The Potential Mechanisms of Berberine in the Treatment of Nonalcoholic Fatty Liver Disease
Abstract
:1. Introduction
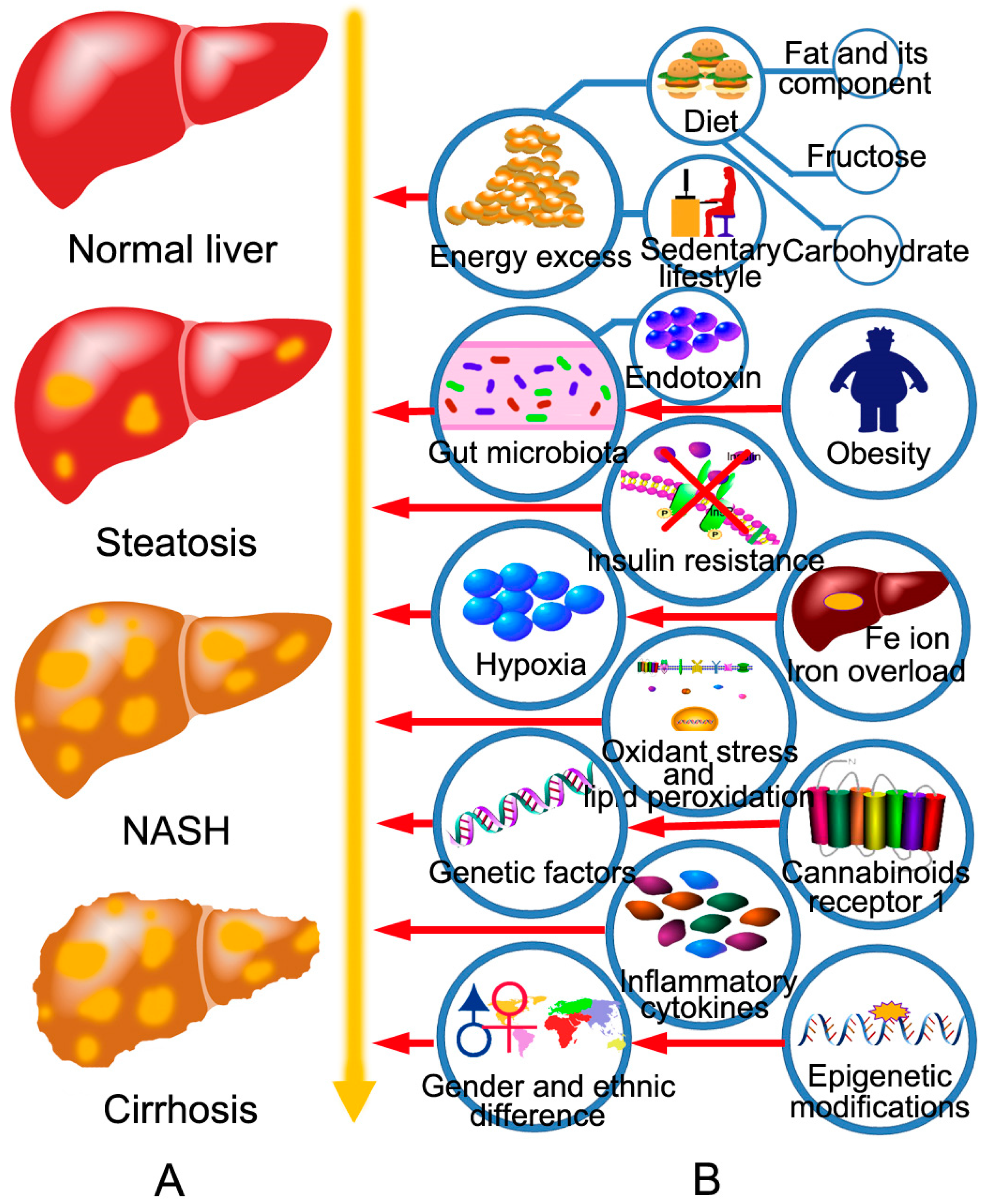
2. The Pathogenesis of NAFLD and the Targets of BBR in the Treatment of NAFLD
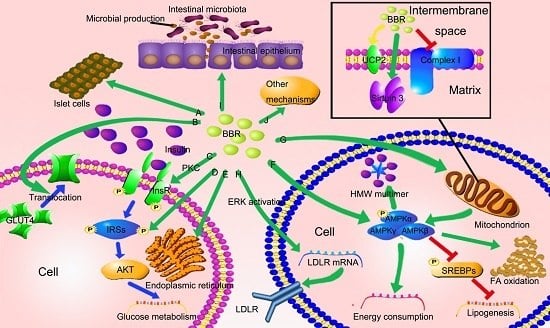
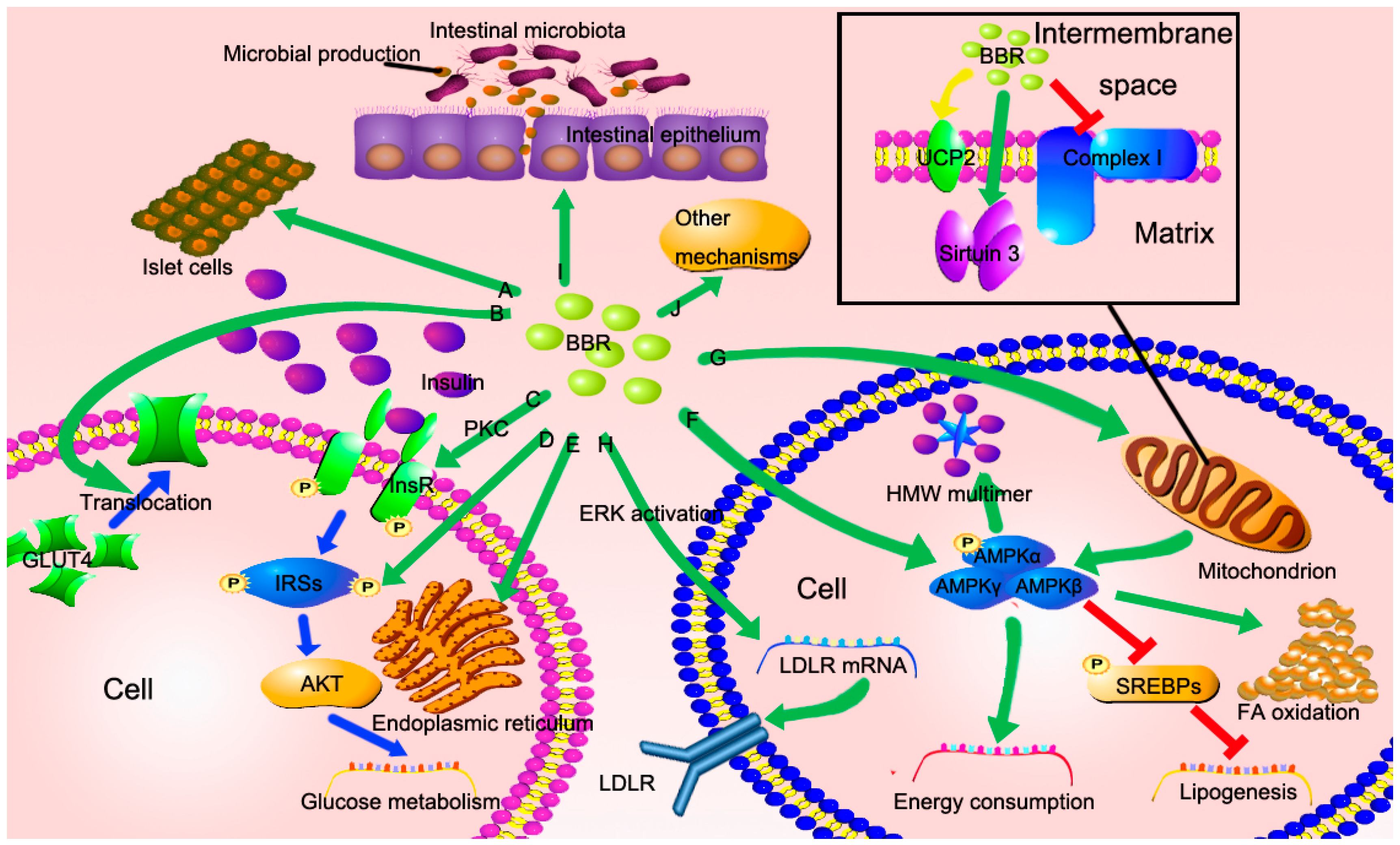
3. The Potential Mechanisms of Berberine in the Treatment of NAFLD
3.1. BBR Improves Insulin Resistance via Multiple Ways
3.2. BBR Reduced Lipid Accumulation via Regulating AMPK Phosphorylation
3.3. BBR Improves Mitochondrial Function and Alleviates Oxidative Stress
3.4. BBR Reduces Serum Cholesterol via A Distinctive Mechanism
3.5. The New Role of BBR in Gut Microenvironment
3.6. Other Potential Mechanisms
4. Future Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human fatty liver disease: Old questions and new insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Kotronen, A.; Yki-Jarvinen, H. Fatty liver: A novel component of the metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the american association for the study of liver diseases, american college of gastroenterology, and the american gastroenterological association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Vanni, E.; Bugianesi, E.; Kotronen, A.; de Minicis, S.; Yki-Jarvinen, H.; Svegliati-Baroni, G. From the metabolic syndrome to NAFLD or vice versa? Dig. Liver Dis. 2010, 42, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Otgonsuren, M.; Henry, L.; Venkatesan, C.; Mishra, A.; Erario, M.; Hunt, S. Association of non-alcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the united states from 2004–2009. Hepatology 2015, 62, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; El-Serag, H.B. Epidemiology of hepatocellular carcinoma: Consider the population. J. Clin. Gastroenterol. 2013, 47. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; El-Serag, H.B.; Sada, Y.H.; Kanwal, F.; Duan, Z.G.; Temple, S.; May, S.B.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Hepatocellular carcinoma in the absence of cirrhosis in united states veterans is associated with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2016, 14, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.R.; Duan, Z.G.; Yu, X.Y.; White, D.; El-Serag, H.B. Trends in the burden of nonalcoholic fatty liver disease in a united states cohort of veterans. Clin. Gastroenterol. Hepatol. 2016, 14, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Adams, L.A.; Canbay, A.; Syn, W.K. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology 2014, 59, 1174–1197. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Ertek, S. Metabolic and cardiovascular effects of berberine: From preclinical evidences to clinical trial results. Clin. Lipidol. 2009, 4, 553–563. [Google Scholar] [CrossRef]
- Wong, V.W.S.; Wong, G.L.H.; Tsang, S.W.C.; Fan, T.; Chu, W.C.W.; Woo, J.; Chan, A.W.H.; Choi, P.C.L.; Chim, A.M.L.; Lau, J.Y.W.; et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut 2011, 60, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Lavine, J.E.; Schwimmer, J.B.; van Natta, M.L.; Molleston, J.P.; Murray, K.F.; Rosenthal, P.; Abrams, S.H.; Scheimann, A.O.; Sanyal, A.J.; Chalasani, N.; et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents the tonic randomized controlled trial. J. Am. Med. Assoc. 2011, 305, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Gouni-Berthold, I.; Papanas, N.; Maltezos, E. The role of oral antidiabetic agents and incretin mimetics in type 2 diabetic patients with non-alcoholic fatty liver disease. Curr. Pharm. Des. 2014, 20, 3705–3715. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V. Starting the battle to control non-alcoholic steatohepatitis. Lancet 2015, 385, 922–924. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B.; et al. Farnesoid x nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (flint): A multicentre, randomised, placebo-controlled trial. Lancet 2015, 385, 956–965. [Google Scholar] [CrossRef]
- Tillhon, M.; Ortiz, L.M.G.; Lombardi, P.; Scovassi, A.I. Berberine: New perspectives for old remedies. Biochem. Pharmacol. 2012, 84, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Birdsall, T.C.; Kelly, G.S. Berberine: Therapeutic potential of an alkaloid found in several medicinal plants. Altern. Med. Rev. 1997, 2, 94–103. [Google Scholar]
- Vuddanda, P.R.; Chakraborty, S.; Singh, S. Berberine: A potential phytochemical with multispectrum therapeutic activities. Expert Opin. Investig. Drugs 2010, 19, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.K.; Dhir, A. Berberine: A plant alkaloid with therapeutic potential for central nervous system disorders. Phytother. Res. 2010, 24, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.H.; Shackel, N.A.; Gorrell, M.D.; McLennan, S.V.; Twigg, S.M. Diabetes and nonalcoholic fatty liver disease: A pathogenic duo. Endocr. Rev. 2013, 34, 84–129. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.F.F.; Fielding, B.A.; Frayn, K.N. Mechanisms for the acute effect of fructose on postprandial lipemia. Am. J. Clin. Nutr. 2007, 85, 1511–1520. [Google Scholar] [PubMed]
- Baidal, J.A.W.; Lavine, J.E. The intersection of nonalcoholic fatty liver disease and obesity. Sci. Transl. Med. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E. Nonalcoholic fatty liver disease a systematic review. J. Am. Med. Assoc. 2015, 313, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Miele, L.; Valenza, V.; la Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Masciana, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Gaebele, E.; Dostert, K.; Hofmann, C.; Wiest, R.; Schoelmerich, J.; Hellerbrand, C.; Obermeier, F. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J. Hepatol. 2011, 55, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.A.; Adegboyega, P.; van Rooijen, N.; Tagalicud, A.; Allman, M.; Wallace, M. Toll-like receptor-4 signaling and kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J. Hepatol. 2007, 47, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.S.; Ehli, E.A.; Kittelsrud, J.; Ronan, P.J.; Munger, K.; Downey, T.; Bohlen, K.; Callahan, L.; Munson, V.; Jahnke, M.; et al. Lipid-lowering effect of berberine in human subjects and rats. Phytomedicine 2012, 19, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; D’Angelo, A.; Bonaventura, A.; Bianchi, L.; Romano, D.; Maffioli, P. Effects of berberine on lipid profile in subjects with low cardiovascular risk. Expert Opin. Biol.Ther. 2013, 13, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.M.; Xia, M.F.; Wang, Y.; Chang, X.X.; Yao, X.Z.; Rao, S.X.; Zeng, M.S.; Tu, Y.F.; Feng, R.; Jia, W.P.; et al. Efficacy of berberine in patients with non-alcoholic fatty liver disease. PLoS ONE 2015, 10, e0134172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Song, H.Y.; Ji, G. Update on berberine in nonalcoholic fatty liver disease. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. Selective versus total insulin resistance: A pathogenic paradox. Cell Metab. 2008, 7, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.M.; Lee, C.H.; Oh, W.K.; Kim, C.T.; et al. Berberine, a natural plant product, activates amp-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.J.; et al. Ampk phosphorylates and inhibits srebp activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, G.; Zhu, H.; Huang, L.; Liu, Y.; Ma, C.; Qin, C. Beneficial effect of berberine on hepatic insulin resistance in diabetic hamsters possibly involves in SREBPS, LXRα and PPARα transcriptional programs. Endocr. J. 2010, 57, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Li, Y.; Wang, Y.W.; Wen, Y.; Sun, C.H. Berberine improves free-fatty-acid-induced insulin resistance in l6 myotubes through inhibiting peroxisome proliferator-activated receptor gamma and fatty acid transferase expressions. Metab. Clin. Exp. 2009, 58, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.J.; Zhang, H.; Song, D.Q.; Xue, R.; Zhao, W.; Wei, J.; Wang, Y.M.; Shan, N.; Zhou, Z.X.; Yang, P.; et al. Berberine reduces insulin resistance through protein kinase c-dependent up-regulation of insulin. Receptor expression. Metab. Clin. Exp. 2009, 58, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Lou, T.J.; Zhang, Z.G.; Xi, Z.L.; Liu, K.; Li, L.; Liu, B.L.; Huang, F. Berberine inhibits inflammatory response and ameliorates insulin resistance in hepatocytes. Inflammation 2011, 34, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.J.; Zhang, L.; Liu, T.; Hua, Y.Q.; Zheng, P.Y.; Ji, G. Berberine reducing insulin resistance by up-regulating IRS-2 mRNA expression in nonalcoholic fatty liver disease (NAFLD) rat liver. Eur. J. Pharmacol. 2011, 668, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.H.; Lu, F.E.; Xu, L.J. Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion. Acta Pharmacol. Sin. 2004, 25, 496–502. [Google Scholar] [PubMed]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating amp-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Waki, H.; Yamauchi, T.; Kamon, J.; Ito, Y.; Uchida, S.; Kita, S.; Hara, K.; Hada, Y.; Vasseur, F.; Froguel, P.; et al. Impaired multimerization of human adiponectin mutants associated with diabetes—Molecular structure and multimer formation of adiponectin. J. Biol. Chem. 2003, 278, 40352–40363. [Google Scholar] [CrossRef] [PubMed]
- Pajvani, U.B.; Du, X.L.; Combs, T.P.; Berg, A.H.; Rajala, M.W.; Schulthess, T.; Engel, J.; Brownlee, M.; Scherer, P.E. Structure-function studies of the adipocyte-secreted hormone ACRP30/adiponectin—Implications for metabolic regulation and bioactivity. J. Biol. Chem. 2003, 278, 9073–9085. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, P.C.; Zhuang, Y.; Lin, H.; Li, Y.H.; Liu, L.; Meng, Q.H.; Cui, T.; Liu, J.; Li, Z. Activation of ampk by berberine promotes adiponectin multimerization in 3T3-L1 adipocytes. Febs Lett. 2011, 585, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Chi, C.W.; Liu, T.Y. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004, 203, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Woo, S.L.; Guo, X.; Li, H.G.; Zheng, J.; Botchlett, R.; Liu, M.Y.; Pei, Y.; Xu, H.; Cai, Y.L.; et al. Berberine ameliorates hepatic steatosis and suppresses liver and adipose tissue inflammation in mice with diet-induced obesity. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-S.; Lu, F.-E.; Xu, L.-J.; Dong, H. Berberine reduces endoplasmic reticulum stress and improves insulin signal transduction in Hep G2 cells. Acta Pharmacol. Sin. 2010, 31, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Li, B.; Meng, X.J.; Yao, S.S.; Jin, L.N.; Yang, J.; Wang, J.Q.; Zhang, H.Z.; Zhang, Z.J.; Cai, D.S.; et al. Berberine prevents progression from hepatic steatosis to steatohepatitis and fibrosis by reducing endoplasmic reticulum stress. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.J.; Jensen, P.B.; Sorensen, R.V.; Larsen, L.K.; Vrang, N.; Wulff, E.M.; Wassermann, K. Differential influences of peroxisonte proliferator-activated receptors gamma and -α on food intake and energy homeostasis. Diabetes 2003, 52, 2249–2259. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yin, J.H.; Gao, H.F.; Xu, L.X.; Wang, Y.; Xu, L.; Li, M. Berberine improves insulin sensitivity by inhibiting fat store and adjusting adipokines profile in human preadipocytes and metabolic syndrome patients. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. Amp-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Krishan, S.; Richardson, D.R.; Sahni, S. Adenosine monophosphate-activated kinase and its key role in catabolism: Structure, regulation, biological activity, and pharmacological activation. Mol. Pharmacol. 2015, 87, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Lee, Y.S.; Cha, S.H.; Jeong, H.W.; Choe, S.S.; Lee, M.R.; Oh, G.T.; Park, H.S.; Lee, K.U.; Lane, M.D.; et al. Berberine improves lipid dysregulation in obesity by controlling central and peripheral ampk activity. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E812–E819. [Google Scholar] [CrossRef] [PubMed]
- Brusq, J.M.; Ancellin, N.; Grondin, P.; Guillard, R.; Martin, S.; Saintillan, Y.; Issandou, M. Inhibition of lipid synthesis through activation of amp kinase: An additional mechanism for the hypolipidemic effects of berberine. J. Lipid Res. 2006, 47, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Li, J.Y.; Gosby, A.; To, S.W.C.; Cheng, Z.; Miyoshi, H.; Taketo, M.M.; Cooney, G.J.; Kraegen, E.W.; James, D.E.; et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: A mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes 2008, 57, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Johnstone, S.R.; Dickerson, K.; Leiper, F.C.; Fryer, L.G.D.; Neumann, D.; Schlattner, U.; Wallimann, T.; Carlson, M.; Carling, D. LKB1 is the upstream kinase in the amp-activated protein kinase cascade. Curr. Biol. 2003, 13, 2004–2008. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Pan, D.A.; Mustard, K.J.; Ross, L.; Bain, J.; Edelman, A.M.; Frenguelli, B.G.; Hardie, D.G. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005, 2, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Momcilovic, M.; Hong, S.-P.; Carlson, M. Mammalian TAK1 activates SNF1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J. Biol. Chem. 2006, 281, 25336–25343. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xiao, Y.Y.; Yin, J.; Hou, W.L.; Yu, X.Y.; Shen, L.; Liu, F.; Wei, L.; Jia, W.P. Berberine promotes glucose consumption independently of AMP-activated protein kinase activation. PLoS ONE 2014, 9, e103702. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.J.; Zhou, Y.; Xu, P.X.; Wang, Y.; Yan, J.K.; Bin, W.; Qiu, F.; Kang, N. Berberine metabolites exhibit triglyceride-lowering effects via activation of AMP-activated protein kinase in Hep G2 cells. J. Ethnopharmacol. 2013, 149, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, J.S.; Duarte, F.V.; Gomes, A.P.; Varela, A.T.; Peixoto, F.M.; Rolo, A.P.; Palmeira, C.M. Berberine reverts hepatic mitochondrial dysfunction in high-fat fed rats: A possible role for SIRT3 activation. Mitochondrion 2013, 13, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.P.; Duarte, F.V.; Nunes, P.; Hubbard, B.P.; Teodoro, J.S.; Varela, A.T.; Jones, J.G.; Sinclair, D.A.; Palmeira, C.M.; Rolo, A.P. Berberine protects against high fat diet-induced dysfunction in muscle mitochondria by inducing SIRT1-dependent mitochondrial biogenesis. Biochim. Biophys. Acta 2012, 1822, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Larrouy, D.; Laharrague, P.; Carrera, G.; ViguerieBascands, N.; LeviMeyrueis, C.; Fleury, C.; Pecqueur, C.; Nibbelink, M.; Andre, M.; Casteilla, L.; et al. Kupffer cells are a dominant site of uncoupling protein 2 expression in rat liver. Biochem. Biophys. Res. Commun. 1997, 235, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Baffy, G. Uncoupling protein-2 and non-alcoholic fatty liver disease. Front. Biosci. 2005, 10, 2082–2096. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xiang, Z.; Chen, Y.-P.; Ma, K.-F.; Ye, Y.-F.; Li, Y.-M. Uncoupling protein and nonalcoholic fatty liver disease. Chin. Med. J. 2013, 126, 3151–3155. [Google Scholar] [PubMed]
- Yang, S.; Zhu, H.; Li, Y.; Lin, H.; Gabrielson, K.; Trush, M.A.; Diehl, A.M. Mitochondrial adaptations to obesity-related oxidant stress. Arch. Biochem. Biophys. 2000, 378, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.H.; Hu, S.P.; Zhang, Y.P.; Xie, W.N.; Li, N.; Ji, G.Y.; Qiao, N.L.; Lin, X.F.; Chen, T.Y.; Liu, H.T. Effect of berberine on expressions of uncoupling protein-2 mRNA and protein in hepatic tissue of non-alcoholic fatty liver disease in rats. Chin. J. Integr. Med. 2011, 17, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Fares, R.; Petta, S.; Lombardi, R.; Grimaudo, S.; Dongiovanni, P.; Pipitone, R.; Rametta, R.; Fracanzani, A.L.; Mozzi, E.; Craxi, A.; et al. The UCP2-866 G>A promoter region polymorphism is associated with nonalcoholic steatohepatitis. Liver Int. 2015, 35, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.J.; Wei, J.; Abidi, P.; Lin, M.H.; Inaba, S.; Li, C.; Wang, Y.L.; Wang, Z.Z.; Si, S.Y.; Pan, H.N.; et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Zhang, M.; Pang, X.; Xu, J.; Kang, C.; Li, M.; Zhang, C.; Zhang, Z.; Zhang, Y.; et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS ONE 2012, 7, e42529. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Li, N.; Li, Q.; Zhang, Q.; Wang, C.; Zhu, W.; Li, J. The effect of berberine in vitro on tight junctions in human CACO-2 intestinal epithelial cells. Fitoterapia 2009, 80, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Li, N.; Gong, J.; Li, Q.; Zhu, W.; Li, J. Berberine ameliorates intestinal epithelial tight-junction damage and down-regulates myosin light chain kinase pathways in a mouse model of endotoxinemia. J. Infect. Dis. 2011, 203, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Abifadel, M.; Varret, M.; Rabes, J.P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.C.; Boerwinkle, E.; Mosley, T.H.; Hobbs, H.H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 2006, 354, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.L.; Xu, L.J.; Dong, H.; Chen, G.; Huang, Z.Y.; Zou, X.; Wang, K.F.; Luo, Y.H.; Lu, F.E. Inhibition of proprotein convertase subtilisin/kexin type 9: A novel mechanism of berberine and 8-hydroxy dihydroberberine against hyperlipidemia. Chin. J. Integr. Med. 2015, 21, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Z.Z.; Guo, M.X.; Xu, K.J.; Jiang, M.; Lu, A.P.; Gao, X.Y. Metabolomics profiling to investigate the pharmacologic mechanisms of berberine for the treatment of high-fat diet-induced nonalcoholic steatohepatitis. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.J.; Liu, F.; Ong, E.S.; Li, S.F.Y. Metabolic profile associated with glucose and cholesterol lowering effects of berberine in sprague-dawley rats. Metabolomics 2012, 8, 1052–1068. [Google Scholar] [CrossRef]
- Yuan, X.L.; Wang, J.; Tang, X.Y.; Li, Y.X.; Xia, P.; Gao, X. Berberine ameliorates nonalcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles. J. Transl. Med. 2015, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.X.; Yan, H.M.; Fei, J.; Jiang, M.H.; Zhu, H.G.; Lu, D.R.; Gao, X. Berberine reduces methylation of the mttp promoter and alleviates fatty liver induced by a high-fat diet in rats. J. Lipid Res. 2010, 51, 2504–2515. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Kesarwala, A.H.; Eggert, T.; Medina-Echeverz, J.; Kleiner, D.E.; Jin, P.; Stroncek, D.F.; Terabe, M.; Kapoor, V.; ElGindi, M.; et al. NAFLD causes selective CD4+ T lymphocyte loss and promotes hepatocarcinogenesis. Nature 2016, 531, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rubio, K.G.; Gonzalez-Ortiz, M.; Martinez-Abundis, E.; Robles-Cervantes, J.A.; Espinel-Bermudez, M.C. Effect of berberine administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2013, 11, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Kong, W.J.; Li, Y.H.; Tang, S.; Li, Z.; Li, Y.B.; Shan, Y.Q.; Bi, C.W.; Jiang, J.D.; Song, D.Q. Synthesis and structure-activity relationship of berberine analogues in LDLR up-regulation and AMPK activation. Bioorg. Med. Chem. 2012, 20, 6552–6558. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Mahajan, S. Berberine and its derivatives: A patent review (2009–2012). Expert Opin. Ther. Pat. 2013, 23, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.M.; Chen, Y.F.; Zidichouski, J.; Zhang, J.Z.; Sun, C.H.; Wang, Y.W. Co-administration of berberine and plant stanols synergistically reduces plasma cholesterol in rats. Atherosclerosis 2008, 201, 101–107. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Bian, H.; Gao, X. The Potential Mechanisms of Berberine in the Treatment of Nonalcoholic Fatty Liver Disease. Molecules 2016, 21, 1336. https://doi.org/10.3390/molecules21101336
Zhu X, Bian H, Gao X. The Potential Mechanisms of Berberine in the Treatment of Nonalcoholic Fatty Liver Disease. Molecules. 2016; 21(10):1336. https://doi.org/10.3390/molecules21101336
Chicago/Turabian StyleZhu, Xiaopeng, Hua Bian, and Xin Gao. 2016. "The Potential Mechanisms of Berberine in the Treatment of Nonalcoholic Fatty Liver Disease" Molecules 21, no. 10: 1336. https://doi.org/10.3390/molecules21101336
APA StyleZhu, X., Bian, H., & Gao, X. (2016). The Potential Mechanisms of Berberine in the Treatment of Nonalcoholic Fatty Liver Disease. Molecules, 21(10), 1336. https://doi.org/10.3390/molecules21101336