (s, 1H), 8.35 (d, J = 8.7 Hz, 2H), 8.08 (d, J = 8.7 Hz, 2H), 7.99 (d, J = 5.2 Hz, 1H), 7.81 (d, J = 8.0 Hz, 2H), 7.38 (d, J = 8.0 Hz, 2H), 4.36 (s, 2H), 3.76 (s, 4H), 3.57 (t, J = 6.4 Hz, 2H), 3.41–3.36 (m, 6H), 2.38 (s, 3H).
N-(4-(4-Morpholino-7,8-dihydro-6,6-dioxide-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(4-ethylphenyl) picolinamide (12h). A pale yellow solid; Yield: 61.8%; m.p.: 276.8–279.1 °C; ESI-MS [M + H] m/z: 570.2; 1H-NMR (DMSO-d6) δ 10.88 (s, 1H), 8.79 (d, J = 5.1 Hz, 1H), 8.42 (d, J = 1.3 Hz, 1H), 8.36 (d, J = 8.8 Hz, 2H), 8.09 (d, J = 8.9 Hz, 2H), 8.01 (dd, J = 5.2, 1.9 Hz, 1H), 7.84 (d, J = 8.2 Hz, 2H), 7.42 (d, J = 8.2 Hz, 2H), 4.37 (s, 2H), 3.80–3.75 (m, 4H), 3.57 (t, J = 6.6 Hz, 2H), 3.40 (s, 6H), 2.70 (dd, J = 15.6, 8.1 Hz, 2H), 1.24 (t, 3H).
N-(4-(4-Morpholino-7,8-dihydro-6,6-dioxide-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(4-methoxy-phenyl) pyridinecarboxamide (12i). A white solid; Yield: 63.1%; m.p.: 289.0–292.1 °C; ESI-MS [M + H] m/z: 572.2; 1H-NMR (DMSO-d6) δ 10.87 (s, 1H), 8.76 (d, J = 5.1 Hz, 1H), 8.40 (d, J = 1.3 Hz, 1H), 8.36 (d, J = 8.8 Hz, 2H), 8.09 (d, J = 8.8 Hz, 2H), 7.98 (dd, J = 5.2, 1.9 Hz, 1H), 7.90 (d, J = 8.9 Hz, 2H), 7.13 (d, J = 8.9 Hz, 2H), 4.37 (s, 2H), 3.85 (s, 3H), 3.80–3.76 (m, 4H), 3.57 (t, J = 6.5 Hz, 2H), 3.40 (t, J = 5.4 Hz, 6H).
N-(4-(4-Morpholino-7,8-dihydro-6,6-dioxide-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-4-(3-methylphenyl) pyridinecarboxamide (12j). A white solid; Yield: 62.5%; m.p.: 287.2–290.0 °C; ESI-MS [M + H] m/z: 556.1; 1H-NMR (CDCl3) δ 10.90 (s, 1H), 8.80 (d, J = 5.1 Hz, 1H), 8.41 (s, 1H), 8.35 (d, J = 8.6 Hz, 2H), 8.08 (d, J = 8.8 Hz, 2H), 8.00 (d, J = 5.2 Hz, 1H), 7.73 (s, 1H), 7.69 (d, J = 7.9 Hz, 1H), 7.46 (t, J = 7.8 Hz, 1H), 7.34 (d, J = 8.2 Hz, 1H), 4.36 (s, 2H), 3.77 (s, 4H), 3.57 (t, J = 6.6 Hz, 2H), 3.38 (d, J = 5.3 Hz, 6H), 2.42 (s, 3H).
N-(4-(4-Morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-6-(p-tolyl)pyrimidine-4-formamide (13a). A pale yellow solid; Yield: 61.8%; m.p.: 257.0–259.7 °C; ESI-MS [M + H] m/z: 525.2; 1H-NMR (CDCl3) δ 10.23 (s, 1H), 9.34 (s, 1H), 8.77 (d, J = 8.4 Hz, 2H), 8.63 (s, 1H), 8.16 (d, J = 7.9 Hz, 2H), 8.03 (d, J = 8.4 Hz, 2H), 7.39 (d, J = 7.8 Hz, 2H), 3.97 (s, 4H), 3.90 (d, J = 13.0 Hz, 6H), 3.64 (s, 2H), 3.05 (s, 2H), 2.48 (s, 3H).
N-(4-(4-Morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-6-(p-methoxyphenyl) pyridine-4-carboxamide (13b). A pale yellow solid; Yield: 64.1%; m.p.: 210.0–212.7 °C; ESI-MS [M + H] m/z: 541.2; 1H-NMR (DMSO-d6) δ 11.01 (s, 1H), 9.39 (s, 1H), 8.56 (s, 1H), 8.35 (t, J = 10.1 Hz, 4H), 8.09 (s, 2H), 7.16 (d, J = 8.6 Hz, 2H), 3.88 (s, 4H), 3.78 (s, 3H), 3.73 (s, 2H), 3.41 (s, 4H), 3.09 (s, 2H), 3.02 (s, 2H).
N-(4-(4-Morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-6-(4-trifluoromethylphenyl) pyridine-4-carboxamide (13c). A pale yellow solid; Yield: 61.0%; m.p.: 244.2–245.8 °C; ESI-MS [M + H] m/z: 579.1; 1H-NMR (CDCl3) δ 10.11 (s, 1H), 9.40 (s, 1H), 8.69 (s, 1H), 8.59 (s, 2H), 8.36 (d, J = 8.1 Hz, 2H), 7.95 (d, J = 8.3 Hz, 2H), 7.83 (d, J = 8.4 Hz, 2H), 3.89 (s, 4H), 3.66 (s, 2H), 3.63 (s, 4H), 3.45 (s, 2H), 3.04 (s, 2H).
N-(4-(4-Morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-6-(4-fluorophenyl) pyridine-4-carboxamide (13d). A pale yellow solid; Yield: 65.1%; m.p.: 237.0–239.3 °C; ESI-MS [M + H] m/z: 529.1; 1H-NMR (CDCl3) δ 10.16 (s, 1H), 9.33 (s, 1H), 8.70 (d, J = 8.6 Hz, 2H), 8.59 (s, 1H), 8.31–8.22 (m, 2H), 7.99 (d, J = 8.6 Hz, 2H), 7.24 (d, J = 8.6 Hz, 2H), 3.88 (s, 8H), 3.75 (s, 2H), 3.63 (s, 2H), 3.03 (s, 2H).
N-(4-(4-Morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-6-(4-bromophenyl) pyridine-4-carboxamide (13e). A yellow solid; Yield: 61.4%; m.p.: 251.8–255.0 °C; ESI-MS [M + H] m/z: 590.1; 1H-NMR (CDCl3) δ 10.19 (s, 1H), 9.35 (s, 1H), 8.75 (s, 2H), 8.61 (s, 1H), 8.12 (d, J = 8.4 Hz, 2H), 8.01 (d, J = 8.2 Hz, 2H), 7.70 (d, J = 8.3 Hz, 2H), 3.96 (s, 4H), 3.90 (s, 4H), 3.87–3.81 (m, 2H), 3.63 (s, 2H), 3.04 (s, 2H).
N-(4-(4-Morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-6-(4-chlorophenyl) pyridine-4-carboxamide (13f). A yellow solid; Yield: 61.5%; m.p.: 281.8–285.0 °C; ESI-MS [M + H] m/z: 546.1; 1H-NMR (DMSO-d6) δ 11.05 (s, 1H), 9.49 (s, 1H), 8.65 (s, 1H), 8.37 (s, 4H), 8.07 (d, J = 8.4 Hz, 2H), 7.68 (d, J = 8.6 Hz, 2H), 3.77 (s, 4H), 3.72 (s, 2H), 3.40 (s, 4H), 3.09 (s, 2H), 3.01 (s, 2H).
N-(4-(4-Morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-6-phenylpyridine-4-carboxamide (13g). A yellow solid; Yield: 63.8%; m.p.: 250.1–252.3 °C; ESI-MS [M + H] m/z: 511.2; 1H-NMR (DMSO-d6) δ 11.04 (s, 1H), 9.48 (s, 1H), 8.63 (s, 1H), 8.35 (t, J = 7.3 Hz, 4H), 8.07 (d, J = 8.7 Hz, 2H), 7.62 (d, J = 6.6 Hz, 3H), 3.77 (s, 4H), 3.72 (s, 2H), 3.39 (s, 4H), 3.08 (d, J = 5.6 Hz, 2H), 3.02 (d, J = 5.6 Hz, 2H).
N-(4-(4-Morpholino-7,8-dihydro-6,6-dioxide-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-6-(p-tolyl) pyrimidine-4-carboxamide (14a). A pale yellow solid; Yield: 62.7%; m.p.: 260.1–263.0 °C; ESI-MS [M + H] m/z: 557.2; 1H-NMR (DMSO-d6) δ 11.05 (s, 1H), 9.43 (s, 1H), 8.58 (s, 1H), 8.37 (d, J = 8.6 Hz, 2H), 8.24 (d, J = 8.0 Hz, 2H), 8.09 (d, J = 8.6 Hz, 2H), 7.41 (d, J = 7.9 Hz, 2H), 4.38 (s, 2H), 3.78 (s, 4H), 3.59 (t, J = 6.0 Hz, 2H), 3.40 (d, J = 14.5 Hz, 6H), 2.41 (s, 3H).
N-(4-(4-Morpholino-7,8-dihydro-6,6-dioxide-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-6-(p-methoxyphenyl) pyridine-4-carboxamide (14b). A yellow solid; Yield: 61.7%; m.p.: 277.0–279.5 °C; ESI-MS [M + H] m/z: 573.1; 1H-NMR (DMSO-d6) δ 11.03 (s, 1H), 9.37 (s, 1H), 8.53 (s, 1H), 8.35 (d, J = 8.4 Hz, 2H), 8.30 (d, J = 9.0 Hz, 2H), 8.07 (d, J = 8.7 Hz, 2H), 7.12 (d, J = 8.9 Hz, 2H), 4.35 (s, 2H), 3.85 (s, 3H), 3.84–3.80 (m, 2H), 3.75 (s, 4H), 3.56 (s, 6H).
N-(4-(4-Morpholino-7,8-dihydro-6,6-dioxide-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-6-(4-trifluoro-methylphenyl) pyridine-4-carboxamide (14c). A pale yellow solid; Yield: 62.5%; m.p.: 287.1–289.2 °C; ESI-MS [M + H] m/z: 611.1; 1H-NMR (DMSO-d6) δ 11.12 (s, 1H), 9.53 (s, 1H), 8.71 (s, 1H), 8.53 (d, J = 7.9 Hz, 2H), 8.35 (d, J = 8.6 Hz, 2H), 8.08 (d, J = 8.6 Hz, 2H), 7.95 (d, J = 7.3 Hz, 2H), 4.36 (s, 2H), 3.75 (s, 6H), 3.54 (s, 6H).
N-(4-(4-Morpholino-7,8-dihydro-6,6-dioxide-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-6-(4-fluoro-phenyl) pyridine-4-carboxamide (14d). A yellow solid; Yield: 61.3%; m.p.: >300 °C; ESI-MS [M + H] m/z: 561.1; 1H-NMR (DMSO-d6) δ 11.07 (s, 1H), 9.44 (s, 1H), 8.61 (s, 1H), 8.40 (s, 2H), 8.35 (d, J = 8.6 Hz, 2H), 8.07 (d, J = 8.2 Hz, 2H), 7.42 (s, 2H), 4.35 (s, 2H), 3.75 (s, 6H), 3.56 (s, 6H).
N-(4-(4-Morpholino-7,8-dihydro-6,6-dioxide-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-6-(4-bromophenyl) pyridine-4-carboxamide (14e). A yellow solid; Yield: 67.1%; m.p.: >300 °C; ESI-MS [M + H] m/z: 622.1; 1H-NMR (DMSO-d6) δ 11.06 (s, 1H), 9.46 (s, 1H), 8.63 (s, 1H), 8.34 (d, J = 8.9 Hz, 2H), 8.27 (d, J = 8.6 Hz, 2H), 8.06 (d, J = 8.8 Hz, 2H), 7.79 (d, J = 8.6 Hz, 2H), 4.35 (s, 2H), 3.75 (s, 4H), 3.55 (d, J = 6.2 Hz, 2H), 3.38 (s, 6H).
N-(4-(4-Morpholino-7,8-dihydro-6,6-dioxide-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-6-(4-chloro-phenyl) pyridine-4-carboxamide (14f). A white solid; Yield: 61.4%; m.p.: >300 °C; ESI-MS [M + H] m/z: 578.1; 1H-NMR (DMSO-d6) δ 11.08 (s, 1H), 9.46 (s, 1H), 8.63 (s, 1H), 8.35 (d, J = 8.5 Hz, 4H), 8.07 (d, J = 8.7 Hz, 2H), 7.65 (d, J = 8.6 Hz, 2H), 4.35 (s, 2H), 3.75 (s, 4H), 3.55 (d, J = 6.4 Hz, 2H), 3.38 (s, 6H).
N-(4-(4-Morpholino-7,8-dihydro-6,6-dioxide-5H-thiopyrano[4,3-d]pyrimidin-2-yl)phenyl)-6-phenylpyridine-4-carboxamide (14g). A green solid; Yield: 62.8%; m.p.: 294.2–296.6 °C; ESI-MS [M + H] m/z: 543.2; 1H-NMR (CDCl3) δ 10.12 (s, 1H), 9.34 (s, 1H), 8.67 (s, 1H), 8.49 (d, J = 7.8 Hz, 2H), 8.25 (s, 2H), 7.93 (d, J = 8.0 Hz, 2H), 7.59 (s, 2H), 7.58–7.55 (m, 1H), 4.18 (s, 2H), 3.89 (s, 4H), 3.61 (s, 2H), 3.45 (s, 6H).