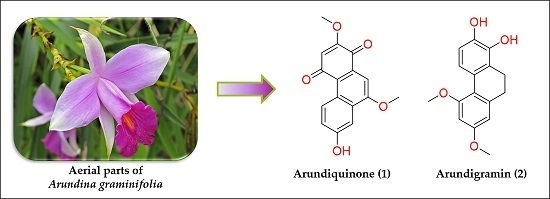
Two New Stilbenoids from the Aerial Parts of Arundina graminifolia (Orchidaceae)
Abstract
:1. Introduction
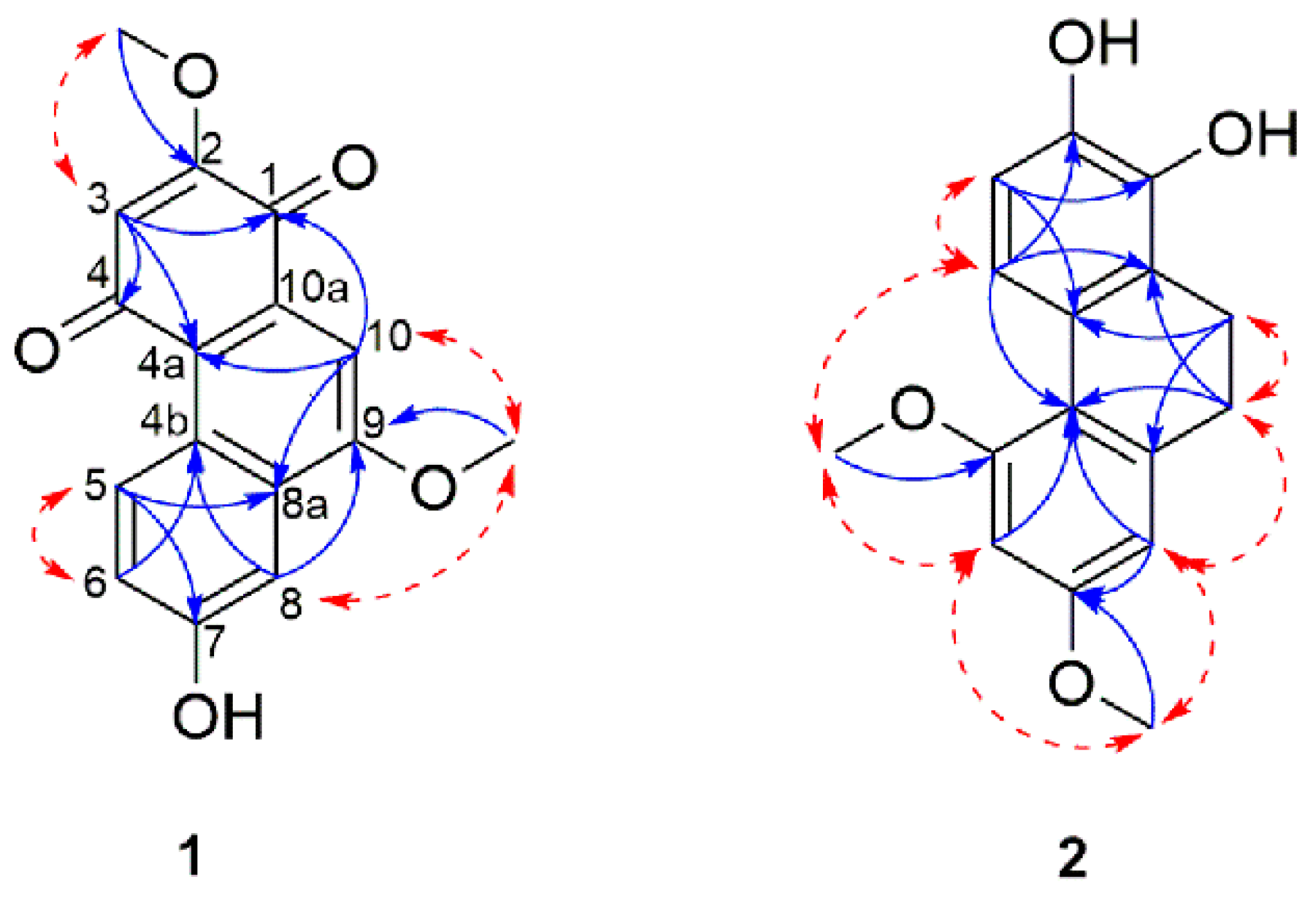
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Compound Characterization
3.5. Cytoprotective Assay
3.5.1. Chemical and Reagents
3.5.2. Cytoprotective Protocol
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hong, D.Y.; Lian, Y.S.; Shen, L.D. Orchidaceae. In Flora of China; Chinese Science Press: Beijing, China, 1983; Volume 73, p. 320. [Google Scholar]
- Liu, M.; Ding, Y.; Du, L. Chemical components of traditional Dai medicine Arundina graminifolia (D. Don) Hochr. Zhong Cao Yao 2007, 38, 676–677. [Google Scholar]
- Hossain, M.M. Therapeutic orchids: Traditional uses and recent advances—An overview. Fitoterapia 2011, 82, 102–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chongsuvivatwong, V.; Keawpradub, N.; Lin, Y. Analysis of prescription database extracted from standard textbooks of traditional Dai medicine. J. Ethnobiol. Ethnomed. 2012, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Xiaohua, D.; Jin, Z.; Hui, W.; Haifeng, C.; Chao, Z.; Zepu, Y. Effect of Yajieshaba, a preparation of Dai indigenous medicine, on enhanced liver detoxification. J. Tradit. Chin. Med. 2015, 35, 197–205. [Google Scholar] [CrossRef]
- Kumar, S. Arundina. In The Medicinal Plants of North-East India; Scientific Publishers: Judhpur, India, 2002; p. 212. [Google Scholar]
- Panda, A.K.; Mandal, D. The folklore medicinal orchids of Sikkim. Anc. Sci. Life 2013, 33, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M. Traditional therapeutic uses of some indigenous orchids of Bangladesh. Med. Aromat. Plant. Sci. Biotechnol. 2009, 42, 101–106. [Google Scholar]
- Hegde, S. Orchid wealth of India. Proc. Indian Natl. Sci. Acad. B 1997, 63, 229–244. [Google Scholar]
- Li, Y.-K.; Zhou, B.; Ye, Y.-Q.; Du, G.; Niu, D.-Y.; Meng, C.-Y.; Gao, X.-M.; Hu, Q.-F. Two new diphenylethylenes from Arundina graminifolia and their cytotoxicity. Bull. Korean Chem. Soc. 2013, 34, 3257–3260. [Google Scholar] [CrossRef]
- Hu, Q.-F.; Zhou, B.; Ye, Y.-Q.; Jiang, Z.-Y.; Huang, X.-Z.; Li, Y.-K.; Du, G.; Yang, G.-Y.; Gao, X.-M. Cytotoxic deoxybenzoins and diphenylethylenes from Arundina graminifolia. J. Nat. Prod. 2013, 76, 1854–1859. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jin, Y.; Yang, S.; Wu, J.; Gao, X.; Hu, Q.; Ma, Y. A new diphenylethylene from Arundina graminifolia and its cytotoxicity. Asian J. Chem. 2014, 26, 3903–3905. [Google Scholar]
- Meng, C.-Y.; Niu, D.-Y.; Li, Y.-K.; Zhou, B.; Ye, Y.-Q.; Du, G.; Hu, Q.-F.; Gao, X.-M. A new cytotoxic stilbenoid from Arundina graminifolia. Asian J. Chem. 2014, 26, 2411–2413. [Google Scholar]
- Yang, J.X.; Wang, H.; Lou, J.; Li, L.; Liu, G.Y.; Gao, X.; Hu, Q.; Ye, Y. A new cytotoxic diphenylethylene from Arundina graminifolia. Asian J. Chem. 2014, 26, 4517–4518. [Google Scholar]
- Majumder, P.L.; Ghosal, S. Two stilbenoids from the orchid Arundina bambusifolia. Phytochemistry 1993, 32, 439–444. [Google Scholar] [CrossRef]
- Liu, M.F.; Han, Y.; Xing, D.M.; Shi, Y.; Xu, L.Z.; Du, L.J.; Ding, Y. A new stilbenoid from Arundina graminifolia. J. Asian Nat. Prod. Res. 2004, 6, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.F.; Han, Y.; Xing, D.M.; Wang, W.; Xu, L.Z.; Du, L.J.; Ding, Y. Chemical constituents from the rhizoma of Arundina graminifolia. Zhongguo Zhong Yao Za Zhi 2004, 29, 147–149. [Google Scholar] [PubMed]
- Du, G.; Shen, Y.; Yang, L.; Shu, L.; Wen, M.-L.; Hu, Q.-F. Bibenzyl derivatives of Arundina graminifolia and their cytotoxicity. Chem. Nat. Compd. 2014, 49, 1019–1022. [Google Scholar] [CrossRef]
- Liu, M.F.; Ding, Y.; Zhang, D.M. Phenanthrene constituents from rhizome of Arundina graminifolia. Zhongguo Zhong Yao Za Zhi 2005, 30, 353–356. [Google Scholar] [PubMed]
- Liu, M.F.; Han, Y.; Xing, D.M.; Wang, W.; Xu, L.Z.; Du, L.J.; Ding, Y. One new benzyldihydrophenanthrene from Arundina graminifolia. J. Asian Nat. Prod. Res. 2005, 7, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yang, L.; Shen, Y.; Shu, L.; Li, X.; Hu, Q.-F. Phenolic compounds from Arundina graminifolia and their anti-tobacco mosaic virus activity. Bull. Korean Chem. Soc. 2012, 33, 2447–2449. [Google Scholar] [CrossRef]
- Niu, D.-Y.; Han, J.-M.; Kong, W.-S.; Cui, Z.-W.; Hu, Q.-F.; Gao, X.-M. Antiviral fluorenone derivatives from Arundina graminifolia. Asian J. Chem. 2013, 25, 9514–9516. [Google Scholar]
- Hu, Q.F.; Zhou, B.; Huang, J.M.; Gao, X.M.; Shu, L.D.; Yang, G.Y.; Che, C.T. Antiviral phenolic compounds from Arundina graminifolia. J. Nat. Prod. 2013, 76, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, L.; Shu, L.; Shen, Y.; Hu, Q.; Xia, Z. Flavonoid compounds from Arundina graminifolia. Asian J. Chem. 2013, 25, 4922–4924. [Google Scholar]
- Shu, L.; Shen, Y.; Yang, L.; Gao, X.; Hu, Q.-F. Flavonoids derivatives from Arundina graminifolia and their cytotoxicity. Asian J. Chem. 2013, 25, 8358–8360. [Google Scholar]
- Li, L.; Xu, W.-X.; Liu, C.-B.; Zhang, C.-M.; Zhao, W.; Shang, S.-Z.; Deng, L.; Guo, Y.-D. A new antiviral phenolic compounds from Arundina graminifolia. Asian J. Chem. 2015, 27, 3525–3526. [Google Scholar] [CrossRef]
- Kong, J.-M.; Goh, N.-K.; Chia, L.-S.; Chia, T.-F. Recent advances in traditional plant drugs and orchids. Acta Pharmacol. Sin. 2003, 24, 7–21. [Google Scholar] [PubMed]
- Chen, L.; Chen, J.-B. Pharmacological research of stilbenoids. Guangdong Yaoxue 2005, 15, 84–86. [Google Scholar]
- Xiao, K.; Zhang, H.-J.; Xuan, L.-J.; Zhang, J.; Xu, Y.-M.; Bai, D.-L. Stilbenoids: Chemistry and bioactivities. Stud. Nat. Prod. Chem. 2008, 34, 453–646. [Google Scholar]
- Kovacs, A.; Vasas, A.; Hohmann, J. Natural phenanthrenes and their biological activity. Phytochemistry 2008, 69, 1084–1110. [Google Scholar] [CrossRef] [PubMed]
- Tzakou, O. Naturally occurring stilbenoids and their biological activity. Pharmakeutike 2009, 22, 132–141. [Google Scholar]
- Williams, R.B.; Martin, S.M.; Hu, J.F.; Garo, E.; Rice, S.M.; Norman, V.L.; Lawrence, J.A.; Hough, G.W.; Goering, M.G.; O’Neil-Johnson, M.; et al. Isolation of apoptosis-inducing stilbenoids from four members of the Orchidaceae family. Planta Med. 2012, 78, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Simmler, C.; Antheaume, C.; Lobstein, A. Antioxidant biomarkers from Vanda coerulea stems reduce irradiated HaCaT PGE-2 production as a result of COX-2 inhibition. PLoS ONE 2010, 5, e13713. [Google Scholar] [CrossRef] [PubMed]
- Simmler, C.; Antheaume, C.; Andre, P.; Bonte, F.; Lobstein, A. Glucosyloxybenzyl eucomate derivatives from Vanda teres stimulate hacat cytochrome c oxidase. J. Nat. Prod. 2011, 74, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Cakova, V.; Urbain, A.; Antheaume, C.; Rimlinger, N.; Wehrung, P.; Bonte, F.; Lobstein, A. Identification of phenanthrene derivatives in Aerides rosea (Orchidaceae) using the combined systems HPLC-ESI-HRMS/MS and HPLC-DAD-MS-SPE-UV-NMR. Phytochem. Anal. 2015, 26, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, K.; Dietrich, H. Syringaresinol, a dehydrogenation product of sinapinyl alcohol. Chem. Ber. 1953, 86, 4–10. [Google Scholar] [CrossRef]
- Cai, X.F.; Lee, I.S.; Dat, N.T.; Shen, G.; Kang, J.S.; Kim, D.H.; Kim, Y.H. Inhibitory lignans against NFAT transcription factor from Acanthopanax koreanum. Arch. Pharm. Res. 2004, 27, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Fisch, M.H.; Flick, B.H.; Arditti, J. Structure and antifungal activity of hircinol, loroglossol and orchinol. Phytochemistry 1973, 12, 437–441. [Google Scholar] [CrossRef]
- Tezuka, Y.; Hirano, H.; Kikuchi, T.; Xu, G. Constituents of orchidaceous plants. X. Constituents of Ephemerantha lonchophylla; isolation and structure elucidation of new phenolic compounds, ephemeranthol-A, ephemeranthol-B, and ephemeranthoquinone, and of a new diterpene glucoside, ephemeranthoside. Chem. Pharm. Bull. 1991, 39, 593–598. [Google Scholar] [CrossRef]
- Fan, C.Q.; Zhao, W.M.; Qin, G.W. New bibenzyl and phenanthrenedione from Dendrobium densiflorum. Chin. Chem. Lett. 2000, 11, 705–706. [Google Scholar]
- Majumder, P.; Laha, S.; Datta, N. Coelonin, a 9,10-dihydrophenanthrene from the orchids Coelogyne ochracea and C. elata. Phytochemistry 1982, 21, 478–480. [Google Scholar] [CrossRef]
- Majumder, P.L.; Lahiri, S. Lusianthrin and lusianthridin, two stilbenoids from the orchid Lusia indivisa. Phytochemistry 1990, 29, 621–624. [Google Scholar] [CrossRef]
- Hashimoto, T.; Hasegawa, K.; Yamaguchi, H.; Saito, M.; Ishimoto, S. Structure and synthesis of batatasins, dormancy-inducing substances of yam bulbils. Phytochemistry 1974, 13, 2849–2852. [Google Scholar] [CrossRef]
- Majumder, P.L.; Banerjee, S. Structure of flavanthrin, the first dimeric 9,10-dihydrophenanthrene derivative from the orchid Eria flava. Tetrahedron 1988, 44, 7303–7308. [Google Scholar] [CrossRef]
- Talapatra, B.; Mukhopadhyay, P.; Chaudhury, P.; Talapatra, S.K. Denbinobin, a new phenanthraquinone from Dendrobium Nobile Lindl (Orchidaceae). Indian J. Chem., Sect. B 1982, 21B, 386–387. [Google Scholar]
- Wu, T.S.; Jong, T.T.; Tien, H.J.; Kuoh, C.S.; Furukawa, H.; Lee, K.H. Annoquinone a, an antimicrobial and cytotoxic principle from Annona montana. Phytochemistry 1987, 26, 1623–1625. [Google Scholar] [CrossRef]
- Harborne, J.B.; Smith, T.A.; Majumder, P.L.; Pal, S. Rotundatin, a new 9,10-didydrophenanthrene derivative from Dendrobium rotundatum. Phytochemistry 1992, 31, 3225–3228. [Google Scholar]
- Majumder, P.L.; Sen, S.; Majumder, S. Phenanthrene derivatives from the orchid Coelogyne cristata. Phytochemistry 2001, 58, 581–586. [Google Scholar] [CrossRef]
- Papastamoulis, Y.; Richard, T.; Nassra, M.; Badoc, A.; Krisa, S.; Harakat, D.; Monti, J.-P.; Merillon, J.-M.; Waffo-Teguo, P. Viniphenol A, a complex resveratrol hexamer from Vitis vinifera stalks: Structural elucidation and protective effects against amyloid-β-induced toxicity in PC12 cells. J. Nat. Prod. 2014, 77, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.



| No. | Compound 1 | Compound 2 | ||||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | HMBC | δH (J in Hz) | δC | HMBC | |
| 1 | 181.4 | 141.9 | ||||
| 2 | 159.3 | 143.9 | ||||
| 3 | 6.10 (s) | 111.6 | 1, 2, 4, 4a | 6.70 (d, 7.3) | 112.8 | 1, 4a |
| 4 | 188.5 | 7.65 (d, 7.3) | 120.8 | 2, 3, 4a, 10a | ||
| 4a | 121.8 | 126.3 | ||||
| 4b | 126.6 | 117.8 | ||||
| 5 | 9.50 (d, 9.4) | 131.2 | 4a, 6, 7, 8a | 158.7 | ||
| 6 | 7.36 (dd, 9.4, 2.3) | 122.7 | 4b, 8 | 6.51 (d, 2.4) | 98.4 | 4b, 5, 7, 8 |
| 7 | 158.4 | 159.5 | ||||
| 8 | 7.63 (d, 2.3) | 105.1 | 4b, 6, 9 | 6.47 (d, 2.4) | 106.0 | 4b, 6, 7, 9 |
| 8a | 131.5 | 141.3 | ||||
| 9 | 158.9 | 2.67 (m) | 31.3 | 4b, 8, 8a, 10, 10a | ||
| 10 | 7.42 (s) | 100.4 | 1, 4a, 8a, 9 | 2.74 (m) | 22.4 | 8a, 9, 10a |
| 10a | 131.5 | 125.6 | ||||
| 2-OCH3 | 3.91 (s) | 56.7 | 2 | |||
| 5-OCH3 | 3.85 (s) | 55.9 | 5 | |||
| 7-OCH3 | 3.81 (s) | 55.6 | 7 | |||
| 9-OCH3 | 4.15 (s) | 56.6 | 9 | |||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auberon, F.; Olatunji, O.J.; Krisa, S.; Antheaume, C.; Herbette, G.; Bonté, F.; Mérillon, J.-M.; Lobstein, A. Two New Stilbenoids from the Aerial Parts of Arundina graminifolia (Orchidaceae). Molecules 2016, 21, 1430. https://doi.org/10.3390/molecules21111430
Auberon F, Olatunji OJ, Krisa S, Antheaume C, Herbette G, Bonté F, Mérillon J-M, Lobstein A. Two New Stilbenoids from the Aerial Parts of Arundina graminifolia (Orchidaceae). Molecules. 2016; 21(11):1430. https://doi.org/10.3390/molecules21111430
Chicago/Turabian StyleAuberon, Florence, Opeyemi Joshua Olatunji, Stéphanie Krisa, Cyril Antheaume, Gaëtan Herbette, Frédéric Bonté, Jean-Michel Mérillon, and Annelise Lobstein. 2016. "Two New Stilbenoids from the Aerial Parts of Arundina graminifolia (Orchidaceae)" Molecules 21, no. 11: 1430. https://doi.org/10.3390/molecules21111430
APA StyleAuberon, F., Olatunji, O. J., Krisa, S., Antheaume, C., Herbette, G., Bonté, F., Mérillon, J.-M., & Lobstein, A. (2016). Two New Stilbenoids from the Aerial Parts of Arundina graminifolia (Orchidaceae). Molecules, 21(11), 1430. https://doi.org/10.3390/molecules21111430