Protective Effect of the Plant Extracts of Erythroxylum sp. against Toxic Effects Induced by the Venom of Lachesis muta Snake
Abstract
:1. Introduction
2. Results and Discussion
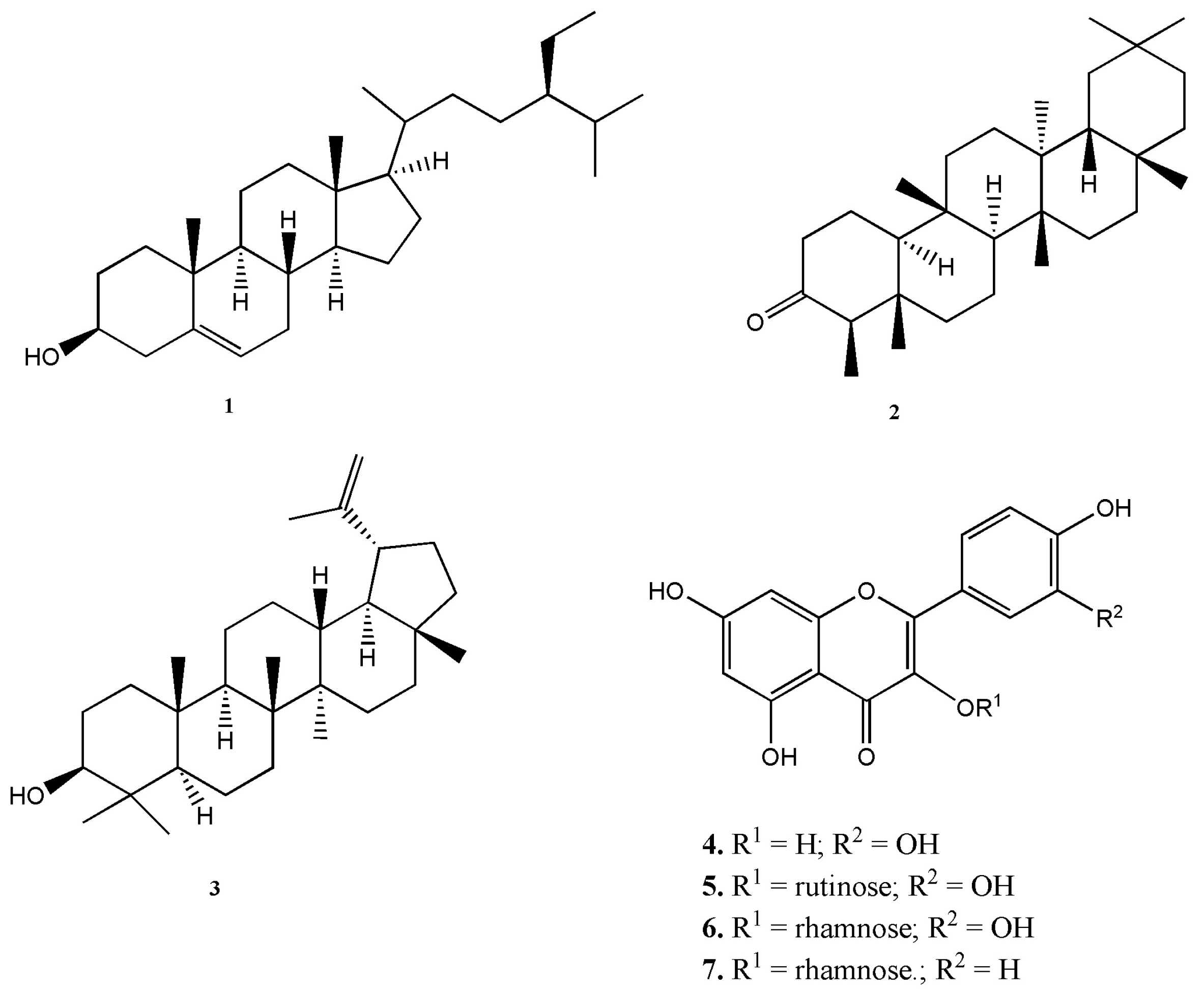
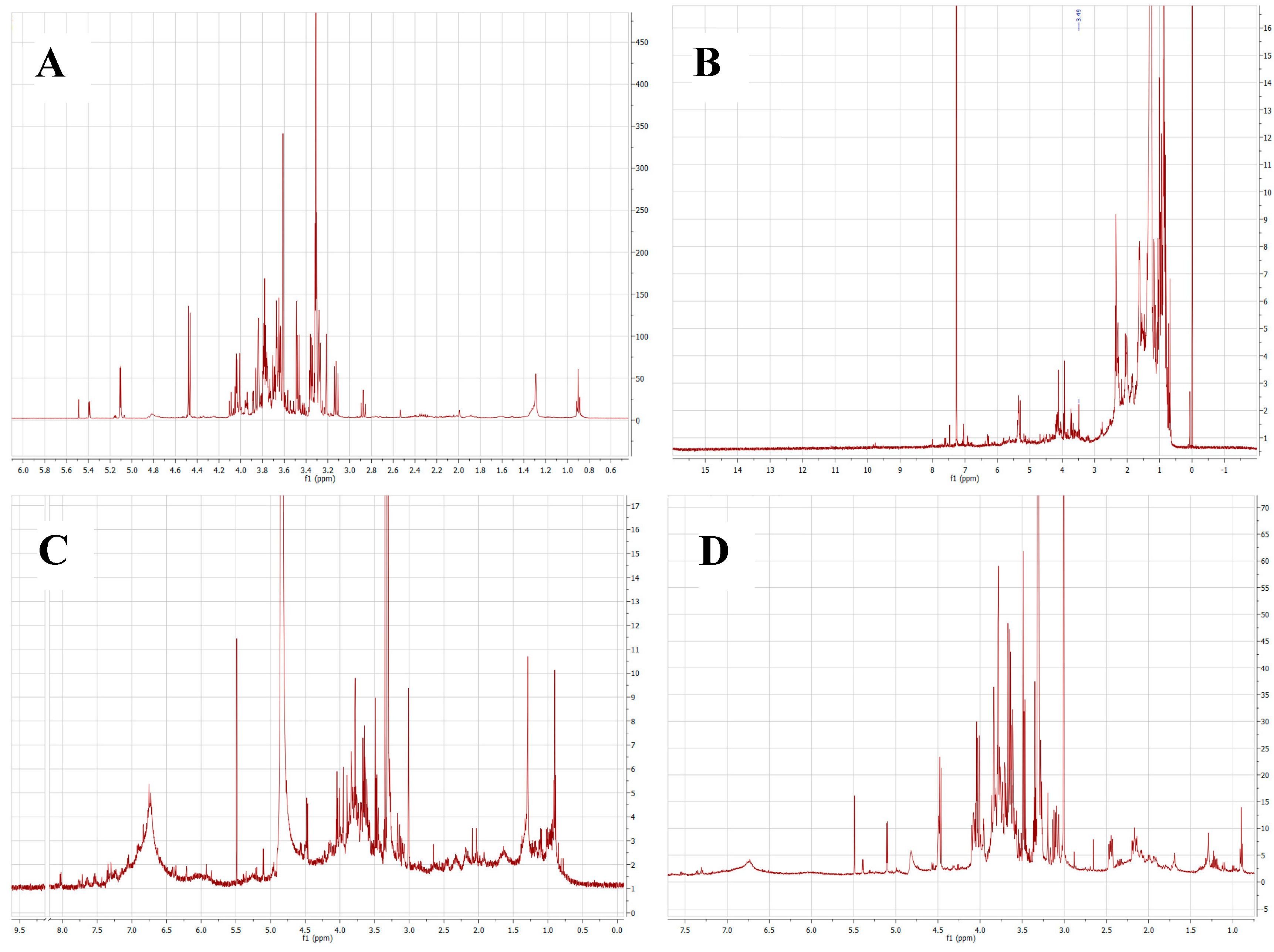
2.1. Chemical Composition of Plant Extracts
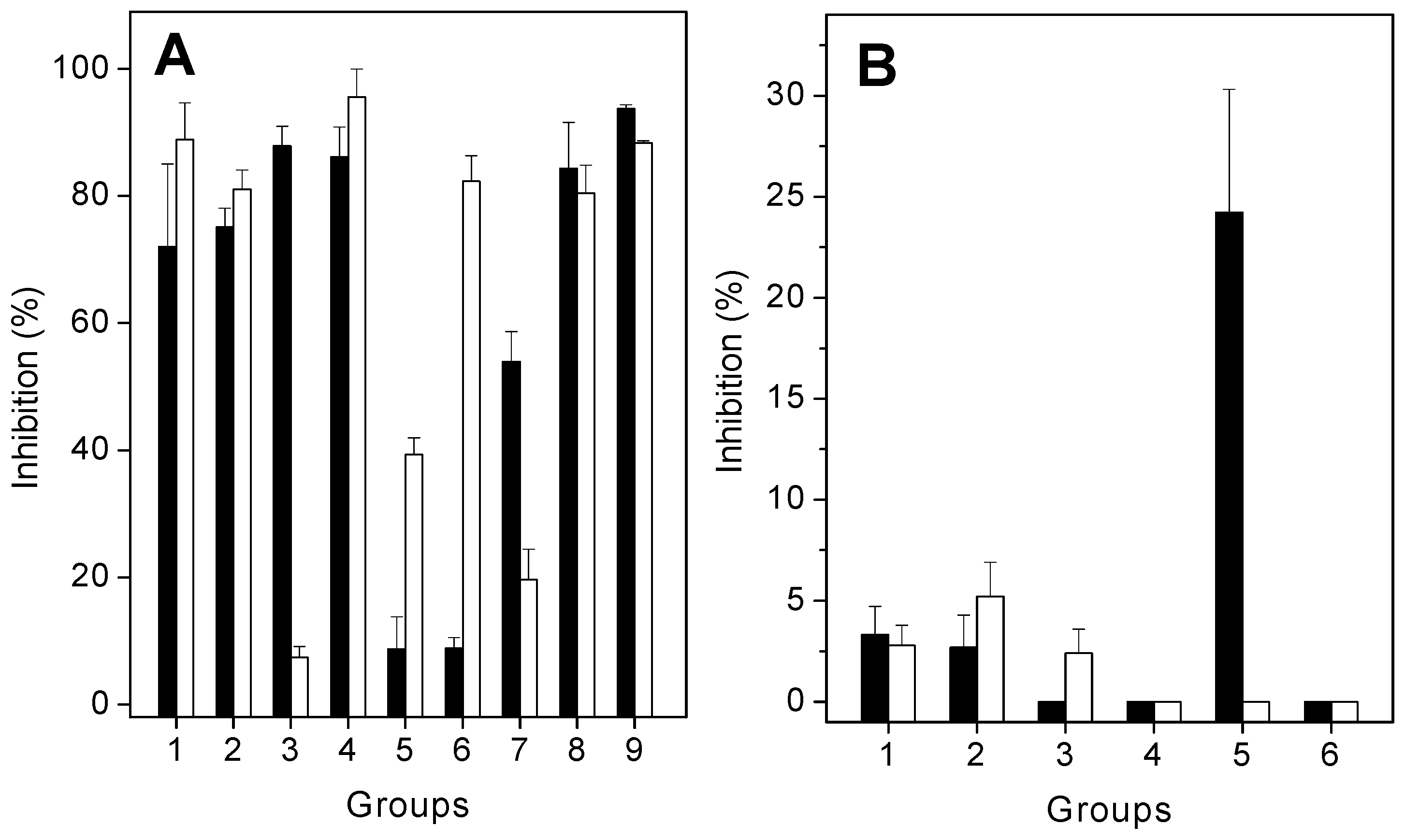
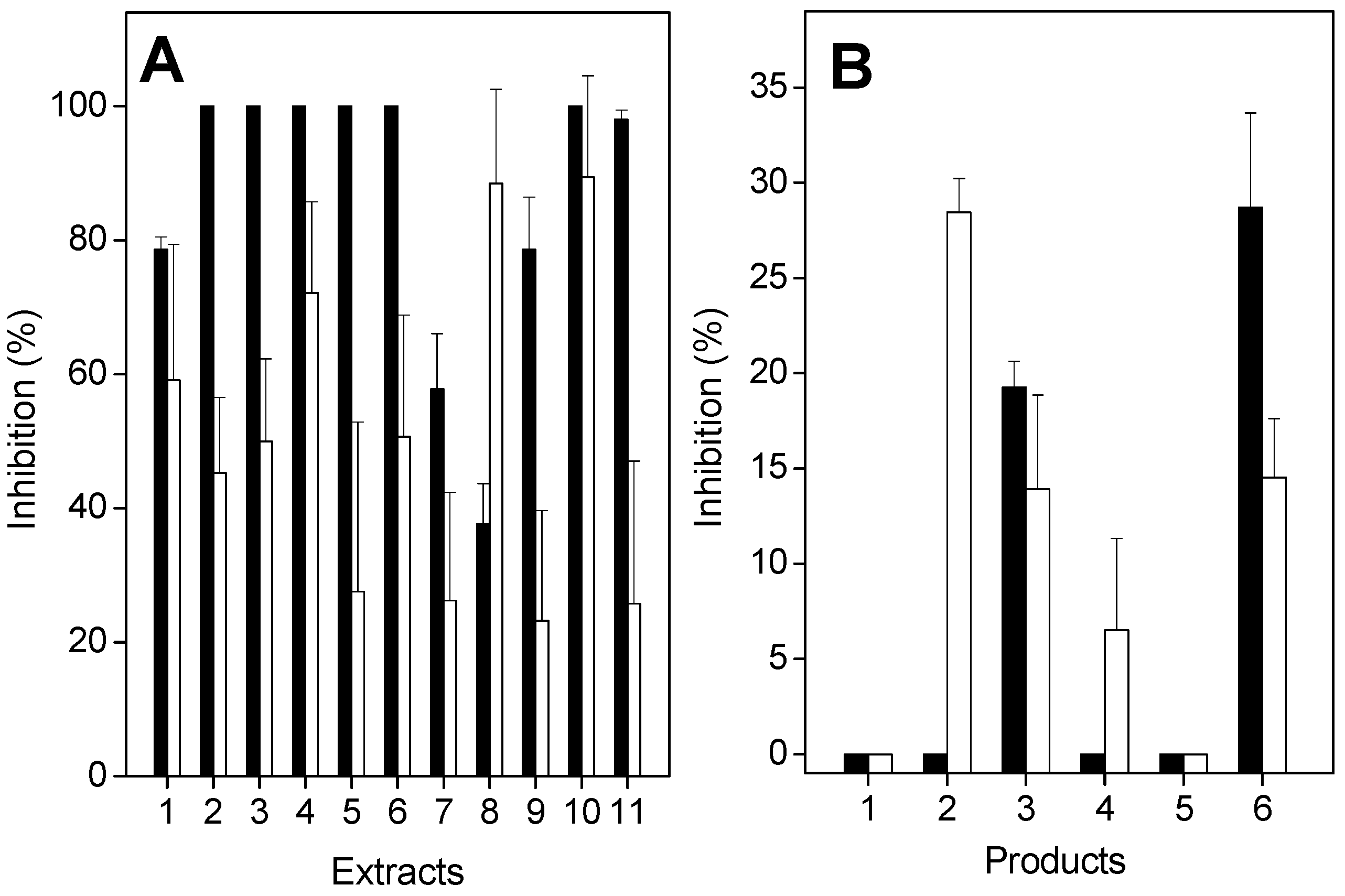
2.2. Antiproteolytic and Antihemolytic Effects of Plant Extracts or Products
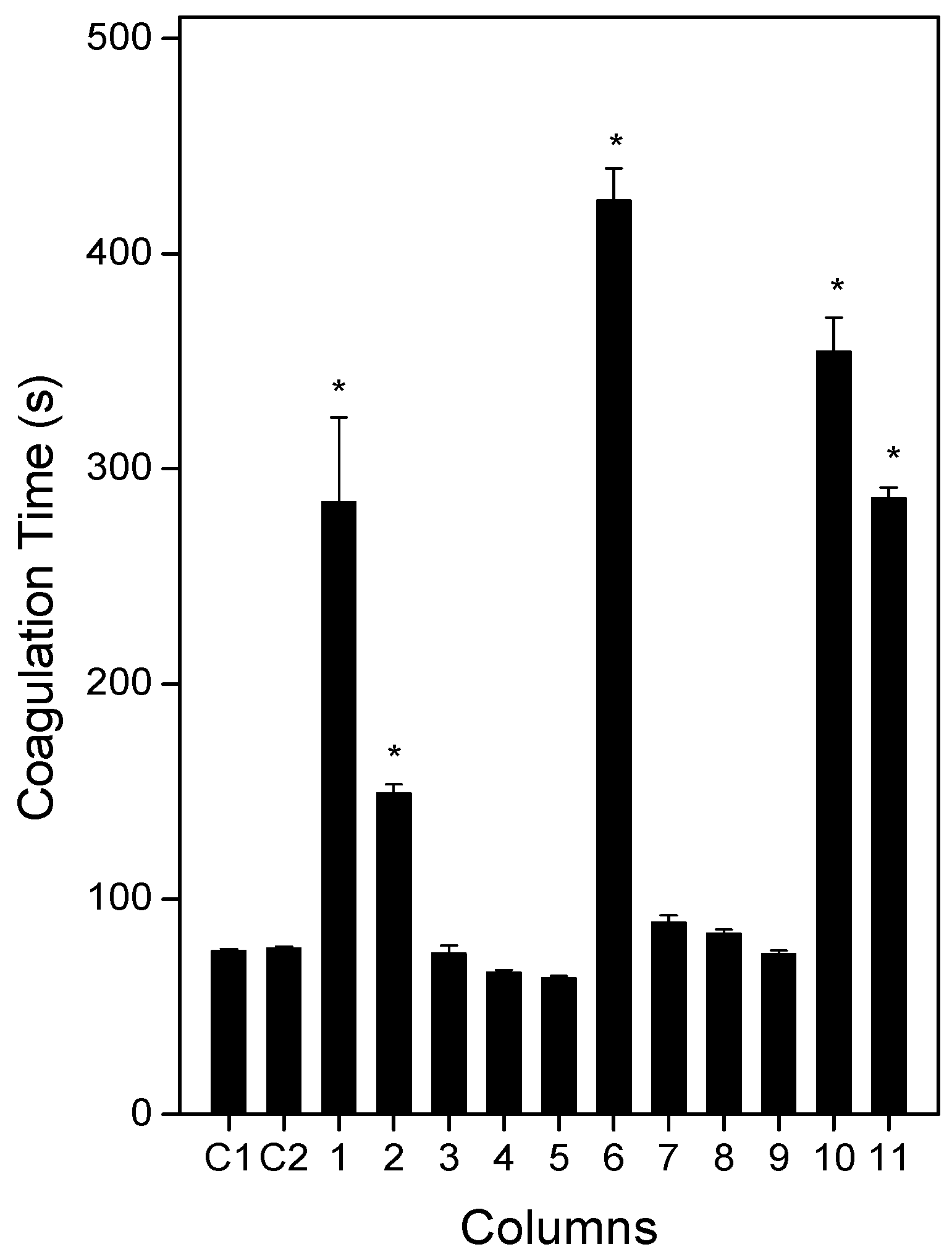
2.3. Anticoagulation Effect of Plants or Products
2.4. Antihemorrhagic and Antiedematogenic Effects of Plants or Products
3. Materials and Methods
3.1. Venom, Animals, and Reagents
3.2. Ethics Statement
3.3. Plant Material
3.4. Preparation of Plant Extracts and Partitions
3.5. Phytochemical Analysis
3.6. Proteolytic Activity
3.7. Coagulating Activity
3.8. Hemolytic Activity
3.9. Hemorrhagic Activity
3.10. Edematogenic Activity
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DMSO | Dimethyl sulfoxide |
| MCD | Minimum Coagulation Dose |
| MHD | Minimum Hemorrhagic Dose |
| MIHD | Minimum Indirect Hemolytic Dose |
| PLA2 | Phospholipase A2 |
| TLC | Thin-layer chromatography |
| E. ovalifolium | Erythroxylum ovalifolium |
| E. subsessile | Erythroxylum subsessile |
| L. muta | Lachesis muta |
References
- Warrel, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Williams, D.; Gutiérrez, J.M.; Harrison, R.; Warrell, D.A.; White, J.; Winkel, K.D.; Gopalakrishnakone, P. The global snake bite initiative: An antidote for snake bite. Lancet 2010, 375, 89–91. [Google Scholar] [CrossRef]
- Gutierrez, J.M. Isolation of bothrasperin, a disintegrin with potent platelet aggregation inhibitory activity, from the venom of the snake Bothrops asper. Rev. Biol. Trop. 2002, 50, 377–394. [Google Scholar] [PubMed]
- Colombini, M.; Fernandes, I.; Cardoso, D.F.; Moura-da-Silva, A.M. Lachesis muta venom: Immunological differences compared with Bothrops atrox venom and importance of specific antivenom therapy. Toxicon 2001, 39, 711–719. [Google Scholar] [CrossRef]
- Adukauskienė, D.; Varanauskienė, E.; Adukauskaitė, A. Venomous snakebites. Medicina (Kaunas) 2011, 47, 461–467. [Google Scholar] [PubMed]
- Lomonte, B.; León, G.; Angulo, Y.; Rucavado, A.; Núñez, V. Neutralization of Bothrops asper venom by antibodies, natural products and synthetic drugs: Contributions to understanding snakebite envenomings and their treatment. Toxicon 2009, 54, 1012–1028. [Google Scholar] [CrossRef] [PubMed]
- Mors, W.B.; do Nascimento, M.C.; Pereira, B.M.R.; Pereira, N.A. Plant natural products active against snake bite–the molecular approach. Phytochemistry 2000, 55, 627–642. [Google Scholar] [CrossRef]
- Oliveira, C.Z.; Maiorano, V.A.; Marcussi, S.; Sant'ana, C.D.; Januário, A.H.; Lourenço, M.V.; Sampaio, S.V.; França, S.C.; Pereira, P.S.; Soares, A.M. Anticoagulant and antifibrinogenolytic properties of the aqueous extract from Bauhinia forficata against snake venoms. J. Ethnopharmacol. 2005, 98, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Gibbons, S. Ethnopharmacology in drug discovery: An analysis of its role and potential contribution. J. Pharm. Pharmacol. 2001, 53, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Ladio, A.; Lozada, M.; Weigandt, M. Comparison of traditional wild plants use between two Mapuche communities inhabiting arid an forest environments in Patagonia, Argentina. J. Arid. Environ. 2007, 69, 695–715. [Google Scholar] [CrossRef]
- Butt, M.A.; Ahmad, M.; Fatima, A.; Sultana, S.; Zafar, M.; Yaseen, G.; Ashraf, M.A.; Shinwari, Z.K.; Kayani, S. Ethnomedicinal uses of plants for the treatment of snake and scorpion bite in Northern Pakistan. J. Ethnopharmacol. 2015, 168, 164–181. [Google Scholar] [CrossRef] [PubMed]
- Molander, M.; Nielsen, L.; Søgaard, S.; Staerk, D.; Ronsted, N.; Diallo, D.; Chifundera, K.Z.; van Staden, J.; Jäger, A.K. Hyaluronidase, phospholipase A2 and protease inhibitory activity of plants used in traditional treatment of snakebite-induced tissue necrosis in Mali, DR Congo and South Africa. J. Ethnopharmacol. 2014, 157, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, S.B. Erythroxylaceae, Erythroxylum subsessile (Mart.) O.E. Schulz in Espírito Santo: Distribution extension. Check List 2012, 1292–1293. [Google Scholar] [CrossRef]
- Aguiar, J.S.; Araújo, R.O.; Rodrigues, M.R.; Sena, K.X.; Batista, A.M.; Guerra, M.M.; Oliveira, S.L.; Tavares, J.F.; Silva, M.S.; Nascimento, S.C.; et al. Antimicrobial, antiproliferative and proapoptotic activities of extract, fractions and isolated compounds from the stem of Erythroxylum caatingae plowman. Int. J. Mol. Sci. 2012, 13, 4124–4140. [Google Scholar] [CrossRef] [PubMed]
- Barreiros, M.L.; David, J.P.; David, J.M.; Lopes, L.M.X.; de Sá, M.S.; Costa, J.F.O.; Almeida, M.Z.; de Queiróz, L.P.; Sant’Ana, A.E.G. Ryanodane diterpenes from two Erythroxylum species. Phytochemistry 2007, 68, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, S.L.; Tavares, J.F.; Branco, M.V.; Lucena, H.F.S.; Barbosa-Filho, J.M.; Agra, M.F.; do Nascimento, S.C.; Aguiar, J.S.; da Silva, T.G.; de Simone, C.A.; et al. Tropane alkaloids from Erythroxylum caatingae Plowman. Chem. Biodiv. 2011, 8, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.J.; Linb, G.D. Chemotaxonomy and geographical distribution of tropane alkaloids. Phytochemistry 2000, 53, 623–637. [Google Scholar] [CrossRef]
- Zanolari, B.; Guilet, D.; Marston, A.; Queiroz, E.F.; Paulo, M.Q.; Hostettmann, K. Tropane alkaloids from the bark of Erythroxylum vacciniifolium. J. Nat. Prod. 2003, 66, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Picot, C.M.N.; Subratty, A.H.; Mahomoodally, M.F. Inhibitory potential of five traditionally used native antidiabetic medicinal plants on α -amylase, α-glucosidase, glucose entrapment, and amylolysis kinetics in vitro. Adv. Pharm. Sci. 2014, 2014, 739834. [Google Scholar]
- Barreiros, M.L.; David, J.M.; Queiroz, L.P.; David, J.P. Flavonoids and triterpenes from leaves of Erythroxylum nummularia. Biochem. Syst. Ecol. 2005, 33, 537–540. [Google Scholar] [CrossRef]
- Katkar, G.D.; Sharma, R.D.; Vishalakshi, G.J.; Naveenkumar, S.K.; Madhur, G.; Thushara, R.M.; Narender, T.; Girish, K.S.; Kemparaju, K. Lupeol derivative mitigates Echis carinatus venom-induced tissue destruction by neutralizing venom toxins and protecting collagen and angiogenic receptors on inflammatory cells. Biochim. Biophys. Acta 2015, 1850, 2393–2409. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.M.; Santos, J.D.; Xavier, B.M.; Almeida, J.R.; Resende, L.M.; Martins, W.; Marcussi, S.; Marangoni, S.; Stábeli, R.G.; Calderon, L.A.; et al. Snake venom PLA2s inhibitors isolated from Brazilian plants: Synthetic and natural molecules. BioMed Res. Int. 2013, 2013, 153045. [Google Scholar] [CrossRef] [PubMed]
- Van Kiem, P.; Van Minh, C.; Huong, H.T.; Nam, N.H.; Lee, J.J.; Kim, Y.H. Pentacyclic triterpenoids from Mallotus apelta. Arch. Pharm. Res. 2004, 27, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Mahato, S.B.; Kundu, A.P. 13C-NMR Spectra of Pentacyclic Triterpenoids—A compilation and some salient features. Phytochemistry 1994, 37, 1517–1575. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Farimani, M.M.; Salahvarzi, S.; Amin, G. Chemical constituents of dichloromethane extract of cultivated Satureja khuzistanica. J. Evid. Based Complement. Altern. Med. 2007, 4, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Barreiros, M.L.; David, J.M.; Pereira, P.A.P.; Guedes, M.L.S.; David, J.P. Fatty acid esters of triterpenes from Erythroxylum passerinum. J. Braz. Chem. Soc. 2002, 5, 669–673. [Google Scholar] [CrossRef]
- Chávez, J.P.; Dos Santos, I.D.; Cruz, F.G.; David, J.M. Flavonoids and triterpene ester derivatives from Erythroxylum leal costae. Phytochemistry 1996, 41, 941–943. [Google Scholar] [CrossRef]
- Albuquerque, C.H.; Tavares, J.F.; Oliveira, S.L.; Silva, T.S.; Gonçalves, G.F.; Costa, V.C.O.; Agra, M.F.; Pessôa, H.L.F.; Silva, M.S. Flavonoides glicosilados de Erythroxylum pulchrum a. st.-hil. (Erythroxylaceae). Quim. Nova 2014, 37, 663–666. [Google Scholar] [CrossRef]
- Perera Córdova, W.H.; Gómez, M.M.; Tabart, J.; Sipel, A.; Kevers, C.; Dommes, J. In vitro characterization of antioxidant properties of cuban endemic varieties of Erythroxylum alaternifolium a. rich. isolation of two flavonol glycosides. J. Chil. Chem. Soc. 2012, 57, 1340–1343. [Google Scholar] [CrossRef]
- Johnson, E.L.; Schmidt, W.F.; Cooper, D. Flavonoids as chemotaxonomic markers for cultivated Amazonian coca. Plant Physiol. Biochem. 2002, 40, 89–95. [Google Scholar] [CrossRef]
- Soares, A.M.; Ticli, F.K.; Marcussi, S.; Lourenço, M.V.; Januário, A.H.; Sampaio, S.V.; Giglio, J.R.; Lomonte, B.; Pereira, O.S. Medicinal plants with inhibitory properties against snake venoms. Cur. Med. Chem. 2005, 12, 2625–2641. [Google Scholar] [CrossRef]
- Samy, R.P.; Gopalakrishnakone, P.; Chow, V.T.K. Therapeutic application of natural inhibitors against snake venom phospholipase A2. Bioinformation 2012, 8, 48–57. [Google Scholar] [CrossRef]
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, E.J.; Kim, Y.M.; Jung, H.A. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem. Toxicol. 2014, 64, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Toyama, D.O.; Marangoni, S.; Diz-Filho, E.B.; Oliveira, S.C.; Toyama, M.H. Effect of umbelliferone (7-hydroxycoumarin, 7-HOC) on the enzymatic, edematogenic and necrotic activities of secretory phospholipase A2 (sPLA2) isolated from Crotalus durissus collilineatus venom. Toxicon 2009, 53, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.Z.; Dalcol, I.I.; Adolpho, L.; Teixidó, M.; Tarragó, T.; Morel, A.; Giralt, E. Chemical composition and inhibitory effects of Hypericum brasiliense and H. connatum on prolyl oligopeptidase and acetylcholinesterase activities. Med. Chem. 2016, 12, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Dal Belo, C.A.; Lucho, A.P.; Vinadé, L.; Rocha, L.; Seibert, H.F.; Marangoni, S.; Rodrigues-Simioni, L. In vitro antiophidian mechanisms of Hypericum brasiliense choisy standardized extract: quercetin-dependent neuroprotection. BioMed Res. Int. 2013, 2013, 943520–943526. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.; Chérigo, L.; Acosta, H.; Otero, R.; Martínez-Luis, S. Evaluation of anti-Bothrops asper venom activity of ethanolic extract of Brownea rosademonte leaves. Acta Pharm. 2014, 64, 475–483. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, E.C.; Fernandes, C.P.; Sanchez, E.F.; Rocha, L.; Fuly, A.L. Inhibitory effect of plant Manilkara subsericea against biological activities of Lachesis muta snake venom. BioMed Res. Int. 2014, 2014, 408068–408074. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.M.; Vieira, S.A.P.; Gomes, M.S.R.; Paula, V.F.; Alcântara, T.M.; Homsi-Brandeburgo, M.I.; dos Santos, J.I.; Magro, A.J.; Fontes, M.R.; Rodrigues, V.M. Triacontyl p-coumarate: An inhibitor of snake venom metalloproteinases. Phytochemistry 2013, 86, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Nazato, V.S.; Rubem-Mauro, L.; Vieira, N.A.G.; Rocha-Junior, D.S.; Silva, M.G.; Lopes, P.S.; Dal-Belo, C.A.; Cogo, J.C.; Santos, M.C.; Cruz-Höfling, M.A.; et al. In vitro antiophidian properties of Dipteryx alata vogel bark extract. Molecules 2010, 15, 5956–5970. [Google Scholar] [CrossRef] [PubMed]
- Puebla, P.; Oshima-Franco, Y.; Franco, L.M.; Santos, M.G.; da Silva, R.V.; Rubem-Mauro, L.; Feliciano, A.S. Chemical constituents of the bark of Dipteryx alata vogel, an active species against Bothrops jararacussu Venom. Molecules 2010, 15, 8193–8204. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.C.; Yoshida, E.H.; Tavares, R.V.S.; Cogo, J.C.; Cintra, A.C.O.; Dal Belo, C.A.; Franco, L.M.; dos Santos, M.G.; Resende, F.A.; Varanda, E.A.; et al. An Isoflavone from Dipteryx alata Vogel is active against the in vitro neuromuscular paralysis of Bothrops jararacussu snake venom and Bothropstoxin I, and prevents venom-induced myonecrosis. Molecules 2014, 19, 5790–5805. [Google Scholar] [CrossRef] [PubMed]
- Domingos, T.F.S.; Vallim, M.A.; Cavalcanti, D.N.; Sanchez, E.F.; Teixeira, V.L.; Fuly, A.L. Effect of diterpenes isolated of the marine alga Canistrocarpus cervicornis against some toxic effects of the venom of the Bothrops jararaca snake. Molecules 2015, 20, 3515–3526. [Google Scholar] [CrossRef] [PubMed]
- Faioli, C.N.; Domingos, T.F.; de Oliveira, E.C.; Sanchez, E.F.; Ribeiro, S.; Muricy, G.; Fuly, A.L. Appraisal of antiophidic potential of marine sponges against Bothrops jararaca and Lachesis muta venom. Toxins 2013, 17, 1799–1813. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.S.; Guimarães, J.A.; Prado, J.L. Purification and characterization of a sulfhydryl-dependent protease from Rhodnius prolixus midgut. Arch. Biochem. Biophys. 1978, 188, 315–322. [Google Scholar] [CrossRef]
- Fuly, A.L.; Machado, O.L.; Alves, E.W.; Carlini, C.R. Mechanism of inhibitory action on platelet activation of a phospholipase A2 isolated from Lachesis muta (Bushmaster) snake venom. Thromb. Haemost. 1997, 78, 1372–1380. [Google Scholar] [PubMed]
- Kondo, H.; Kondo, S.; Ikezawa, H.; Murata, R. Studies on the quantitative method for determination of hemorrhagic activity of Habu snake venom. Jpn. J. Med. Sci. Biol. 1960, 13, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, M.; Nozani, M.; Hokama, Z. Toxins: Animal, Plant and Microbial; Ohsaka, A., Hayashi, K., Sawai, Y., Eds.; Plenum Press: New York, NY, USA, 1976; pp. 97–120. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
| Sample | % Inhibition | |||
|---|---|---|---|---|
| Hemorrhage | Edema | |||
| i.p. | oral | i.p. | oral | |
| 1 | 8 ± 9 | 0 | 0 | 22 ± 7 |
| 2 | 5 ± 7 | 0 | 18 ± 9 | 19 ± 9 |
| 3 | 10 ± 8 | 3 ± 15 | 0 | 0 |
| 4 | 40 ± 4 | 4 ± 12 | 12 ± 6 | 17 ± 4 |
| 5 | 7 ± 9 | 0 | 0 | 29 ± 8 |
| 6 | 18 ± 5 | 0 | 12 ± 4 | 5 ± 2 |
| 7 | 14 ± 9 | 7 ± 1 | 0 | 30 ± 9 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coriolano de Oliveira, E.; Alves Soares Cruz, R.; De Mello Amorim, N.; Guerra Santos, M.; Carlos Simas Pereira Junior, L.; Flores Sanchez, E.O.; Pinho Fernandes, C.; Garrett, R.; Machado Rocha, L.; Lopes Fuly, A. Protective Effect of the Plant Extracts of Erythroxylum sp. against Toxic Effects Induced by the Venom of Lachesis muta Snake. Molecules 2016, 21, 1350. https://doi.org/10.3390/molecules21101350
Coriolano de Oliveira E, Alves Soares Cruz R, De Mello Amorim N, Guerra Santos M, Carlos Simas Pereira Junior L, Flores Sanchez EO, Pinho Fernandes C, Garrett R, Machado Rocha L, Lopes Fuly A. Protective Effect of the Plant Extracts of Erythroxylum sp. against Toxic Effects Induced by the Venom of Lachesis muta Snake. Molecules. 2016; 21(10):1350. https://doi.org/10.3390/molecules21101350
Chicago/Turabian StyleCoriolano de Oliveira, Eduardo, Rodrigo Alves Soares Cruz, Nayanna De Mello Amorim, Marcelo Guerra Santos, Luiz Carlos Simas Pereira Junior, Eladio Oswaldo Flores Sanchez, Caio Pinho Fernandes, Rafael Garrett, Leandro Machado Rocha, and André Lopes Fuly. 2016. "Protective Effect of the Plant Extracts of Erythroxylum sp. against Toxic Effects Induced by the Venom of Lachesis muta Snake" Molecules 21, no. 10: 1350. https://doi.org/10.3390/molecules21101350
APA StyleCoriolano de Oliveira, E., Alves Soares Cruz, R., De Mello Amorim, N., Guerra Santos, M., Carlos Simas Pereira Junior, L., Flores Sanchez, E. O., Pinho Fernandes, C., Garrett, R., Machado Rocha, L., & Lopes Fuly, A. (2016). Protective Effect of the Plant Extracts of Erythroxylum sp. against Toxic Effects Induced by the Venom of Lachesis muta Snake. Molecules, 21(10), 1350. https://doi.org/10.3390/molecules21101350










