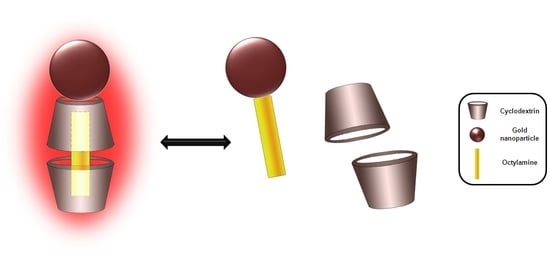
Evidence of the Disassembly of α-Cyclodextrin-octylamine Inclusion Compounds Conjugated to Gold Nanoparticles via Thermal and Photothermal Effects
Abstract
:1. Introduction
2. Results and Discussion
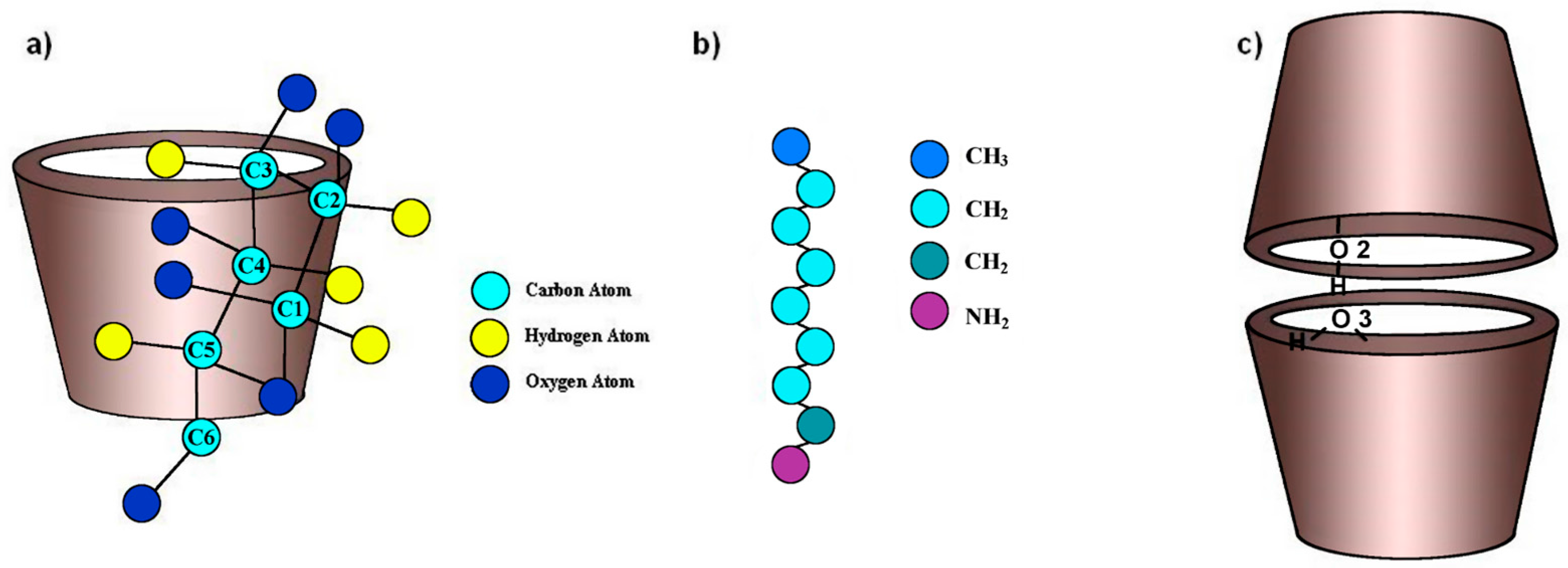
2.1. Inclusion-Compound Preparation, Stoichiometric and Binding Constant Determination
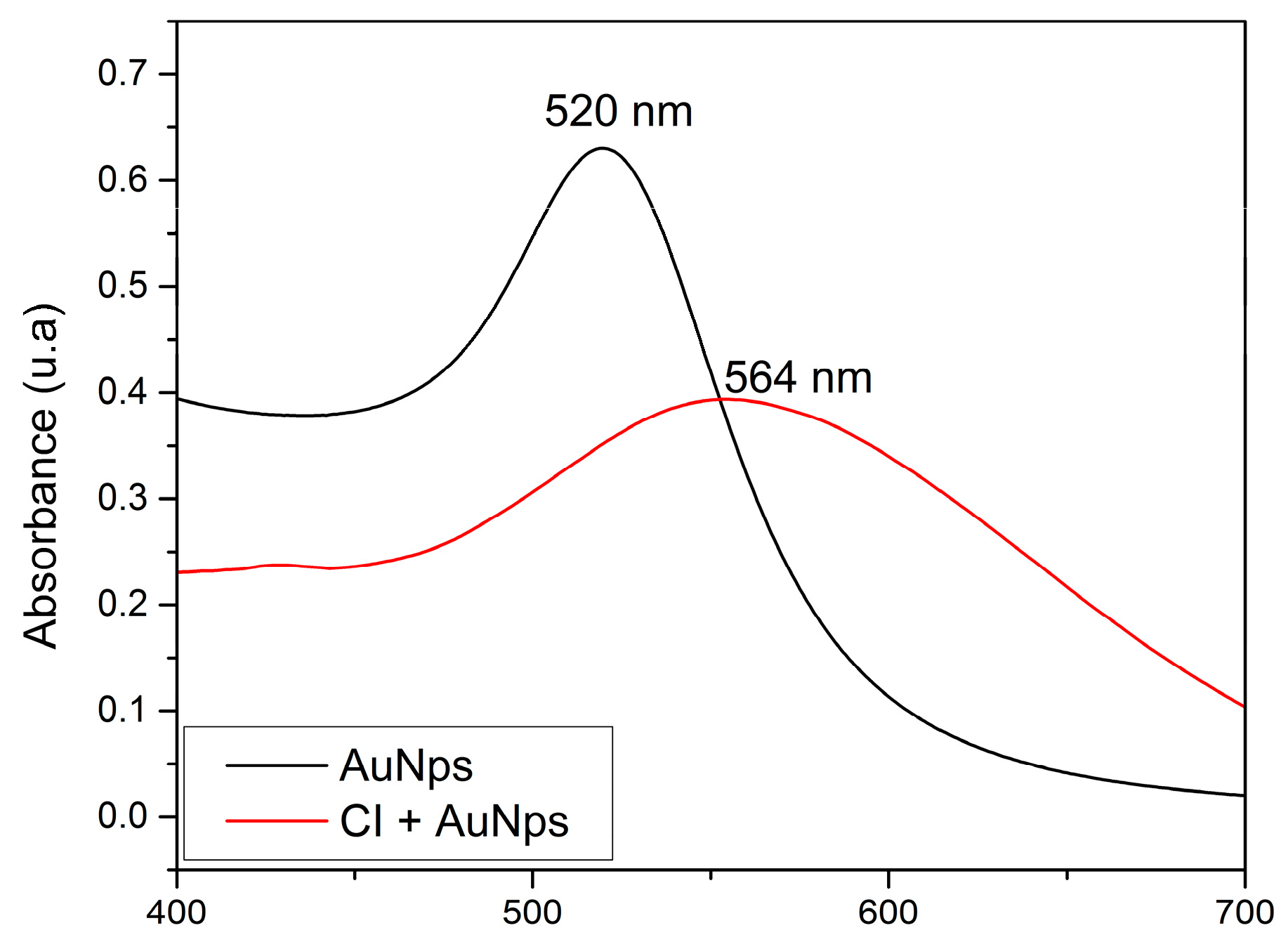
2.2. UV-Visible Absorption Spectrophotometry
2.3. Transmission Electronic Microscopy (TEM)
2.4. Scanning Electronic Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS)
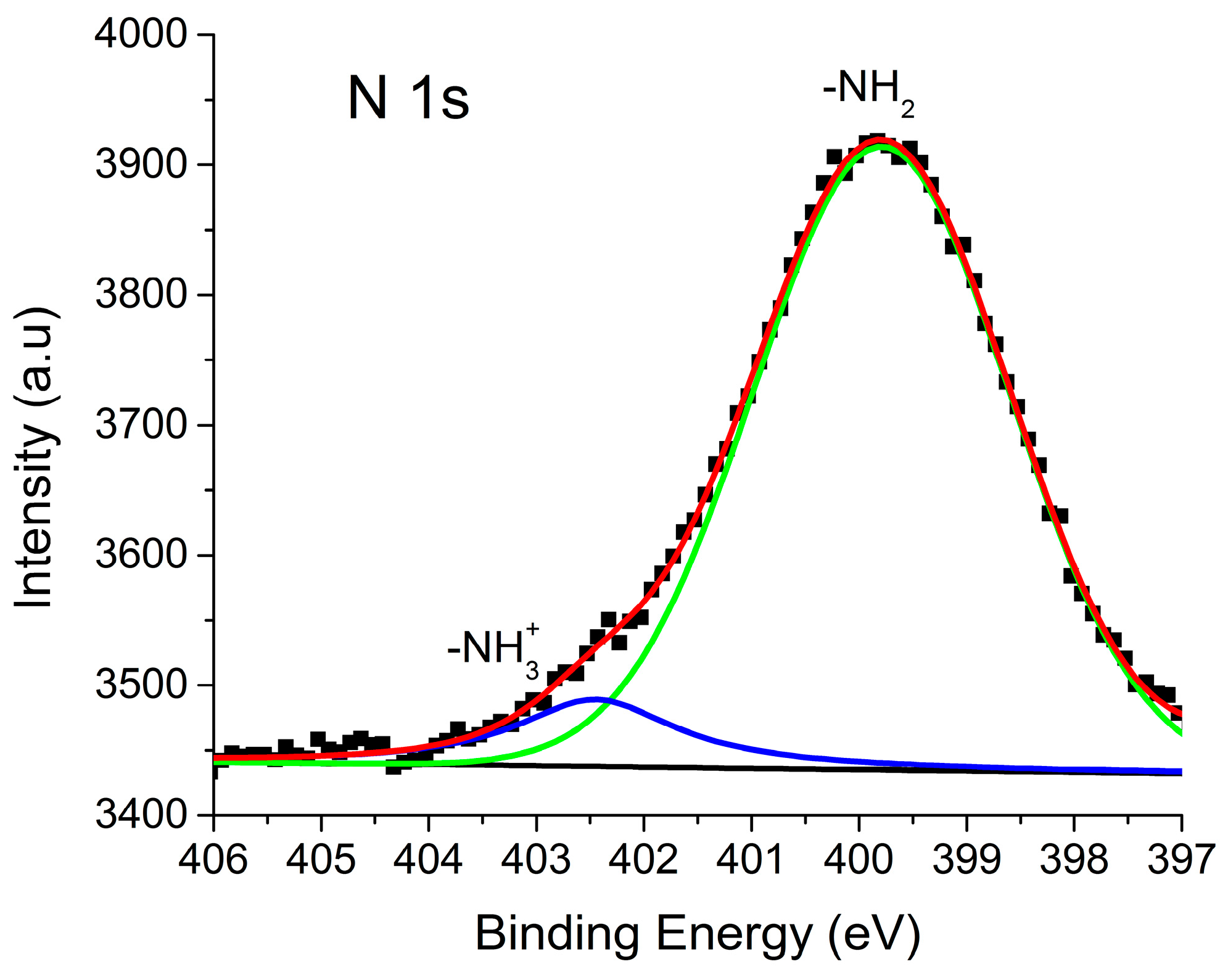
2.5. X-ray Photoelectron Spectroscopy (XPS)
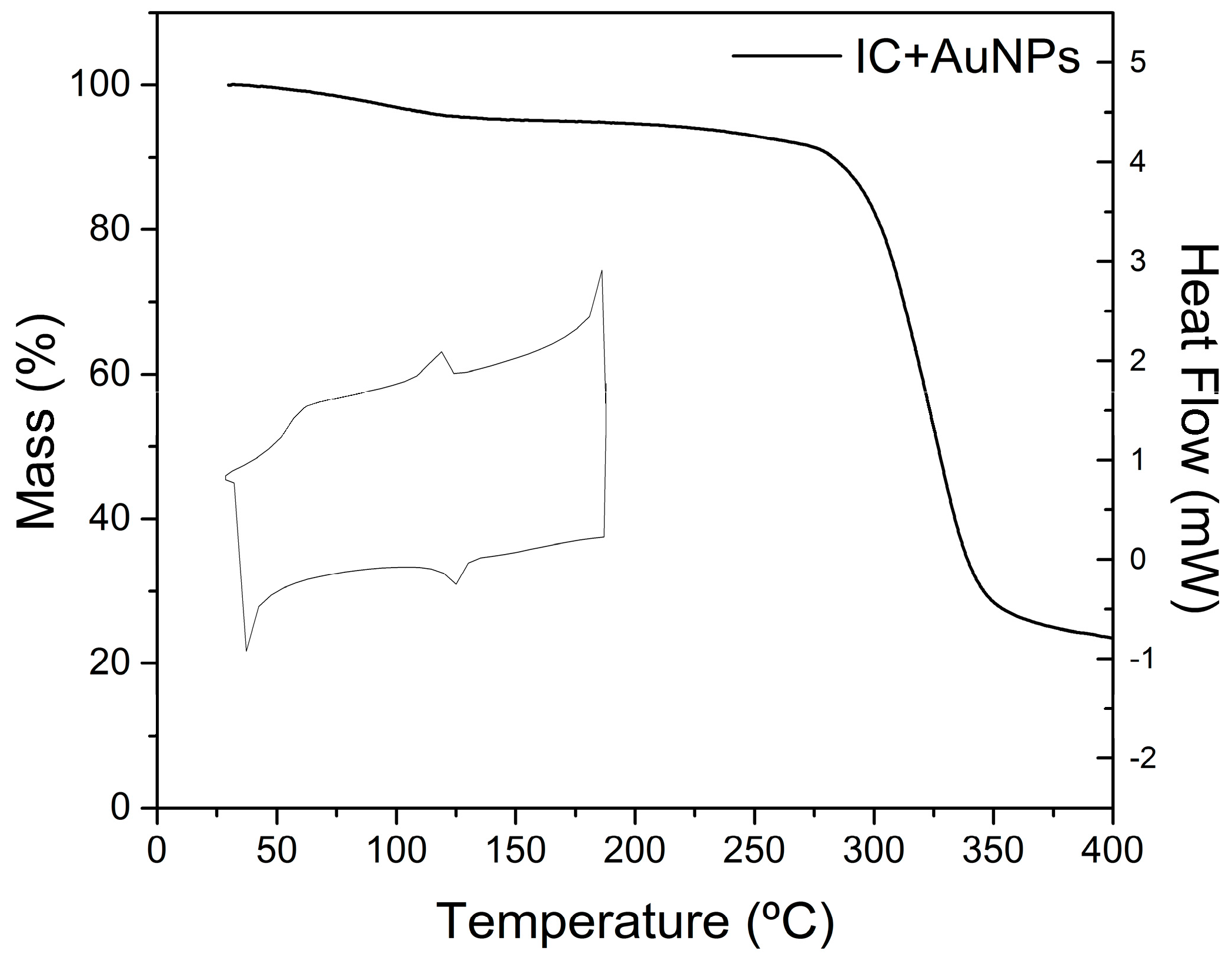
2.6. Thermal Analysis
2.7. Variable-Temperature 1H-NMR Study
2.8. 1H-NMR Study of Photothermal Effects
3. Materials and Methods
3.1. Inclusion-Compound Preparation, Stoichiometric and Binding Constant Determination
3.2. UV-Visible Absorption Spectroscopy
3.3. Transmission Electronic Microscopy
3.4. Scanning Electronic Microscopy and Energy-Dispersive X-ray Spectrometry
3.5. X-ray Photoelectron Spectroscopy
3.6. Thermal Analysis
3.7. 1H Nuclear Magnetic Resonance (1H-NMR)
3.8. 1H-NMR Study of Photothermal Effects
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
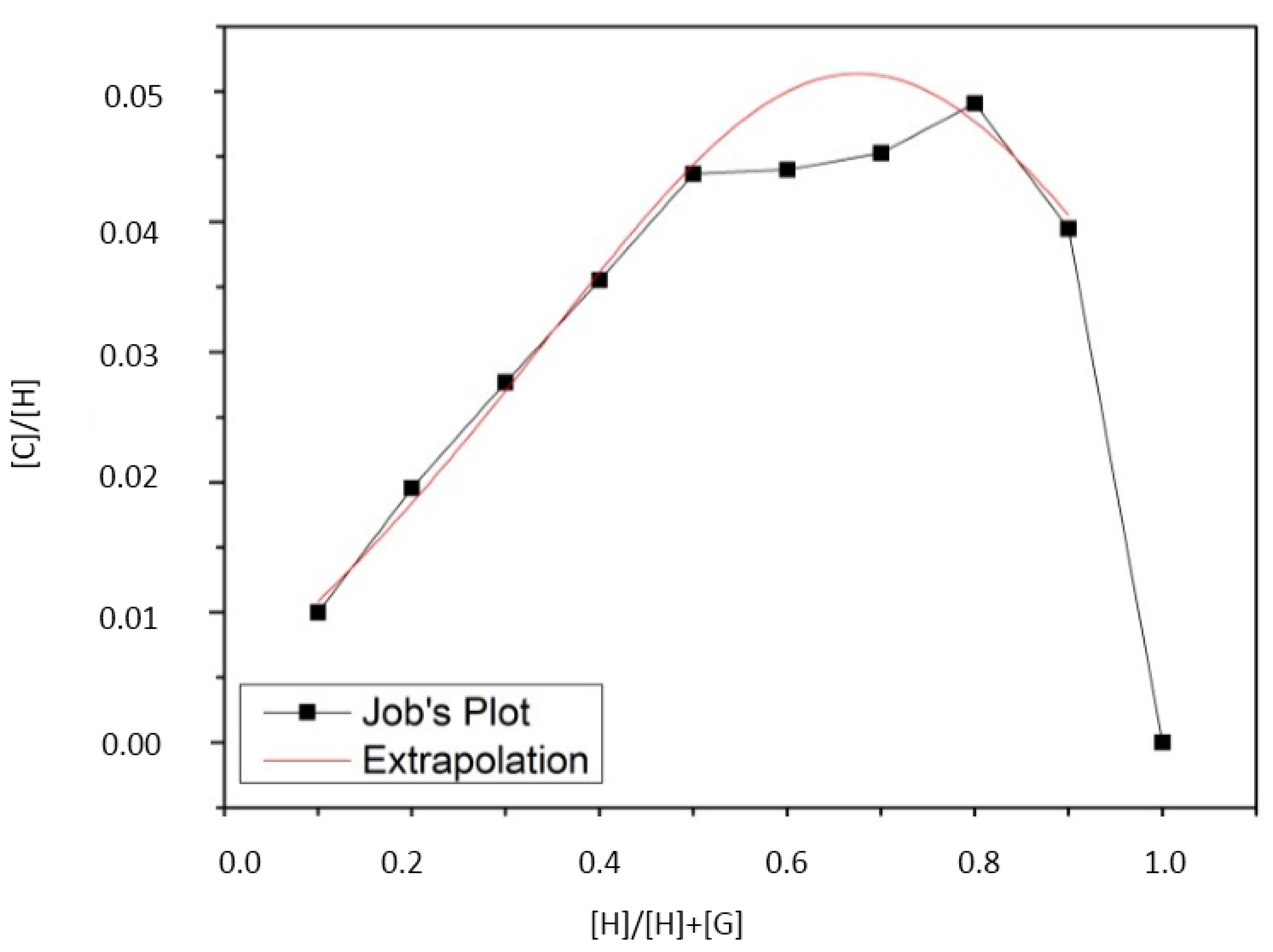
| [H] M | [G] M | [H]/[G] | k·M−2 |
|---|---|---|---|
| 0.01 | 0.09 | 0.11 | 8.90 |
| 0.02 | 0.08 | 0.25 | 4.56 |
| 0.03 | 0.07 | 0.43 | 3.23 |
| 0.04 | 0.06 | 0.67 | 2.53 |
| 0.05 | 0.05 | 1.00 | 2.06 |
| 0.06 | 0.04 | 1.50 | 2.06 |
| 0.07 | 0.03 | 2.33 | 2.01 |
| 0.08 | 0.02 | 4.00 | 1.87 |
| 0.09 | 0.01 | 9.00 | 2.38 |
References
- Sá Couto, A.; Salústio, P.; Cabral-Marques, H. Polysaccharides: Bioactivity and Biotechnology; Ramawat, G.K., Mérillon, J.M., Eds.; Springer: Cham, Switzerland, 2015; pp. 1–36. [Google Scholar]
- D’Souza, V.; Lipkowitz, K. Cyclodextrins. Chem. Rev. 1998, 98, 1741–1742. [Google Scholar] [CrossRef] [PubMed]
- Cabral Marques, H.M. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Rodríquez-Llamazares, S.; Yutronic, N.; Jara, P.; Englert, U.; Noyong, M.; Simon, U. The Structure of the First Supramolecular a-Cyclodextrin Complex with an Aliphatic Monofunctional Carboxylic Acid. Eur. J. Org. Chem. 2007, 26, 4298–4300. [Google Scholar] [CrossRef]
- Herrera, B.; Adura, C.; Yutronic, N.; Kogan, M.; Jara, P. Selective nanodecoration of modified cyclodextrin crystals with gold nanorods. J. Colloid Interface Sci. 2013, 389, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Grandelli, H.E.; Stickle, B.; Whitetington, A.; Kiran, E. Inclusion complex formation of β-cyclodextrin and Naproxen: A study on exothermic complex formation by differential scanning calorimetry. J. Incl. Phenom. Macrocycl. Chem. 2013, 77, 269–277. [Google Scholar] [CrossRef]
- Lauro, M.R.; Carbone, C.; Auditore, R.; Musumeci, T.; Santagati, N.A.; Aquino, R.P.; Puglisi, G. A new inclusion complex of amlodipine besylate and soluble β-cyclodextrin polymer: Preparation, characterization and dissolution profile. J. Incl. Phenom. Macrocycl. Chem. 2013, 76, 19–28. [Google Scholar] [CrossRef]
- Liu, B.; Li, W.; Zhao, J.; Liu, Y.; Zhu, X.; Liang, G. Physicochemical characterization of the supramolecular structure of luteolin/ cyclodextrin inclusion complex. Food Chem. 2013, 141, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Martin Del Valle, E.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Aiassa, V.; Zoppi, A.; Albesa, I.; Longhi, M.R. Inclusion complexes of chloramphenicol with B-cyclodextrin and aminoacids as a way to increase drug solubility and modulate ROS production. Carbohydr. Polym. 2015, 121, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Male, K.B.; Bouvrette, P.; Luong, J.H.T. Control of the size and distribution of gold nanoparticles by unmodified cyclodextrins. Chem. Mater. 2003, 15, 4172–4180. [Google Scholar] [CrossRef]
- Barrientos, L.; Yutronic, N.; Del Monte, F.; Gutiérrez, M.C.; Jara, P. Ordered arrangement of gold nanoparticles on an α-cyclodextrin-dodecanethiol inclusion compound produced by magnetron sputtering. New J. Chem. 2007, 31, 1400–1402. [Google Scholar] [CrossRef]
- Silva, N.; Moris, S.; Herrera, B.; Díaz, M.; Kogan, M.; Barrientos, L.; Yutronic, N.; Jara, P. Formation of Copper Nanoparticles Supported onto Inclusion Compounds of a-cyclodextrin: A New Route to Obtain Copper Nanoparticles. Mol. Cryst. Liq. Cryst. 2010, 521, 246–252. [Google Scholar] [CrossRef]
- Díaz, M.; Silva, N.; Yutronic, N.; Peña, E.; Chornik, B.; Jara, P. γ-Cyclodextrin/alkylthiol inclusion compounds crystals as substrates for the formation and immobilization of gold nanoparticles produced by magnetron sputtering. J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 133–138. [Google Scholar] [CrossRef]
- Silva, N.; Arellano, E.; Castro, C.; Yutronic, N.; Lang, E.; Chornik, B.; Jara, P. Cyclodextrin inclusion compound crystals for growth of Cu-Au core-shell nanoparticles. J. Incl. Phenom. Macrocycl. Chem. 2015, 82, 497–504. [Google Scholar] [CrossRef]
- Silva, N.; Muñoz, C.; Diaz-Marcos, J.; Samitier, J.; Yutronic, N.; Kogan, M.J.; Jara, P. In Situ Visualization of the Local Photothermal Effect Produced on α-Cyclodextrin Inclusion Compound Associated with Gold Nanoparticles. Nanoscale Res. Lett. 2016, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Skirtach, A.G.; Dejugnat, C.; Braun, D.; Susha, A.S.; Rogach, A.L.; Parak, W.J.; Möhwald, J.; Sukhorukov, G.B. The Role of Metal Nanoparticles in Remote Release of Encapsulated Materials. Nano Lett. 2005, 5, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Pissuwan, D.; Valenzuela, S.M.; Cortie, M.B. Therapeutic possibilities of plasmonically heated gold nanoparticles. Trends Biotechnol. 2006, 24, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Au nanoparticles target cancer. Nano Today 2007, 2, 18–29. [Google Scholar] [CrossRef]
- Hirose, K. A Practical Guide for the Determination of Binding Constants. J. Incl. Phenom. Macrocycl. Chem. 2001, 39, 193–209. [Google Scholar] [CrossRef]
- Simova, S.; Berger, S. Diffusion measurements vs. chemical shift titration for determination of association constants on the example of camphor-cyclodextrin complexes. J. Incl. Phenom. 2005, 53, 163–170. [Google Scholar] [CrossRef]
- Dodziuk, H.; Nowinski, K.S.; Kozminski, W.; Dolgonos, G. On the impossibility of determination of stepwise binding constants for the 1:2 complex of (+)-camphor with α-cyclodextrin. Org. Biomol. Chem. 2003, 1, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Llamazares, S.; Yutronic, N.; Jara, P.; Noyong, M.; Bretschneider, J.; Simon, U. Face Preferred Deposition of Gold Nanoparticles on α-Cyclodextrin/Octanethiol Inclusion Compound. J. Colloid Interface Sci. 2007, 316, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Wojciech Fabianowski, S.L.R.; Coyle, L.C.; Weber, B.A.; Granata, R.D.; Castner, D.G.; Sadownik, A. Spontaneous assembly of phosphatidylcholine monolayers via chemisorption onto gold. Langmuir 1989, 5, 35–41. [Google Scholar] [CrossRef]
- Petrovykh, D.Y.; Kimura-Suda, H.; Whitman, L.J.; Tarlov, M.J. Quantitative analysis and characterization of DNA immobilized on gold. J. Am. Chem. Soc. 2003, 125, 5219–5226. [Google Scholar] [CrossRef] [PubMed]
- Techane, S.D.; Gamble, L.J.; Castner, D.G. X-ray photoelectron spectroscopy characterization of gold nanoparticles functionalized with amine-terminated alkanethiols. Biointerphases 2011, 6, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Baio, J.E.; Weidner, T.; Brison, J.; Graham, D.J.; Gamble, L.J.; Castner, D.G. Amine Terminated SAMs: Investigating Why Oxygen is Present in these Films. J. Electron Spectrosc. Relat. Phenom. 2009, 172, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Pong, B.K.; Lee, J.Y.; Trout, B.L. First principles computational study for understanding the interactions between ssdna and gold nanoparticles: Adsorption of methylamine on gold nanoparticulate surfaces. Langmuir 2005, 21, 11599–11603. [Google Scholar] [CrossRef] [PubMed]
- Atkins, P.; de Paula, J. Physical Chemistry; Oxford University Press: Oxford, UK, 2009; Chapter 18. [Google Scholar]
- Liu, J.; Alvarez, J.; Ong, W.; Román, E.; Kaifer, A.E. Phase transfer of hydrophilic, cyclodextrin-modified gold nanoparticles to chloroform solutions. J. Am. Chem. Soc. 2001, 123, 11148–11154. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, M.J.; Wingate, J.E.; Zhong, C.J.; Harris, J.E.; Vachet, R.W.; Clark, M.R.; Londono, J.D.; Green, S.J.; Stokes, J.J.; Wignall, G.D.; et al. Alkanethiolate gold cluster molecules with core diameters from 1.5 to 5.2 nm: core and monolayer properties as a function of core size. Langmuir 1998, 14, 17–30. [Google Scholar] [CrossRef]
- Barrientos, L.; Lang, E.; Zapata-Torres, G.; Celis-Barros, C.; Orellana, C.; Jara, P.; Yutronic, N. Structural elucidation of supramolecular alpha-cyclodextrin dimer/aliphatic monofunctional molecules complexes. J. Mol. Model. 2013, 19, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, L.; Allende, P.; Orellana, C.; Jara, P. Ordered arrangements of metal nanoparticles on alpha-cyclodextrin inclusion complexes by magnetron sputtering. Inorg. Chim. Acta 2012, 18, 11–13. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. The size and shape factor in colloidal systems. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Sample Availability: Sample of the compound α-CD/OA IC is available from the authors.

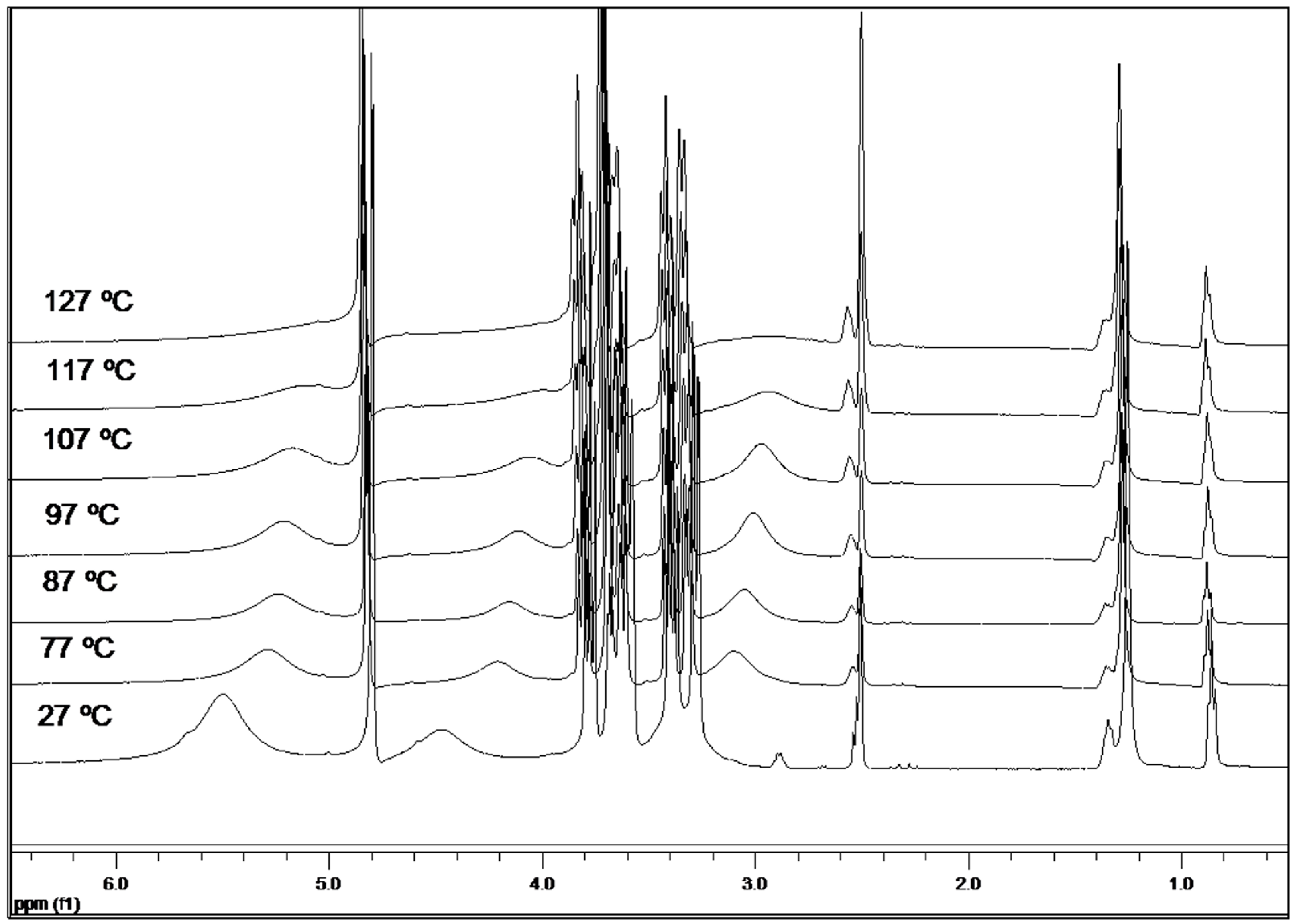


| Temperature | H1 | H2 | H3 | H4 | H5 | H6 | OH(2) | OH(4) | OH(6) |
|---|---|---|---|---|---|---|---|---|---|
| (°C) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) |
| 27 | 4.79 | 3.27 | 3.77 | 3.38 | 3.59 | 3.65 | 5.49 | 5.49 | 4.47 |
| 77 | 4.82 | 3.31 | 3.81 | 3.40 | 3.62 | 3.61 | 5.28 | 5.28 | 4.20 |
| 87 | 4.82 | 3.32 | 3.81 | 3.41 | 3.63 | 3.70 | 5.24 | 5.24 | 4.15 |
| 97 | 4.83 | 3.34 | 3.82 | 3.41 | 3.64 | 3.70 | 5.20 | 5.20 | 4.11 |
| 107 | 4.83 | 3.33 | 3.82 | 3.41 | 3.64 | 3.71 | 5.15 | 5.15 | 4.06 |
| 117 | 4.84 | 3.34 | 3.83 | 3.42 | 3.65 | 3.72 | 5.05 | 5.05 | 4.01 |
| 127 | 4.84 | 3.35 | 3.83 | 3.42 | 3.66 | 3.73 |
| Sample | Temperature (°C) | CH3 (ppm) | -(CH2)n- (ppm) | -CH2- (ppm) | NH2 (ppm) |
|---|---|---|---|---|---|
| α-CD/OA | 27 | 0.85 | 1.23 | 1.31 | 2.53 |
| 77 | 0.89 | 1.28 | 1.36 | 2.54 | |
| 87 | 0.88 | 1.28 | 1.36 | 2.55 | |
| 97 | 0.88 | 1.29 | 1.36 | 2.55 | |
| 107 | 0.89 | 1.29 | 1.37 | 2.56 | |
| 117 | 0.89 | 1.30 | 1.37 | 2.56 | |
| 127 | 0.89 | 1.30 | 1.37 | 2.57 | |
| OA | 27 | 0.89 | 1.30 | 1.37 | 2.56 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, N.; Moris, S.; Díaz, M.; Yutronic, N.; Lang, E.; Chornik, B.; Kogan, M.J.; Jara, P. Evidence of the Disassembly of α-Cyclodextrin-octylamine Inclusion Compounds Conjugated to Gold Nanoparticles via Thermal and Photothermal Effects. Molecules 2016, 21, 1444. https://doi.org/10.3390/molecules21111444
Silva N, Moris S, Díaz M, Yutronic N, Lang E, Chornik B, Kogan MJ, Jara P. Evidence of the Disassembly of α-Cyclodextrin-octylamine Inclusion Compounds Conjugated to Gold Nanoparticles via Thermal and Photothermal Effects. Molecules. 2016; 21(11):1444. https://doi.org/10.3390/molecules21111444
Chicago/Turabian StyleSilva, Nataly, Silvana Moris, Maximiliano Díaz, Nicolás Yutronic, Erika Lang, Boris Chornik, Marcelo J. Kogan, and Paul Jara. 2016. "Evidence of the Disassembly of α-Cyclodextrin-octylamine Inclusion Compounds Conjugated to Gold Nanoparticles via Thermal and Photothermal Effects" Molecules 21, no. 11: 1444. https://doi.org/10.3390/molecules21111444
APA StyleSilva, N., Moris, S., Díaz, M., Yutronic, N., Lang, E., Chornik, B., Kogan, M. J., & Jara, P. (2016). Evidence of the Disassembly of α-Cyclodextrin-octylamine Inclusion Compounds Conjugated to Gold Nanoparticles via Thermal and Photothermal Effects. Molecules, 21(11), 1444. https://doi.org/10.3390/molecules21111444