2-(2-Phenylethyl)chromone Derivatives of Agarwood Originating from Gyrinops salicifolia
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Bioassay of Cytotoxic Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflict of interest
References
- Borris, R.P.; Blaskó, G.; Cordell, G.A. Ethnopharmacologic and phytochemical studies of the Thymelaeaceae. J. Ethnopharmacol. 1988, 24, 41–91. [Google Scholar] [CrossRef]
- Compton, J.; Ishihara, A. The Use and Trade of Agarwood in Japan. A TRAFFIC report to the CITES Secretariat. Available online: https://cites.org/sites/default/files/common/com/pc/15/X-PC15-06-Inf.pdf (accessed on 1 September 2016).
- Persoon, G.A.; Van Beek, H.H. Growing ‘the wood of the gods’: Agarwood production in Southeast Asia. In Smallholder Tree Growing for Rural Development and Environmental Services; Springer: Dordrecht, The Netherlands, 2008; Volume 5, pp. 245–262. [Google Scholar]
- China Pharmacopoeia Editorial Board. Pharmacopoeia of the People’s Republic of China, (Part 1); China Medical Science and Technology Press: Beijing, China, 2010; p. 172. [Google Scholar]
- Naef, R. The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species: A review. Flavour. Frag. J. 2011, 26, 73–87. [Google Scholar] [CrossRef]
- Chen, H.Q.; Wei, J.H.; Yang, J.S.; Zhang, Z.; Yang, Y.; Gao, Z.H.; Sui, C.; Gong, B. Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem. Biodivers. 2012, 9, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Kenmotsu, Y.; Ogita, S.; Katoh, Y.; Yamamura, Y.; Takao, Y.; Tatsuo, Y.; Kurosaki, F. Methyl jasmonate-induced enhancement of expression activity of Am-FaPS-1, a putative farnesyl diphosphate synthase gene from Aquilaria microcarpa. J. Nat. Med. 2011, 65, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Kumeta, Y.; Ito, M. Characterization of δ-guaiene synthases from cultured cells of Aquilaria, responsible for the formation of the sesquiterpenes in agarwood. Plant. Physiol. 2010, 154, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.H.; Wei, J.H.; Yang, Y.; Zhang, Z.; Zhao, W.T. Selection and validation of reference genes for studying stress-related agarwood formation of Aquilaria sinensis. Plant. Cell. Rep. 2012, 31, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Mohamed, R. The origin and domestication of Aquilaria, an important agarwood-producing genus. In Agarwood; Springer Singapore: Singapore, 2016; pp. 1–20. [Google Scholar]
- Mulyaningsih, T.; Yamada, I. Notes on some species of agarwood in Nusa Tenggara, Celebes and West Papua. In Natural Resource Management and Socio-Economic Transformation under the Decentralization in Indonesia: Toward Sulawesi Area Studies; CSEAS Kyoto University: Kyoto, Japan, 2008; pp. 365–372. [Google Scholar]
- Schun, Y.; Cordell, G.A.; Cox, P.J.; Howie, R.A. Wallenone, a C32 triterpenoid from the leaves of Gyrinops walla. Phytochemistry 1986, 25, 753–755. [Google Scholar] [CrossRef]
- Subasinghe, S.M.C.U.P.; Hettiarachchi, D.S. Characterisation of agarwood type resin of Gyrinops walla Gaertn growing in selected populations in Sri Lanka. Ind. Crop. Prod. 2015, 69, 76–79. [Google Scholar] [CrossRef]
- Yang, D.L.; Mei, W.L.; Zeng, Y.B.; Guo, Z.K.; Zhao, Y.X.; Wang, H.; Zuo, W.J.; Dong, W.H.; Wang, Q.H.; Dai, H.F. 2-(2-phenylethyl)chromone derivatives in Chinese Agarwood “Qi-Nan” from Aquilaria sinensis. Planta. Med. 2013, 79, 1329–1334. [Google Scholar] [PubMed]
- Li, W.; Cai, C.H.; Guo, Z.K.; Wang, H.; Zuo, W.J.; Dong, W.H.; Mei, W.L.; Dai, H.F. Five new eudesmane-type sesquiterpenoids from Chinese agarwood induced by artificial holing. Fitoterapia 2015, 100, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qiao, L.R.; Xie, D.; Yuan, Y.H.; Chen, N.H.; Dai, J.G.; Guo, S.X. 2-(2-Phenylethyl)chromones from Chinese eaglewood. Phytochemistry 2012, 76, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T.; Konoshima, T.; Shimada, Y.; Kiyosawa, S. Six new 2-(2-phenylethyl)chromones from Agarwood. Chem. Pharm. Bull. 2002, 50, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Wang, Y.L.; Su, Y.L. Studies on the chemical constituents of Aquilaria sinensis (Lour.) Gilg. V. Isolation and characterization of three 2-(2-phenylethyl)chromone derivatives. Acta Pharmaceutica Sinica 1989, 25, 186–190. [Google Scholar]
- Yoon, J.S.; Lee, M.K.; Sung, S.H.; Kim, Y.C. Neuroprotective 2-(2-Phenylethyl) chromones of Imperata cylindrical. J. Nat. Prod. 2006, 69, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ferrari, M.; Fornasiero, M.C.; Isetta, A.M. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J. Immunol. Methods 1990, 131, 165–172. [Google Scholar] [CrossRef]
- Liu, S.; Liu, S.B.; Zuo, W.J.; Guo, Z.K.; Mei, W.L.; Dai, H.F. New sesquiterpenoids from Aglaiaodorata var. microphyllina and their cytotoxic activity. Fitoterapia 2014, 92, 93–99. [Google Scholar] [PubMed]
- Shao, H.; Mei, W.L.; Li, W.; Gai, C.J.; Zhu, G.P.; Dai, H.F. Chemical constituents of agarwood originating from Gyrinops salicifolia. Nat. Prod. Res. Dev. 2015, 27, 2046–2049. [Google Scholar]
- Shao, H.; Mei, W.L.; Kong, F.D.; Dong, W.H.; Gai, C.J.; Li, W.; Dai, H.F. Sesquiterpenes of agarwood from Gyrinops salicifolia. Fitoterapia 2016, 113, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
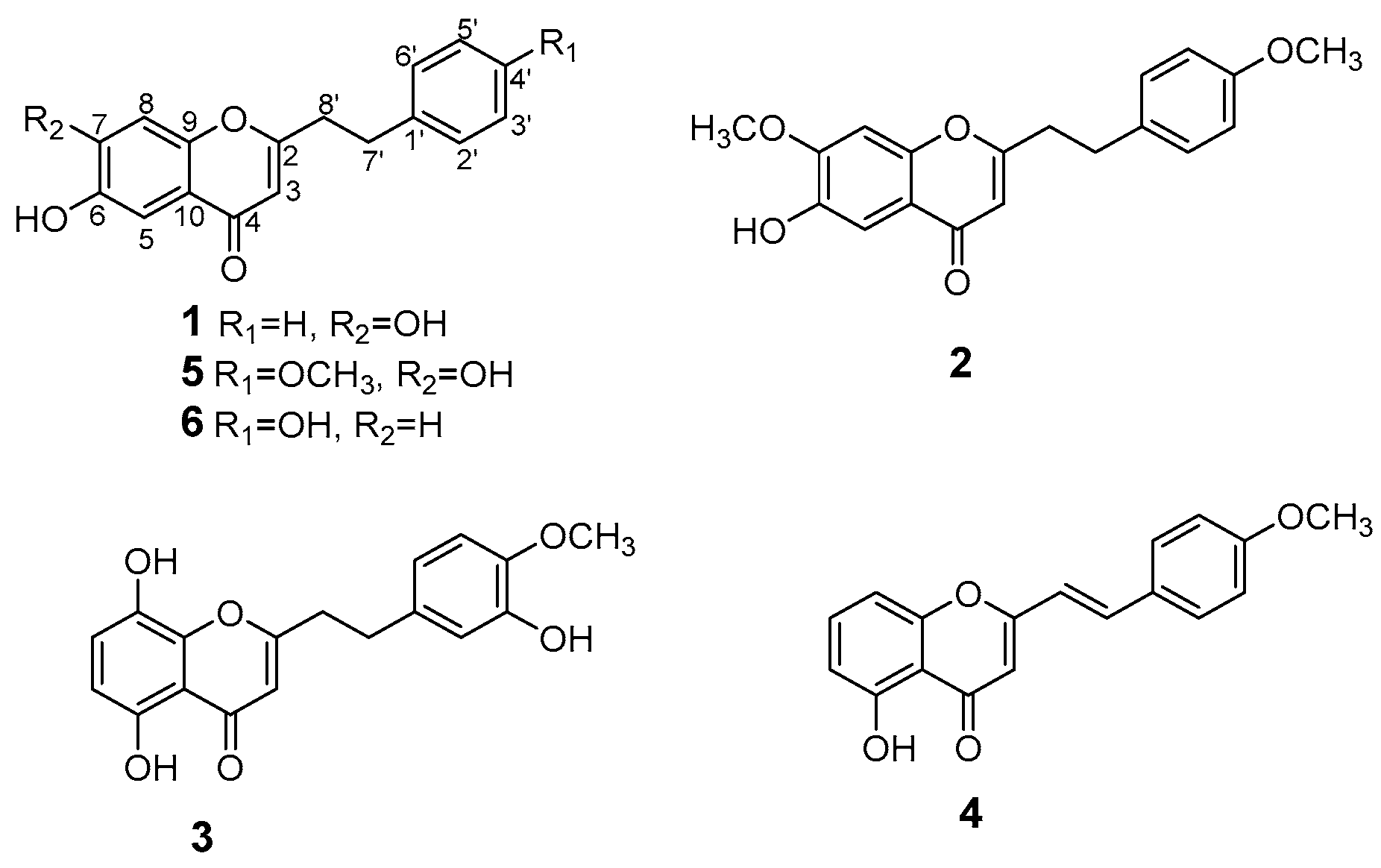
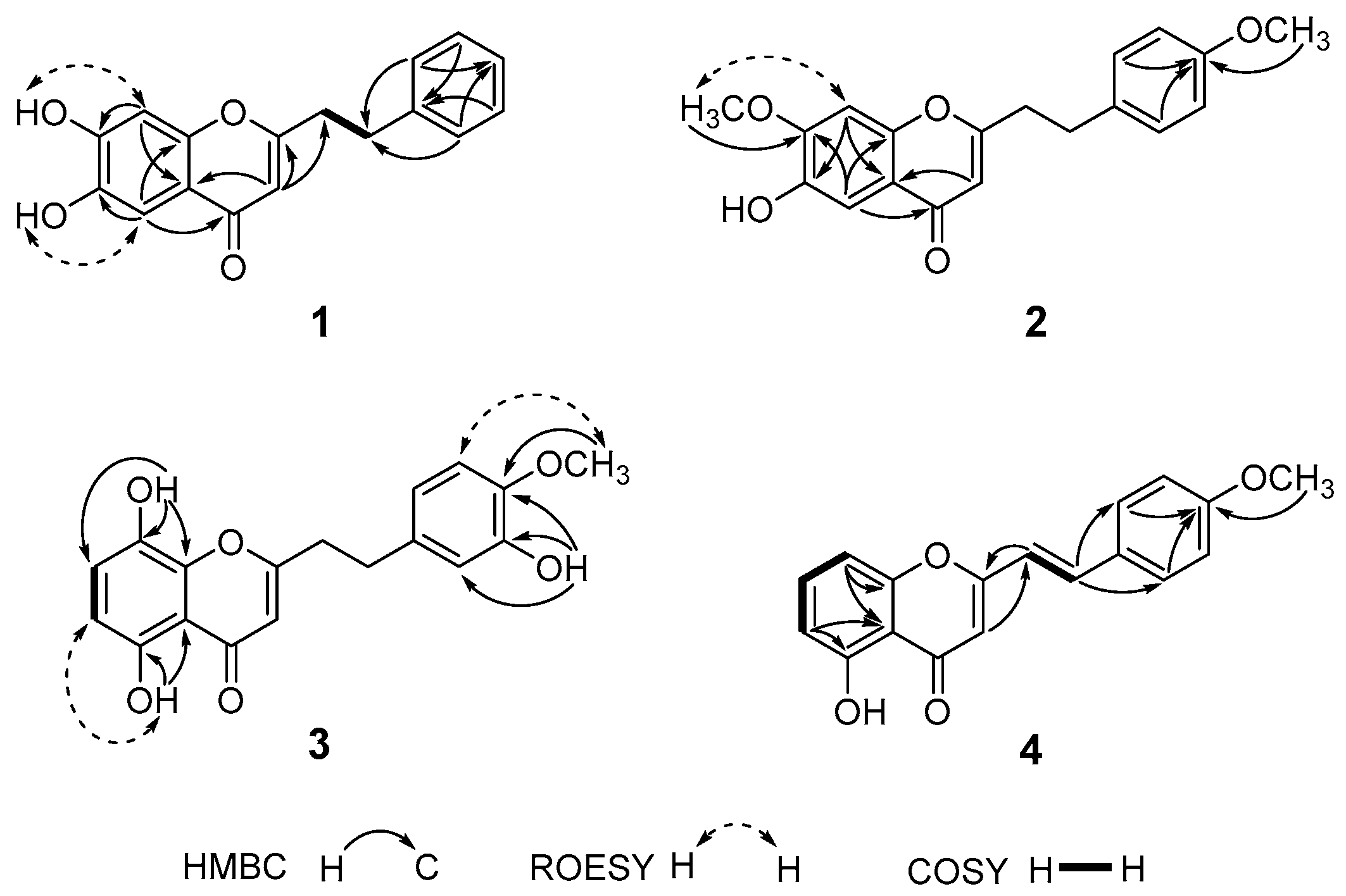
| No. | 1 a | 2 a | 3 a | 4 b | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | δC | δH | |
| 2 | 167.2 | 167.8 | 170.8 | 163.6 | ||||
| 3 | 108.6 | 5.99 s | 108.9 | 6.03 s | 108.4 | 6.22 s | 108.4 | 6.22 s |
| 4 | 176.0 | 176.3 | 183.1 | 183.7 | ||||
| 5 | 107.6 | 7.22 s | 107.3 | 7.23 s | 151.2 | 161.5 | ||
| 6 | 144.3 | 145.1 | 109.8 | 6.61 d (8.2), overlap | 111.4 | 6.79 d (8.3) | ||
| 7 | 152.0 | 153.6 | 121.8 | 7.17 d (8.2) | 135.4 | 7.51 t (8.3) | ||
| 8 | 102.8 | 6.84 s | 100.4 | 7.12 s | 137.6 | 106.9 | 6.96 d (8.3) | |
| 9 | 151.0 | 151.1 | 144.5 | 156.4 | ||||
| 10 | 115.8 | 116.6 | 110.4 | 111.1 | ||||
| 1′ | 140.2 | 132.1 | 132.5 | 127.7 | ||||
| 2′ | 128.4 | 7.26 m | 129.4 | 7.15 d (8.5) | 115.7 | 6.67 d (2.2) | 129.6 | 7.54 d (8.1) |
| 3′ | 128.3 | 7.26 m | 113.9 | 6.83 d (8.5) | 146.2 | 114.7 | 6.95 d (8.1) | |
| 4′ | 126.2 | 7.19 m | 157.8 | 146.4 | 161.0 | |||
| 5′ | 128.3 | 7.26 m | 113.9 | 6.83 d (8.5) | 112.3 | 6.80 d (8.2) | 114.7 | 6.95 d (8.1) |
| 6′ | 128.4 | 7.26 m | 129.4 | 7.15 d (8.5) | 118.8 | 6.61 d (2.2, 8.2), overlap | 129.6 | 7.54 d (8.1) |
| 7′ | 32.1 | 2.97 m | 31.4 | 2.90 m | 31.2 | 2.92 m | 137.9 | 7.59 d (16.0) |
| 8′ | 34.7 | 2.90 m | 35.2 | 2.90 m | 35.2 | 2.92 m | 117.3 | 6.63 d (16.0) |
| 5-OH | 11.85 s | |||||||
| 6-OH | 9.70 s | 9.72 s | ||||||
| 7-OH | 10.35 s | |||||||
| 8-OH | 9.67 s | |||||||
| 3′-OH | 8.85 s | |||||||
| 7-OCH3 | 56.3 | 3.89 s | ||||||
| 4′-OCH3 | 55.0 | 3.70 s | 55.6 | 3.71 s | 55.6 | 3.86 s | ||
| Compound | IC50 (μM) | ||
|---|---|---|---|
| SGC-7901 | K-562 | BEL-7402 | |
| 1 | >50 | 18.1 | 20.1 |
| 2 | 17.8 | 13.9 | 31.9 |
| 3 | >50 | >50 | >50 |
| 4 | >50 | >50 | >50 |
| 5 | >50 | 8.36 | 5.76 |
| 6 | >50 | 47.0 | >50 |
| Paclitaxel b | 1.80 | 7.20 | 2.40 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, H.; Mei, W.-L.; Dong, W.-H.; Gai, C.-J.; Li, W.; Zhu, G.-P.; Dai, H.-F. 2-(2-Phenylethyl)chromone Derivatives of Agarwood Originating from Gyrinops salicifolia. Molecules 2016, 21, 1313. https://doi.org/10.3390/molecules21101313
Shao H, Mei W-L, Dong W-H, Gai C-J, Li W, Zhu G-P, Dai H-F. 2-(2-Phenylethyl)chromone Derivatives of Agarwood Originating from Gyrinops salicifolia. Molecules. 2016; 21(10):1313. https://doi.org/10.3390/molecules21101313
Chicago/Turabian StyleShao, Hang, Wen-Li Mei, Wen-Hua Dong, Cui-Juan Gai, Wei Li, Guo-Peng Zhu, and Hao-Fu Dai. 2016. "2-(2-Phenylethyl)chromone Derivatives of Agarwood Originating from Gyrinops salicifolia" Molecules 21, no. 10: 1313. https://doi.org/10.3390/molecules21101313
APA StyleShao, H., Mei, W.-L., Dong, W.-H., Gai, C.-J., Li, W., Zhu, G.-P., & Dai, H.-F. (2016). 2-(2-Phenylethyl)chromone Derivatives of Agarwood Originating from Gyrinops salicifolia. Molecules, 21(10), 1313. https://doi.org/10.3390/molecules21101313






