Renal Protective Effects of 17β-Estradiol on Mice with Acute Aristolochic Acid Nephropathy
Abstract
:1. Introduction
2. Results
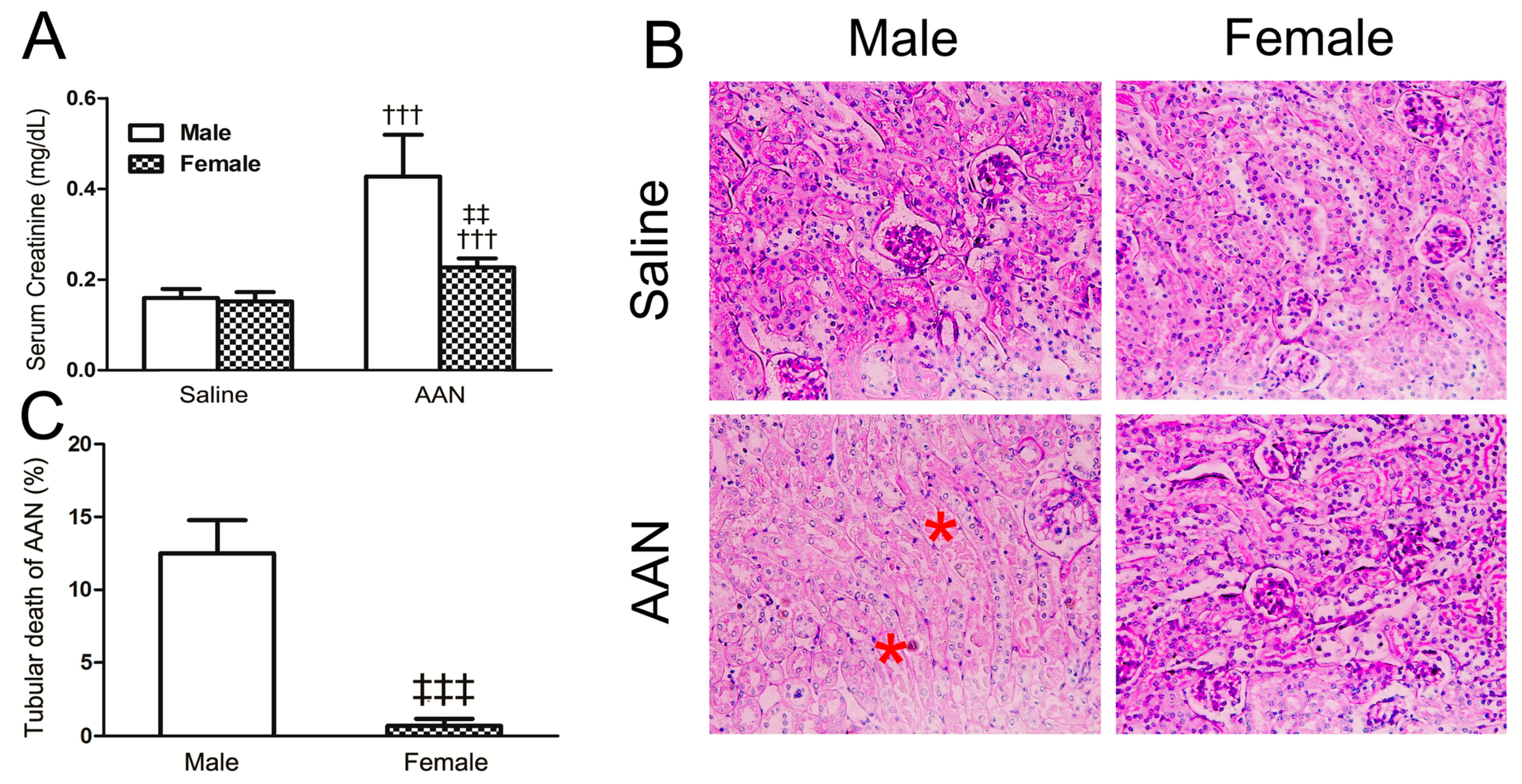
2.1. AA-Induced Acute AAN Was More Severe in Male than in Female Mice
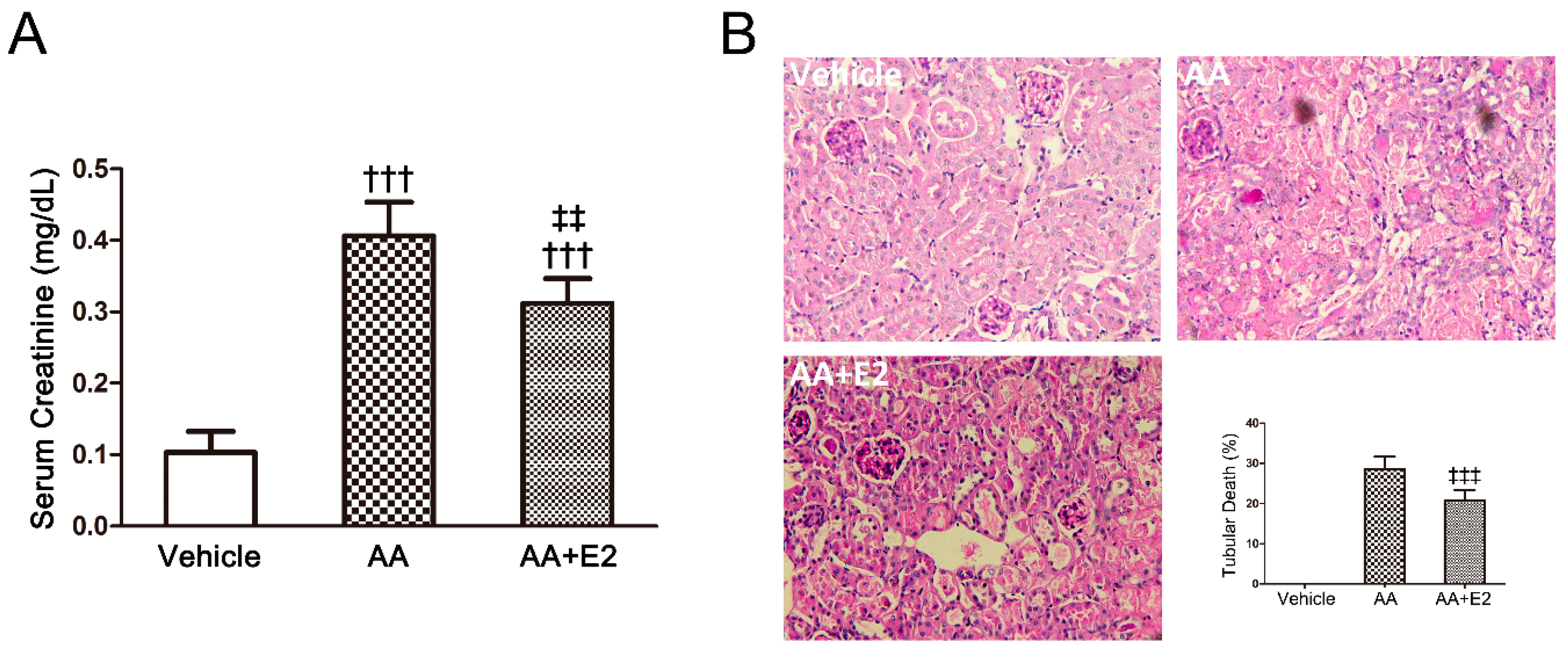
2.2. E2 Had a Protective Effect on Renal Injury in Male AAN Mice
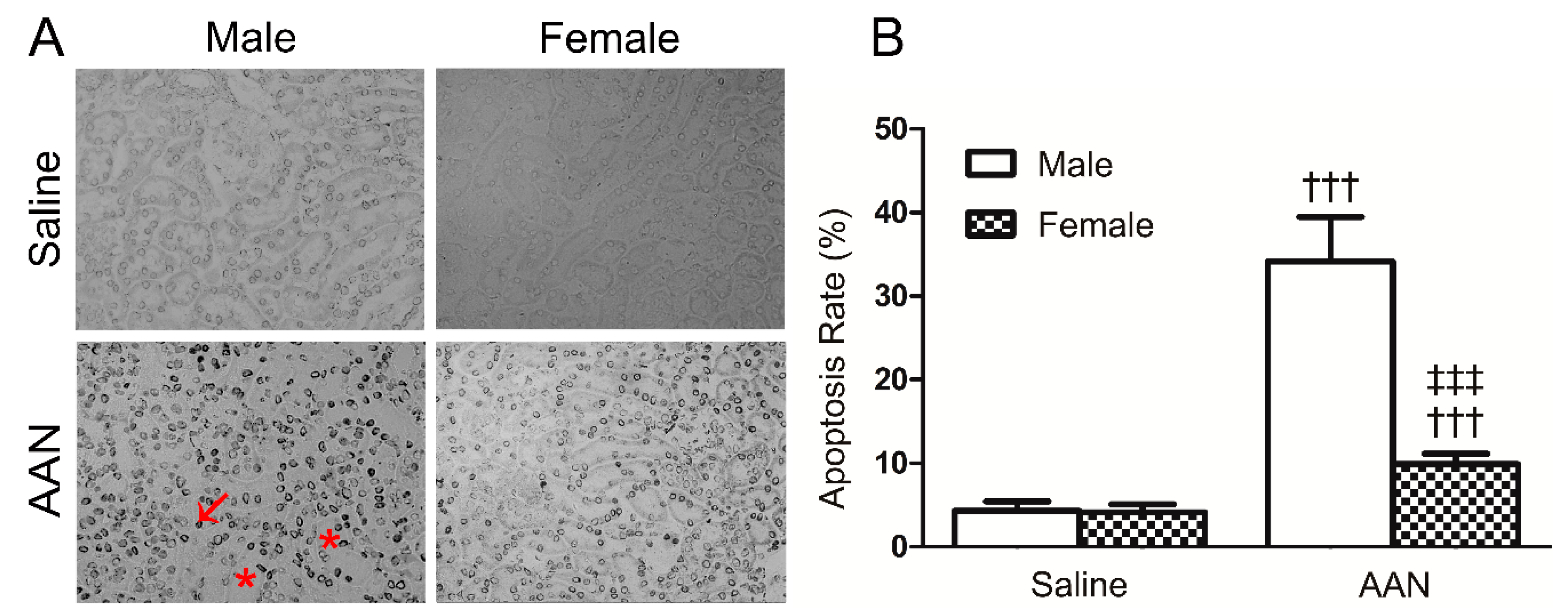
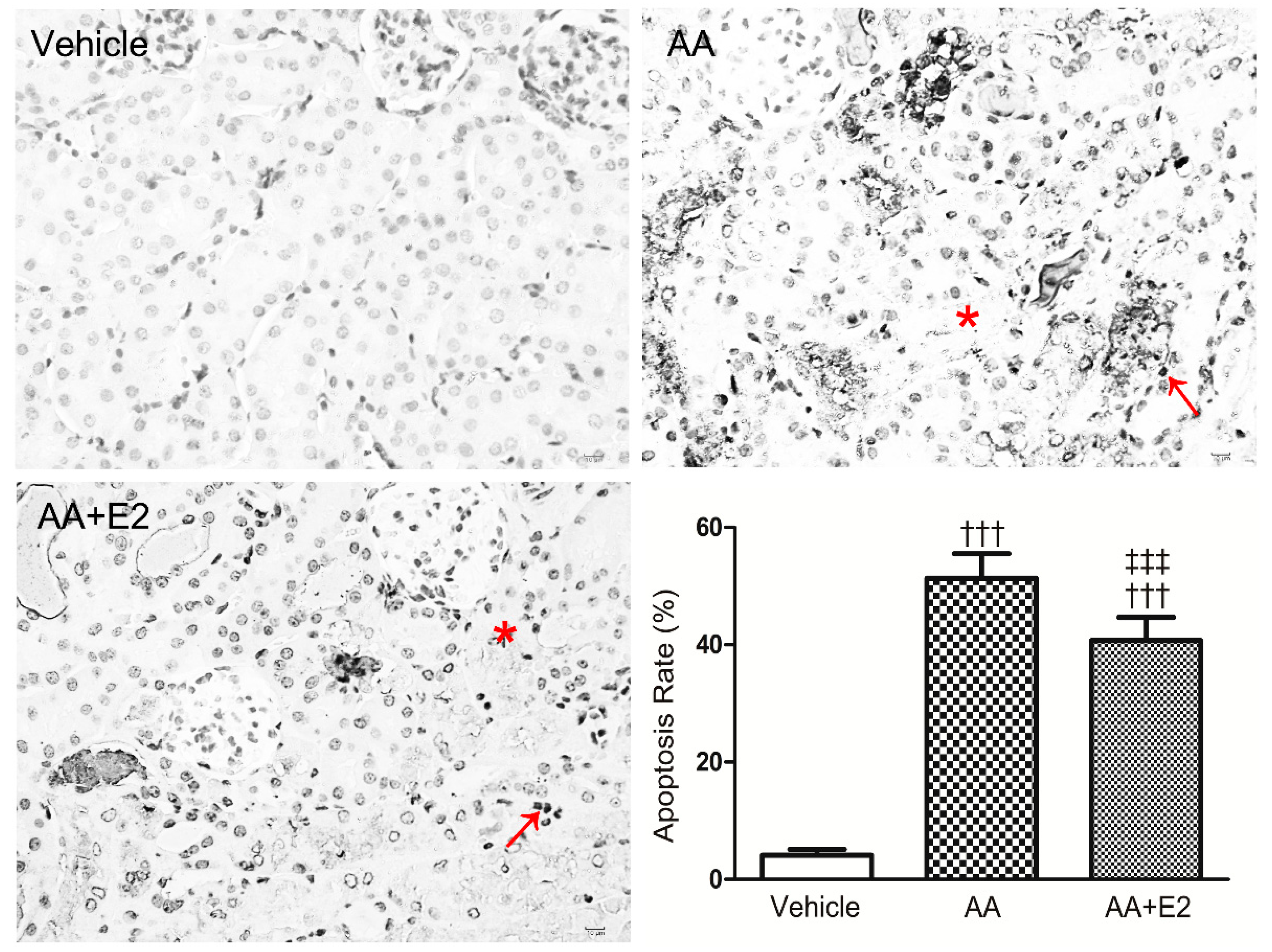
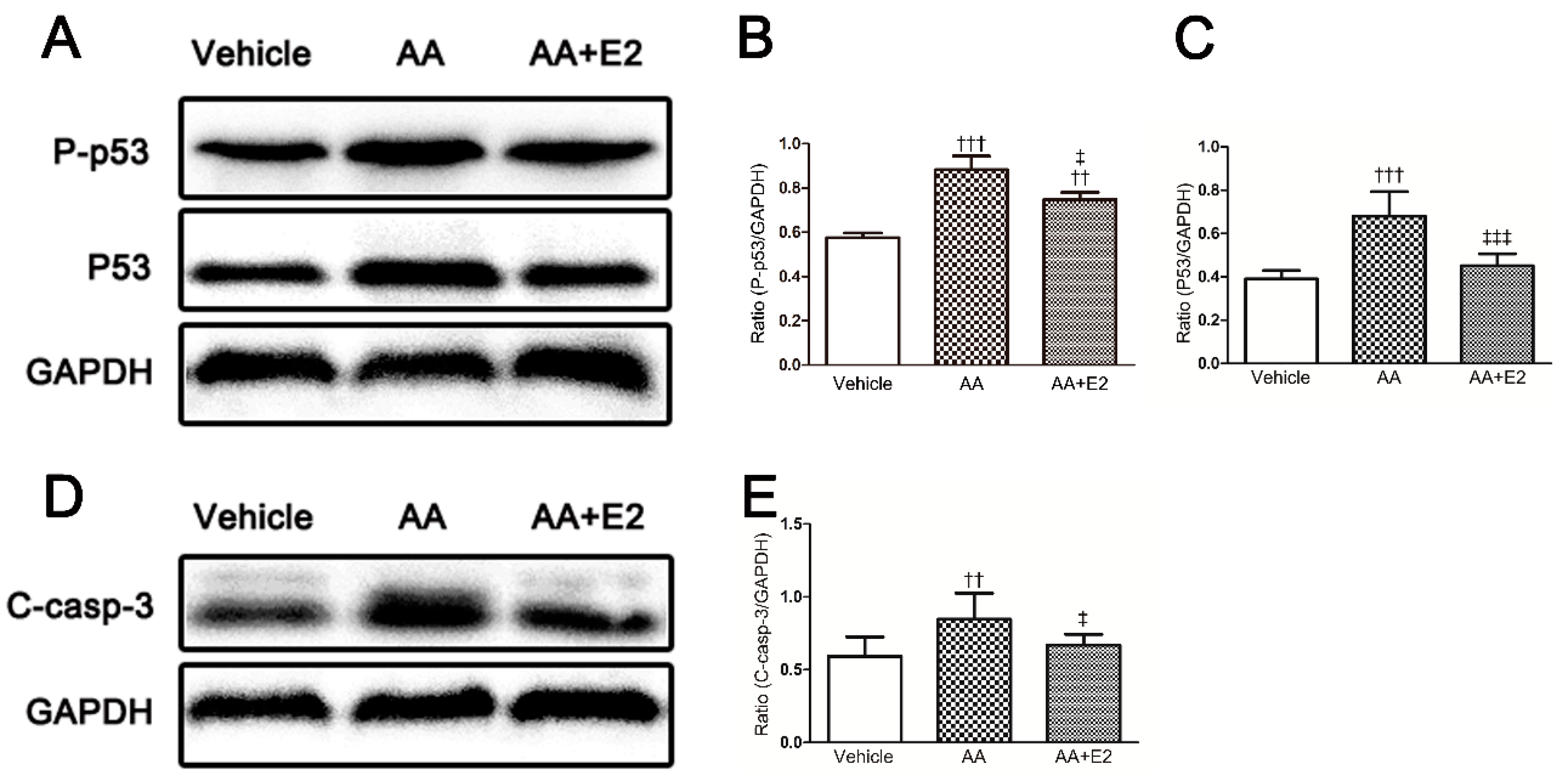
2.3. E2 Prevented p53-Related Apoptosis in Renal Tissue of Acute AAN Mice
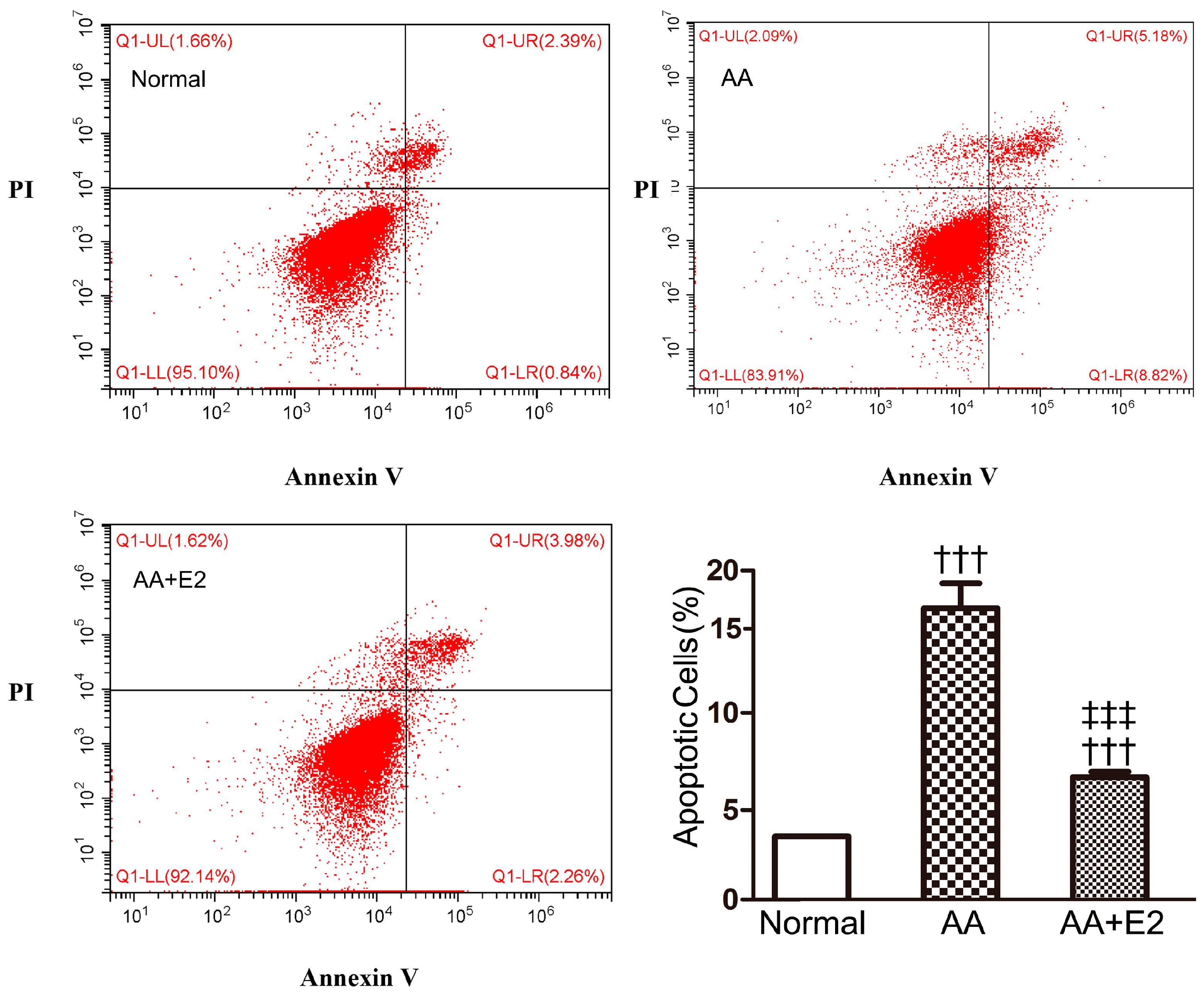
2.4. E2 Reduced AA-Induced Apoptosis in HK-2 Cells
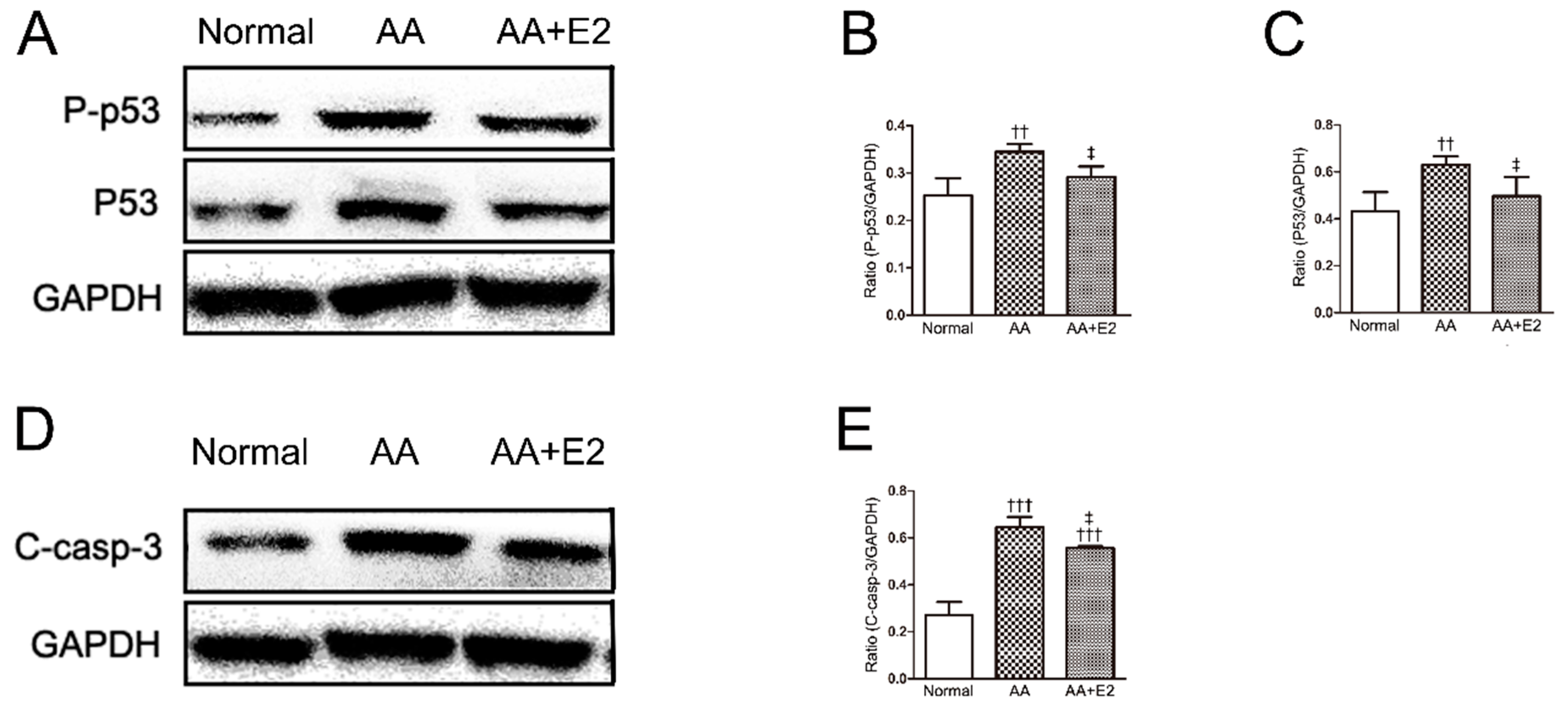
2.5. E2 Prevented p53-Dependent Apoptosis in HK-2 Cells
3. Discussion
4. Materials and Methods
4.1. Animal Model of Acute AAN
4.2. Measurement of Renal Function
4.3. Histological Analysis
4.4. TUNEL Staining for Apoptosis
4.5. Cell Culture
4.6. Annexin V-FITC/Propidium Iodide (PI) Staining
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vanherweghem, J.L.; Depierreux, M.; Tielemans, C.; Abramowicz, D.; Dratwa, M.; Jadoul, M.; Richard, C.; Vandervelde, D.; Verbeelen, D.; Vanhaelen-Fastre, R.; et al. Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including Chinese herbs. Lancet (London England) 1993, 341, 387–391. [Google Scholar] [CrossRef]
- Debelle, F.D.; Vanherweghem, J.L.; Nortier, J.L. Aristolochic acid nephropathy: A worldwide problem. Kidney Int. 2008, 74, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Gillerot, G.; Jadoul, M.; Arlt, V.M.; Van Ypersele De Strihou, C.; Schmeiser, H.H.; But, P.P.; Bieler, C.A.; Cosyns, J.P. Aristolochic acid nephropathy in a Chinese patient: Time to abandon the term “Chinese herbs nephropathy”? Am. J. Kidney Dis. 2001, 38, E26. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Scarpellini, A.; Funck, M.; Verderio, E.A.; Johnson, T.S. Development of a chronic kidney disease model in C57BL/6 mice with relevance to human pathology. Nephron Extra 2013, 3, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Depierreux, M.; Van Damme, B.; Vanden Houte, K.; Vanherweghem, J.L. Pathologic aspects of a newly described nephropathy related to the prolonged use of Chinese herbs. Am. J. Kidney Dis. 1994, 24, 172–180. [Google Scholar] [CrossRef]
- Vanhaelen, M.; Vanhaelen-Fastre, R.; But, P.; Vanherweghem, J.L. Identification of aristolochic acid in Chinese herbs. Lancet (London England) 1994, 343, 174. [Google Scholar] [CrossRef]
- Cosyns, J.P.; Jadoul, M.; Squifflet, J.P.; Van Cangh, P.J.; Van Ypersele de Strihou, C. Urothelial malignancy in nephropathy due to Chinese herbs. Lancet (London England) 1994, 344, 188. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, P.; Huang, X.R.; Liu, F.; Lai, K.N.; Lan, H.Y. Activation of p53 promotes renal injury in acute aristolochic acid nephropathy. JASN 2010, 21, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yang, X.; Wang, J.; Fan, J.; Kong, Q.; Yu, X. Aristolochic acid I induced autophagy extenuates cell apoptosis via ERK 1/2 pathway in renal tubular epithelial cells. PLoS ONE 2012, 7, e30312. [Google Scholar] [CrossRef] [PubMed]
- Neugarten, J. Gender and the progression of renal disease. JASN 2002, 13, 2807–2809. [Google Scholar] [CrossRef] [PubMed]
- Antus, B.; Hamar, P.; Kokeny, G.; Szollosi, Z.; Mucsi, I.; Nemes, Z.; Rosivall, L. Estradiol is nephroprotective in the rat remnant kidney. Nephrol. Dial. Transplant. 2003, 18, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Swide, T.; Vayl, A.; Lahm, T.; Anderson, S.; Hutchens, M.P. Estrogen administered after cardiac arrest and cardiopulmonary resuscitation ameliorates acute kidney injury in a sex- and age-specific manner. Crit. Care (London England) 2015, 19, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.L.; Daniels, F.; Star, R.A.; Kimmel, P.L.; Eggers, P.W.; Molitoris, B.A.; Himmelfarb, J.; Collins, A.J. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. JASN 2006, 17, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Kim, J.I.; Ahn, Y.; Bonventre, A.J.; Bonventre, J.V. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J. Boil. Chem. 2004, 279, 52282–52292. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kil, I.S.; Seok, Y.M.; Yang, E.S.; Kim, D.K.; Lim, D.G.; Park, J.W.; Bonventre, J.V.; Park, K.M. Orchiectomy attenuates post-ischemic oxidative stress and ischemia/reperfusion injury in mice. A role for manganese superoxide dismutase. J. Boil. Chem. 2006, 281, 20349–20356. [Google Scholar] [CrossRef] [PubMed]
- Iran-Nejad, A.; Nematbakhsh, M.; Eshraghi-Jazi, F.; Talebi, A. Preventive role of estradiol on kidney injury induced by renal ischemia-reperfusion in male and female rats. Int. J. Prev. Med. 2015, 6, 22–26. [Google Scholar] [PubMed]
- Tanaka, R.; Tsutsui, H.; Kobuchi, S.; Sugiura, T.; Yamagata, M.; Ohkita, M.; Takaoka, M.; Yukimura, T.; Matsumura, Y. Protective effect of 17beta-estradiol on ischemic acute kidney injury through the renal sympathetic nervous system. Eur. J. Pharmacol. 2012, 683, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Inada, A.; Inada, O.; Fujii, N.L.; Nagafuchi, S.; Katsuta, H.; Yasunami, Y.; Matsubara, T.; Arai, H.; Fukatsu, A.; Nabeshima, Y.I. Adjusting the 17beta-Estradiol-to-Androgen Ratio Ameliorates Diabetic Nephropathy. JASN 2016, 27, 3035–3050. [Google Scholar] [CrossRef] [PubMed]
- Garovic, V.D.; August, P. Sex Differences and Renal Protection: Keeping in Touch with Your Feminine Side. JASN 2016, 27, 2921–2924. [Google Scholar] [CrossRef] [PubMed]
- Satake, A.; Takaoka, M.; Nishikawa, M.; Yuba, M.; Shibata, Y.; Okumura, K.; Kitano, K.; Tsutsui, H.; Fujii, K.; Kobuchi, S.; et al. Protective effect of 17beta-estradiol on ischemic acute renal failure through the PI3K/Akt/eNOS pathway. Kidney Int. 2008, 73, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Negulescu, O.; Bognar, I.; Lei, J.; Devarajan, P.; Silbiger, S.; Neugarten, J. Estradiol reverses TGF-beta1-induced mesangial cell apoptosis by a casein kinase 2-dependent mechanism. Kidney Int. 2002, 62, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Doublier, S.; Lupia, E.; Catanuto, P.; Periera-Simon, S.; Xia, X.; Korach, K.; Berho, M.; Elliot, S.J.; Karl, M. Testosterone and 17beta-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int. 2011, 79, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Verzola, D.; Gandolfo, M.T.; Salvatore, F.; Villaggio, B.; Gianiorio, F.; Traverso, P.; Deferrari, G.; Garibotto, G. Testosterone promotes apoptotic damage in human renal tubular cells. Kidney Int. 2004, 65, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Aufhauser, D.D., Jr.; Wang, Z.; Murken, D.R.; Bhatti, T.R.; Wang, Y.; Ge, G.; Redfield, R.R., III; Abt, P.L.; Wang, L.; Svoronos, N.; et al. Improved renal ischemia tolerance in females influences kidney transplantation outcomes. J. Clin. Investig. 2016, 126, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Hsin, Y.H.; Cheng, C.H.; Tzen, J.T.; Wu, M.J.; Shu, K.H.; Chen, H.C. Effect of aristolochic acid on intracellular calcium concentration and its links with apoptosis in renal tubular cells. Apoptosis 2006, 11, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, P.; Wei, F.; Lin, R.C.; Khan, I.A.; Pasco, D.S. Structure activity relationships of aristolochic acid analogues: Toxicity in cultured renal epithelial cells. Kidney Int. 2005, 67, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Pozdzik, A.A.; Salmon, I.J.; Debelle, F.D.; Decaestecker, C.; Van den Branden, C.; Verbeelen, D.; Deschodt-Lanckman, M.M.; Vanherweghem, J.L.; Nortier, J.L. Aristolochic acid induces proximal tubule apoptosis and epithelial to mesenchymal transformation. Kidney Int. 2008, 73, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Cai, Y.; Gong, L.; Liu, L.; Chen, F.; Xiao, Y.; Wu, X.; Li, Y.; Xue, X.; Ren, J. Role of mitochondrial permeability transition in human renal tubular epithelial cell death induced by aristolochic acid. Toxicol. Appl. Pharmacol. 2007, 222, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, C.; Arlt, V.M.; Schmeiser, H.H.; Boom, A.; Verroust, P.J.; Devuyst, O.; Beauwens, R. Aristolochic acid impedes endocytosis and induces DNA adducts in proximal tubule cells. Kidney Int. 2001, 60, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Appella, E.; Anderson, C.W. Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem. 2001, 268, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, K.; Herrera, J.E.; Saito, S.; Miki, T.; Bustin, M.; Vassilev, A.; Anderson, C.W.; Appella, E. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 1998, 12, 2831–2841. [Google Scholar] [CrossRef] [PubMed]
- Micheli, F.; Palermo, R.; Talora, C.; Ferretti, E.; Vacca, A.; Napolitano, M. Regulation of proapoptotic proteins Bak1 and p53 by miR-125b in an experimental model of Alzheimer's disease: Protective role of 17beta-Estradiol. Neurosci. Lett. 2016, 629, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.T.; Shin, H.; Westerling, T.; Liu, X.S.; Brown, M. Estrogen receptor prevents p53-dependent apoptosis in breast cancer. PNAS 2012, 109, 18060–18065. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of AAI sodium salt and 17β-estradiol are available from the authors.

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.; Ma, L.; Zhou, L.; Fu, P. Renal Protective Effects of 17β-Estradiol on Mice with Acute Aristolochic Acid Nephropathy. Molecules 2016, 21, 1391. https://doi.org/10.3390/molecules21101391
Shi M, Ma L, Zhou L, Fu P. Renal Protective Effects of 17β-Estradiol on Mice with Acute Aristolochic Acid Nephropathy. Molecules. 2016; 21(10):1391. https://doi.org/10.3390/molecules21101391
Chicago/Turabian StyleShi, Min, Liang Ma, Li Zhou, and Ping Fu. 2016. "Renal Protective Effects of 17β-Estradiol on Mice with Acute Aristolochic Acid Nephropathy" Molecules 21, no. 10: 1391. https://doi.org/10.3390/molecules21101391
APA StyleShi, M., Ma, L., Zhou, L., & Fu, P. (2016). Renal Protective Effects of 17β-Estradiol on Mice with Acute Aristolochic Acid Nephropathy. Molecules, 21(10), 1391. https://doi.org/10.3390/molecules21101391





