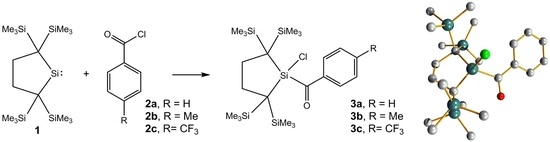
Reactions of an Isolable Dialkylsilylene with Aroyl Chlorides. A New Route to Aroylsilanes
Abstract
:1. Introduction

2. Results and Discussion
2.1. Synthesis and Characterization

2.2. NMR Spectroscopy
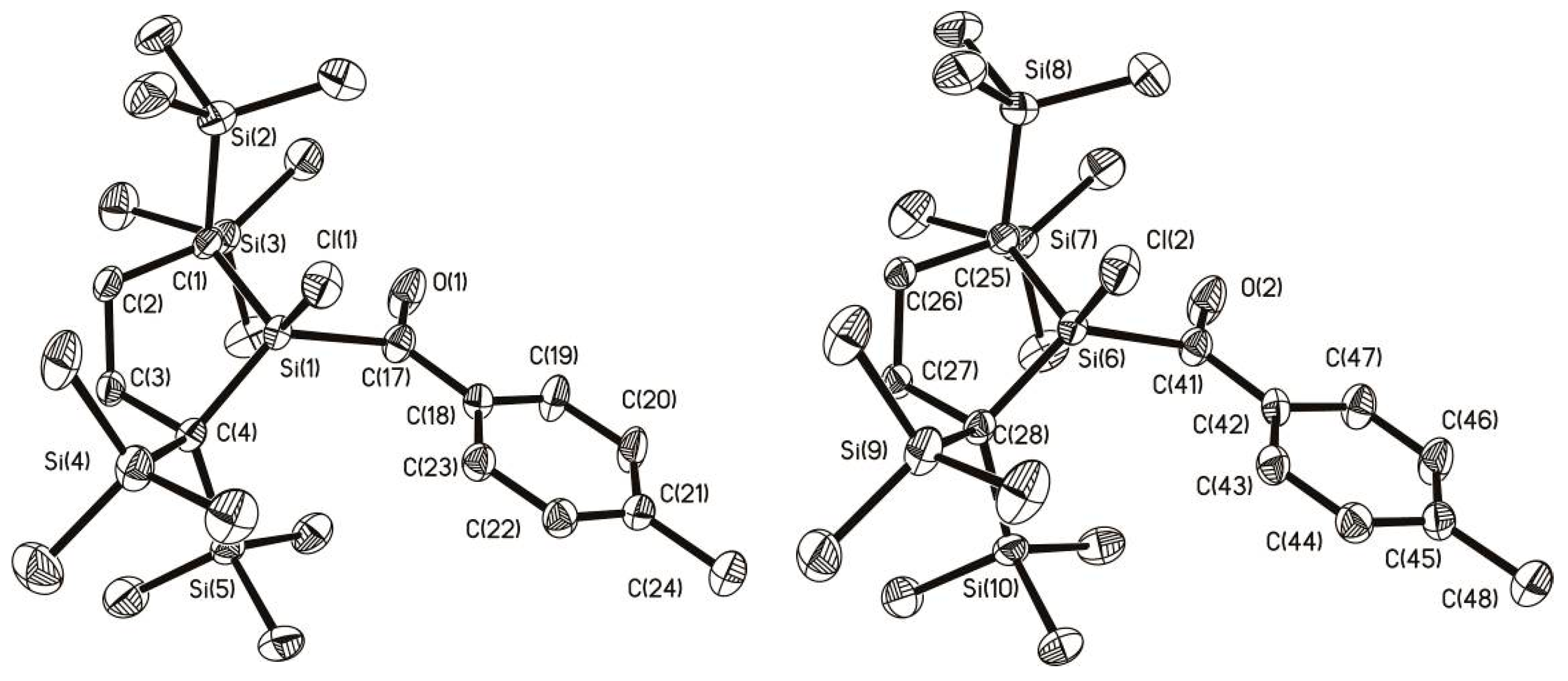
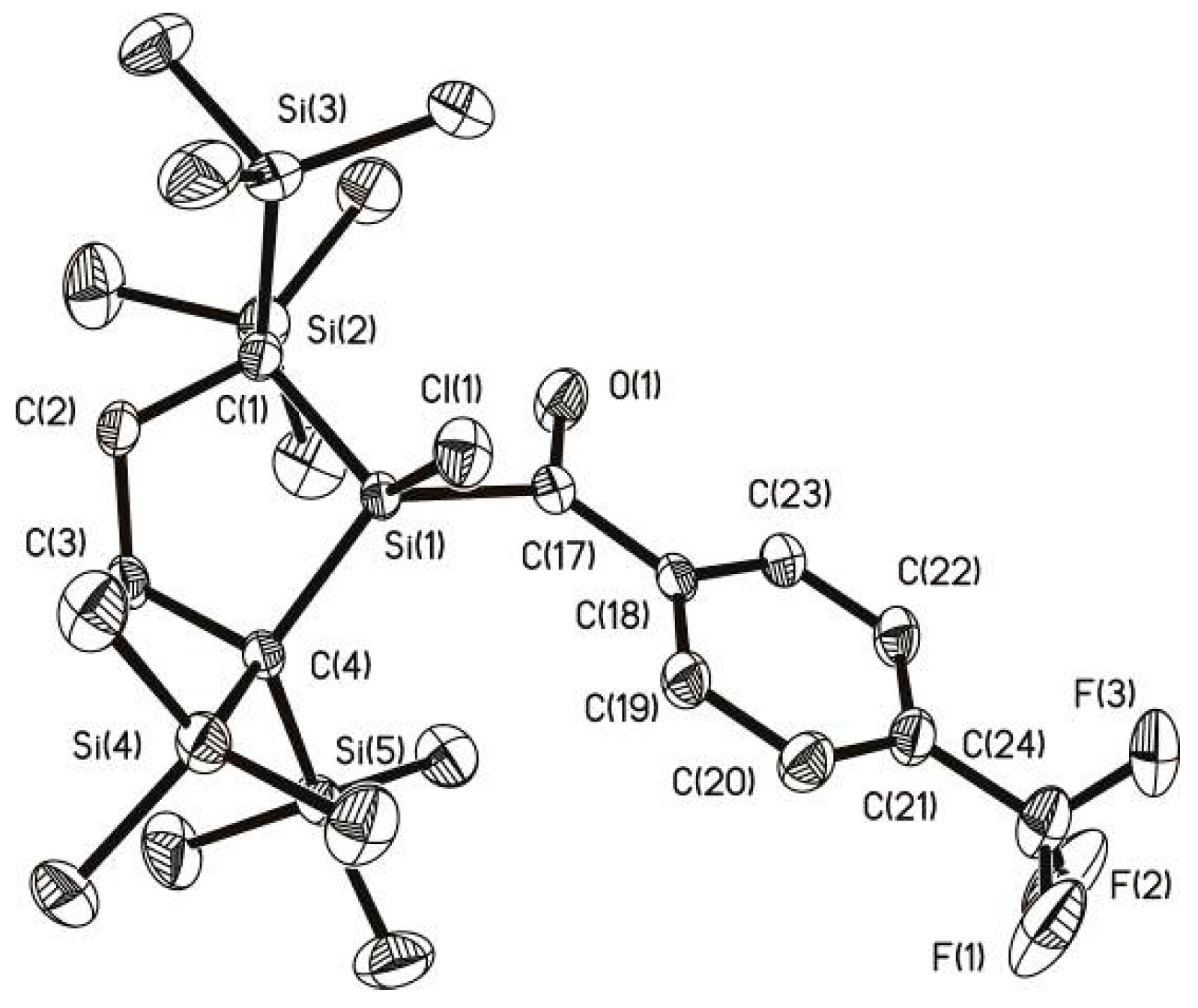
2.3. Molecular Structure Analysis
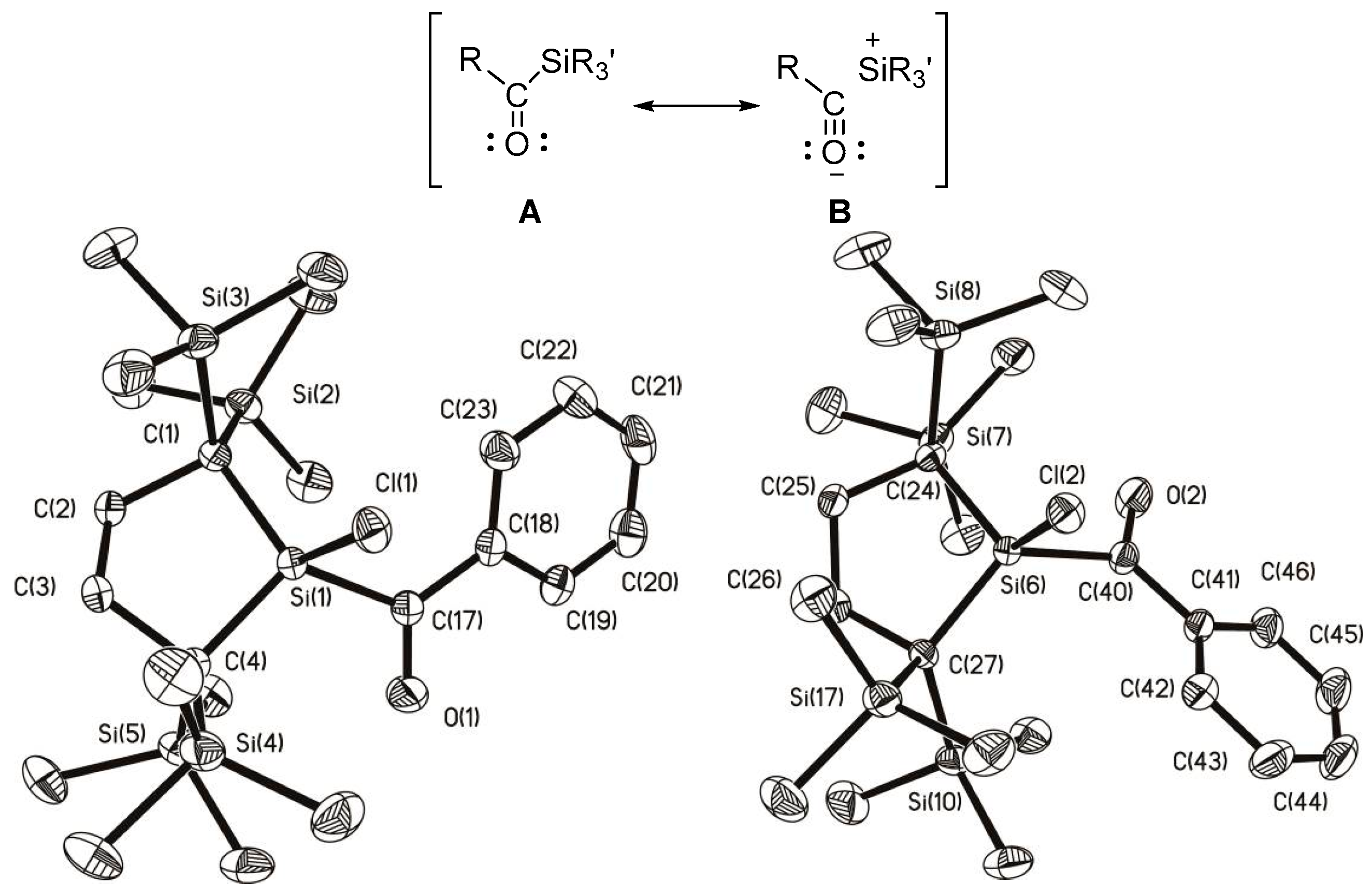
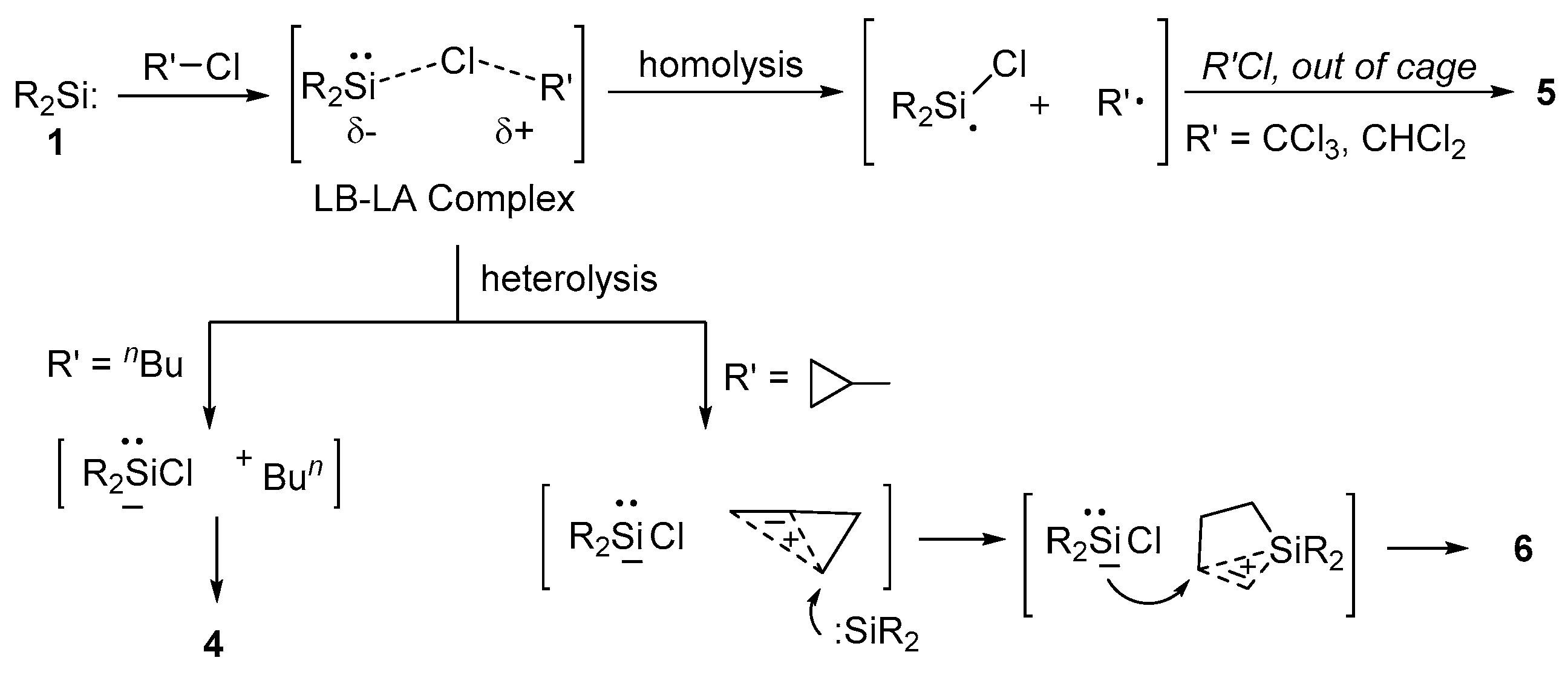
2.4. Mechanistic Aspects




3. Materials and Methods
3.1. General Procedures
3.2. Synthesis
3.2.1. Synthesis of 3a
3.2.2. Synthesis of 3b
3.2.3. Synthesis of 3c
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References and Notes
- Brook, A.G. Keto Derivatives of Group IV Organometalloids. In Advances in Organometallic Chemistry; Stone, F.G.A., Robert, W., Eds.; Academic Press: Cambridge, MA, USA, 1969; Volume 7, pp. 95–155. [Google Scholar]
- Page, P.C.B.; Klair, S.S.; Rosenthal, S. Synthesis and chemistry of acyl silanes. Chem. Soc. Rev. 1990, 19, 147–195. [Google Scholar] [CrossRef]
- Page, P.C.B.; Mckenzie, M.J.; Klair, S.S.; Rosenthal, S. Acyl Silanes. In The Chemistry of Organic Silicon Compounds; Rappoport, Z., Apeloig, Y., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1998; pp. 1599–1665. [Google Scholar]
- Cirillo, P.F.; Panek, J.S. Recent progress in the chemistry of acylsilanes. A review. Org. Prep. Proced. Int. 1992, 24, 553–582. [Google Scholar] [CrossRef]
- Patrocínio, A.F.; Moran, P.J.; Brazil, J. Acylsilanes and their applications in organic chemistry. Chem. Soc. 2001, 12, 7–31. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Priebbenow, D.L.; Bolm, C. Acylsilanes: valuable organosilicon reagents in organic synthesis. Chem. Soc. Rev. 2013, 42, 8540–8571. [Google Scholar] [CrossRef] [PubMed]
- Page, P.C.B.; McKenzie, M.J. Product Subclass 25: Acylsilanes. In Science of Synthesis; Fleming, I., Ed.; Thieme: Stuttgart, Germany, 2001; Volume 4, pp. 513–568. [Google Scholar]
- Garrett, M.N.; Johnson, J.S. Product Subclass 4: Silicon Compounds. In Science of Synthesis Knowledge Updates 2012/2; Fleming, I., Ed.; Thieme: Stuttgart, Germany, 2012; Volume 4, pp. 1–85. [Google Scholar]
- Boyce, G.R.; Grezler, S.N.; Johnson, J.S.; Linghu, X.; malinovski, J.T.; Nicewicz, D.A.; Satterfield, A.D.; Schmitt, D.C.; Steward, K.M. Silyl Glyoxylates. Conception and Realization of Flexible Conjunctive Reagents for Multicomponent Coupling. J. Org. Chem. 2012, 77, 4503–4515. [Google Scholar] [CrossRef] [PubMed]
- Tsubouchi, A.; Sasaki, N.; Enatsu, S.; Takeda, T. Regio-and stereoselective preparation of (Z)-silyl enol ethers by three-component coupling using α, β-unsaturated acylsilanes as core building blocks. Tetrahedron Lett. 2013, 54, 1264–1267. [Google Scholar] [CrossRef]
- Honda, M.; Nakajima, T.; Okada, M.; Yamaguchi, K.; Suda, M.; Kunimoto, K.K.; Segi, M. Reaction of acylsilanes with α-sulfinyl carbanions: Regioselective synthesis of silyl enol ethers. Tetrahedron Lett. 2011, 52, 3740–3742. [Google Scholar] [CrossRef]
- Honda, M.; Iwamoto, R.; Nogami, Y.; Segi, M. Stereoselective tandem Aldol-Tishchenko reaction with acylsilanes. Chem. Lett. 2005, 34, 466–467. [Google Scholar] [CrossRef]
- Zhang, H.J.; Becker, P.; Huang, H.; Pirwerdjan, R.; Pan, F.F.; Bolm, C. Photochemically induced silylacylations of alkynes with acylsilanes. Adv. Synth. Catal. 2012, 354, 2157–2161. [Google Scholar] [CrossRef]
- Wu, L.; Li, G.X.; Fu, Q.Q.; Yu, L.T.; Tang, Z. Organocatalytic asymmetric Michael reaction with acylsilane donors. Org. Biomol. Chem. 2013, 11, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Ding, C.H.; Liu, W.; Hou, X.L.; Dai, L.X. Palladium-catalyzed regio-, diastereo-, and enantioselective allylic alkylation of acylsilanes with monosubstituted allyl substrates. J. Am. Chem. Soc. 2010, 132, 15493–15495. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.H.; Nicewicz, D.A.; Johnson, J.S. Tandem Carbon−Carbon Bond Constructions via Catalyzed Cyanation/Brook Rearrangement/C-Acylation Reactions of Acylsilanes. Org. Lett. 2002, 4, 2957–2960. [Google Scholar]
- Mattson, A.E.; Scheidt, K.A. Catalytic Additions of Acylsilanes to Imines: An Acyl Anion Strategy for the Direct Synthesis of α-Amino Ketones. Org. Lett. 2004, 6, 4363–4366. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Karre, N.; Roisnel, T.; Chandrasekhar, S.; Gree, R. From Protected β-Hydroxy Acylsilanes to Functionalized Silyl Enol Ethers and Applications in Mukaiyama Aldol Reactions. Eur. J. Org. Chem. 2016, 773–779. [Google Scholar] [CrossRef]
- Murthy, A.S.; Roisnel, T.; Chandrasekhar, S.; Gree, R. New β-Hydroxy Acylsilane-Derived Building Blocks and Their Use in the Synthesis of Oxygen-Containing Heterocycles. Synlett 2013, 24, 2216–2220. [Google Scholar] [CrossRef]
- Brook, A.G.; Abdesaken, F.; Gutekunst, B.; Gutekunst, G.; Kallury, R.K. A Solid Silaethene: Isolation and Characterization. J. Chem. Soc. Chem. Commun. 1981, 191–192. [Google Scholar] [CrossRef]
- Brook, A.G.; Nyburg, S.C.; Abdesaken, F.; Gutekunst, B.; Gutekunst, G.; Krishna, R.; Kallury, M.R.; Poon, Y.C.; Chang, Y.-M.; Wong-Ng, W. Stable Solid Silaethylenes. J. Am. Chem. Soc. 1982, 104, 5667–5612. [Google Scholar] [CrossRef]
- Brook, A.G. Triphenylsilyl phenyl ketone. J. Am. Chem. Soc. 1957, 79, 4373–4375. [Google Scholar] [CrossRef]
- Wittenberg, D.; Gilman, H. Reactions of Silyllithium Compounds with Derivatives of Carboxylic Acids. I. Triphenylsilyllithium and Acetyl Chloride. J. Am. Chem. Soc. 1958, 80, 4529–4531. [Google Scholar] [CrossRef]
- Brook, A.G.; Duff, J.M.; Jones, P.F.; Davis, N.R. Synthesis of Silyl and Germyl Ketones. J. Am. Chem. Soc. 1967, 89, 431–434. [Google Scholar] [CrossRef]
- Corey, E.J.; Seebach, D.; Freedman, R. Synthesis of α-Silyl Ketones via 1,3-Dithianes. J. Am. Chem. Soc. 1967, 89, 434–436. [Google Scholar] [CrossRef]
- Chen, W.F.; Wang, L.L.; Li, Z.F.; Lin, A.Q.; Lai, G.Q.; Xiao, X.Q.; Deng, Y.; Kira, M. Diverse reactivity of an isolable dialkylsilylene toward imines. Dalton Trans. 2013, 42, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.P.; Xiao, X.Q.; Xu, Z.; Yang, X.M.; Li, Z.F.; Dong, Z.W.; Yan, C.T.; Lai, G.Q.; Kira, M. Reactions of an Isolable Dialkylsilylene with Carbon Dioxide and Related Heterocumulenes. Organometallics 2014, 33, 5434–5439. [Google Scholar] [CrossRef]
- Wang, L.L.; Chen, W.F.; Li, Z.F.; Xiao, X.Q.; Lai, G.Q.; Liu, X.P.; Xu, Z.; Kira, M. Reactions of an Isolable Dialkylsilylene with Aromatic Nitriles Providing a New Type of Heterosilole. Chem. Commun. 2013, 49, 9776–9778. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Xiao, X.Q.; Li, Z.; Lu, Q.; Lai, G.; Kira, M. Elusive 2H-1,2-Oxasiletes Through Reactions of an Isolable Dialkylsilylene with Diazocarbonyl Compounds. Org. Biomol. Chem. 2015, 13, 9471–9476. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.Q.; Dong, Z.; Li, Z.; Yan, C.; Lai, G.; Kira, M. 1,3-Diazasilabicyclo[1.1.0]butane with a Long Bridging N−N Bond. Angew. Chem. Int. Ed. 2016, 55, 3758–3762. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, D.; Mathew, J.; Kaushansky, A.; Bravo-Zhivotovskii, D.; Apeloig, Y. Isolation and Characterization, Including by X-ray Crystallography, of Contact and Solvent-Separated Ion Pairs of Silenyl Lithium Species. Angew. Chem. Int. Ed. 2016, 55, 10258–10262. [Google Scholar] [CrossRef] [PubMed]
- Kira, M.; Ishida, S.; Iwamoto, T.; Kabuto, C. The first isolable dialkylsilylene. J. Am. Chem. Soc. 1999, 121, 9722–9723. [Google Scholar] [CrossRef]
- Kira, M. Isolable silylene, disilenes, trisilaallene, and related compounds. J. Organomet. Chem. 2004, 689, 4475–4488. [Google Scholar] [CrossRef]
- Kira, M.; Ishida, S.; Iwamoto, T. Comparative chemistry of isolable divalent compounds of silicon, germanium, and tin. Chem. Rec. 2004, 4, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Kira, M.; Iwamoto, T.; Ishida, S. A Helmeted Dialkylsilylene. Bull. Chem. Soc. Jpn. 2007, 80, 258–275. [Google Scholar] [CrossRef]
- Kira, M. An isolable dialkylsilylene and its derivatives. A step toward comprehension of heavy unsaturated bonds. Chem. Commun. 2010, 46, 2893–2903. [Google Scholar] [CrossRef] [PubMed]
- Kira, M. Reactions of a Stable Dialkylsilylene and Their Mechanisms. J. Chem. Sci. 2012, 124, 1205–1215. [Google Scholar] [CrossRef]
- Ishida, S.; Iwamoto, T.; Kira, M. Reactions of an Isolable Dialkylsilylene with Ketones. Organometallics 2010, 29, 5526–5534. [Google Scholar] [CrossRef] and references cited therein.
- Ohshita, J.; Tokunaga, Y.; Sakurai, H.; Kunai, A. Reactions of lithium silenolates with acyl halides. First synthesis of di-and tetraacylsilanes. J. Am. Chem. Soc. 1999, 121, 6080–6081. [Google Scholar] [CrossRef]
- Ohshita, J.; Kawamoto, H.; Kunai, A.; Ottosson, H. Formation of Acylsilenolates from Bis (acyl) trisilanes as the Silicon Analogues of Acylenolates. Organometallics 2010, 29, 4199–4202. [Google Scholar] [CrossRef]
- Chieh, P.; Trotter, J. The structure of acetyltriphenylsilane. J. Chem. Soc. A 1969, 1778–1783. [Google Scholar] [CrossRef]
- Ramsey, B.G.; Brook, A.; Bassindale, A.R.; Bock, H. σ→π*, A reassignment of the long wavelength uv transition in acyl-silanes and-germanes by photoelectron spectroscopy. J. Organomet. Chem. 1974, 74, C41–C45. [Google Scholar] [CrossRef]
- Ishida, S.; Iwamoto, T.; Kabuto, C.; Kira, M. Unexpected reactions of an isolable dialkylsilylene with haloalkanes. Chem. Lett. 2001, 1102–1103. [Google Scholar] [CrossRef]
- Ishikawa, M.; Nakagawa, K.-I.; Katayama, S.; Kumada, M. Photolysis of organopolysilanes. The reaction of photochemically generated trimethylsilyphenylsilylene with alkyl chlorides. J. Organomet. Chem. 1981, 216, C48–C50. [Google Scholar] [CrossRef]
- Nakao, R.; Oka, K.; Dohmaru, T.; Nagata, Y.; Fukumoto, T. Chlorine abstraction from chloromethanes by dimethylsilanediyls. J. Chem. Soc. Chem. Commun. 1985, 766–768. [Google Scholar] [CrossRef]
- Oka, K.; Nakao, R. Reaction of phenyl (trimethylsily) silylene with chloromethanes; insertion into the C-Cl bond and abstraction of chlorine and HCI. J. Organomet. Chem. 1990, 390, 7–18. [Google Scholar] [CrossRef]
- Moser, D.F.; Bosse, T.; Olson, J.; Moser, J.L.; Guzei, I.A.; West, R. Halophilic Reactions of a Stable Silylene with Chloro and Bromocarbons. J. Am. Chem. Soc. 2002, 124, 4186–4187. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yao, S.; Driess, M. Reactivity of a Zwitterionic Stable Silylene toward Halosilanes and Haloalkanes. Organometallics 2009, 28, 1927–1933. [Google Scholar] [CrossRef]
- Ishida, S.; Iwamoto, T.; Kabuto, C.; Kira, M. A stable silicon-based allene analogue with a formally sp-hybridized silicon atom. Nature 2003, 421, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Iwamoto, T.; Kabuto, C.; Kira, M. Insertion of a stable dialkylsilylene into silicon-chlorine bonds. Silicon Chem. 2003, 2, 137–140. [Google Scholar] [CrossRef]
- Chen, Y.S.; Gaspar, P.P. Octakis(triemthylsilyl)cyclotetrasilane. A stable cyclotetrasilane from a silylene precursor. Organometallics 1982, 1, 1410–1412. [Google Scholar] [CrossRef]
- Belzner, J.; Dehnert, U.; Ihmels, H.; Hübner, M.; Müller, P.; Uson, I. New Dichlorosilanes, Cyclotrisilanes, and Silacyclopropanes as Precursors of Intramolecularly Coordinated Silylenes. Chem. Eur. J. 1998, 4, 852–863. [Google Scholar] [CrossRef]
- Belzner, J.; Dehnert, U.; Schar, D.; Rohde, B.; Muller, P.; Uson, I. Synthesis of di- and trisilanes with potentially chelating substituents. J. Organomet. Chem. 2002, 649, 25–42. [Google Scholar] [CrossRef]
- Koecher, J.; Lehnig, M.; Neumann, W.P. Chemistry of heavy carbene analogs R2M (M = Si, Ge, Sn). 12. Concerted and nonconcerted insertion reactions of dimethylgermylene into the carbon-halogen bond. Organometallics 1988, 7, 1201–1207. [Google Scholar] [CrossRef]
- Lai, G.; Xu, Z.; Li, Z.; Jiang, J.; Kira, M.; Qiu, H. Stereoelectronic Substituent Effects on Silylene Insertion into the Si-Cl Bond. Organometallics 2009, 28, 3591–3593. [Google Scholar] [CrossRef]
- Xu, Z.; Jin, J.; Li, Z.; Qiu, H.; Jiang, J.; Lai, G.; Kira, M. Remarkable Substituent Effects on the Activation Energy of Silylene Insertion into Silicon-Chlorine Bonds. Chem. Eur. J. 2009, 15, 8605–8612. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jin, J.; Zhang, H.; Li, Z.; Jiang, J.; Lai, G.; Kira, M. Insertion of Silylenes into Si-H and Si-Cl Bonds. Comparison of Mechanism and Substituent Effects. Organometallics 2011, 30, 3311–3317. [Google Scholar] [CrossRef]
- SHELXTL version 6. 10; Bruker AXS Inc.: Madison, WI, USA, 2003.
- Sheldrick, G.M. SADABS Program for Empirical X-ray Absorption Correction; University of Goettingen: Göttingen, Germany, 1996. [Google Scholar]
- Sample Availability: Samples of the compounds 3a–3c are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.-Q.; Liu, X.; Lu, Q.; Li, Z.; Lai, G.; Kira, M. Reactions of an Isolable Dialkylsilylene with Aroyl Chlorides. A New Route to Aroylsilanes. Molecules 2016, 21, 1376. https://doi.org/10.3390/molecules21101376
Xiao X-Q, Liu X, Lu Q, Li Z, Lai G, Kira M. Reactions of an Isolable Dialkylsilylene with Aroyl Chlorides. A New Route to Aroylsilanes. Molecules. 2016; 21(10):1376. https://doi.org/10.3390/molecules21101376
Chicago/Turabian StyleXiao, Xu-Qiong, Xupeng Liu, Qiong Lu, Zhifang Li, Guoqiao Lai, and Mitsuo Kira. 2016. "Reactions of an Isolable Dialkylsilylene with Aroyl Chlorides. A New Route to Aroylsilanes" Molecules 21, no. 10: 1376. https://doi.org/10.3390/molecules21101376
APA StyleXiao, X.-Q., Liu, X., Lu, Q., Li, Z., Lai, G., & Kira, M. (2016). Reactions of an Isolable Dialkylsilylene with Aroyl Chlorides. A New Route to Aroylsilanes. Molecules, 21(10), 1376. https://doi.org/10.3390/molecules21101376