Constituents of Cryptotaenia japonica Inhibit Melanogenesis via CREB- and MAPK-Associated Signaling Pathways in Murine B16 Melanoma Cells
Abstract
:1. Introduction
2. Results and Discussion
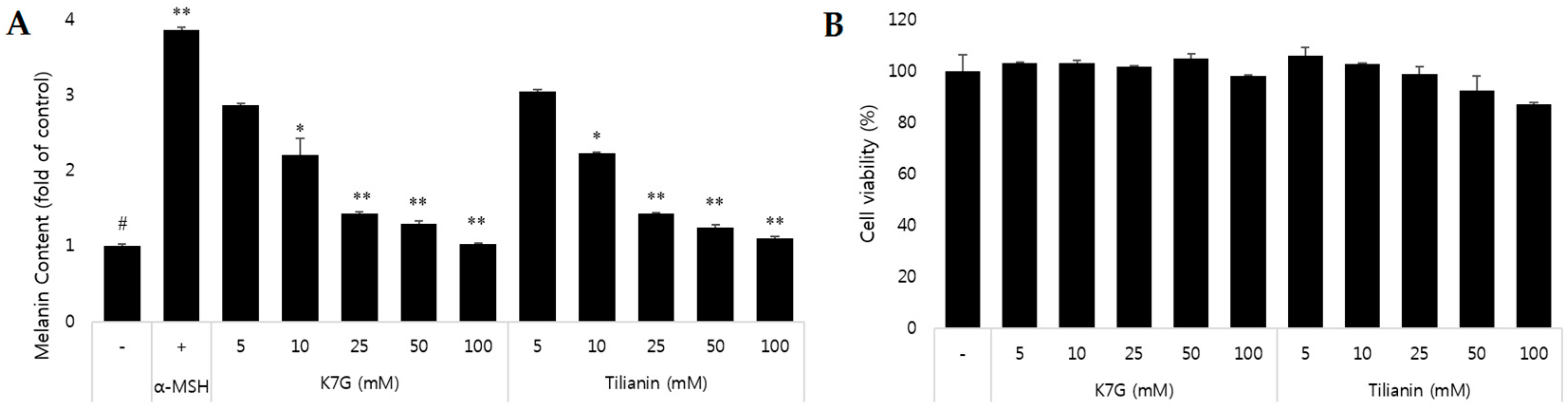
2.1. Effect on Melanin Content and Cytotoxicity of Compounds Isolated from the Leaves of C. japonica
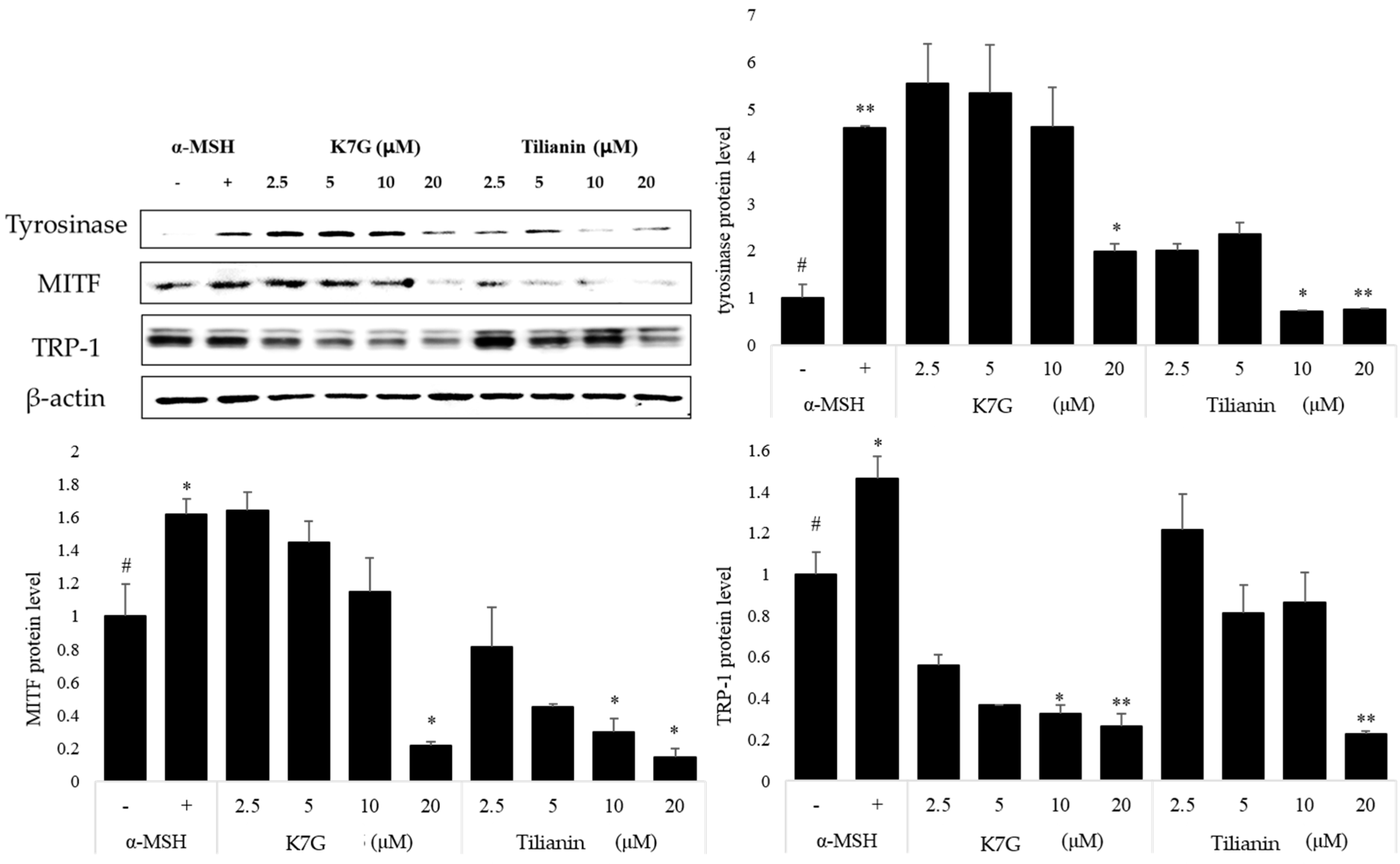
2.2. Effects of K7G and Tilianin on the Expression of Melanogenesis-Related Proteins
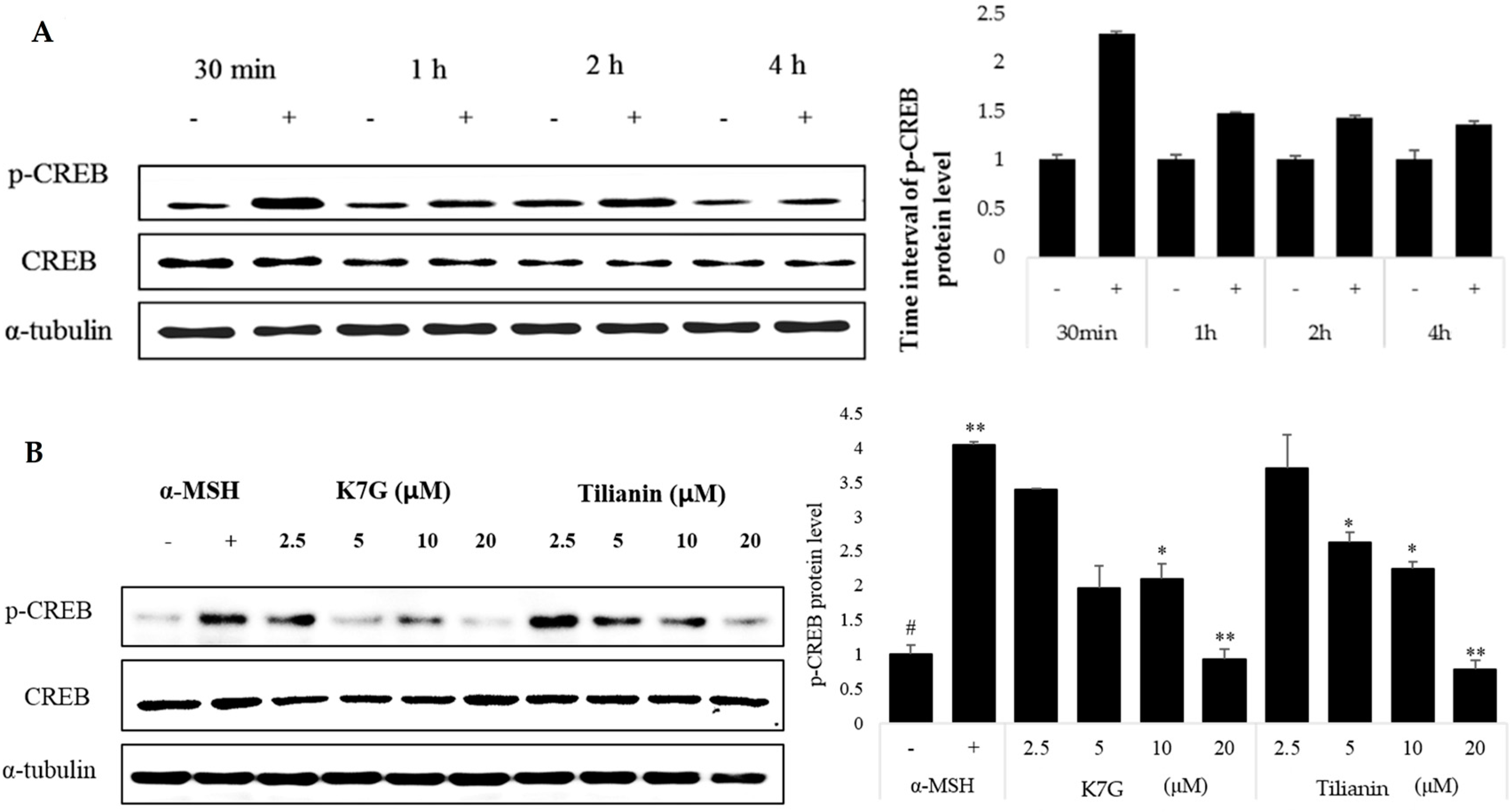
2.3. Effects of K7G and Tilianin on CREB Phosphorylation
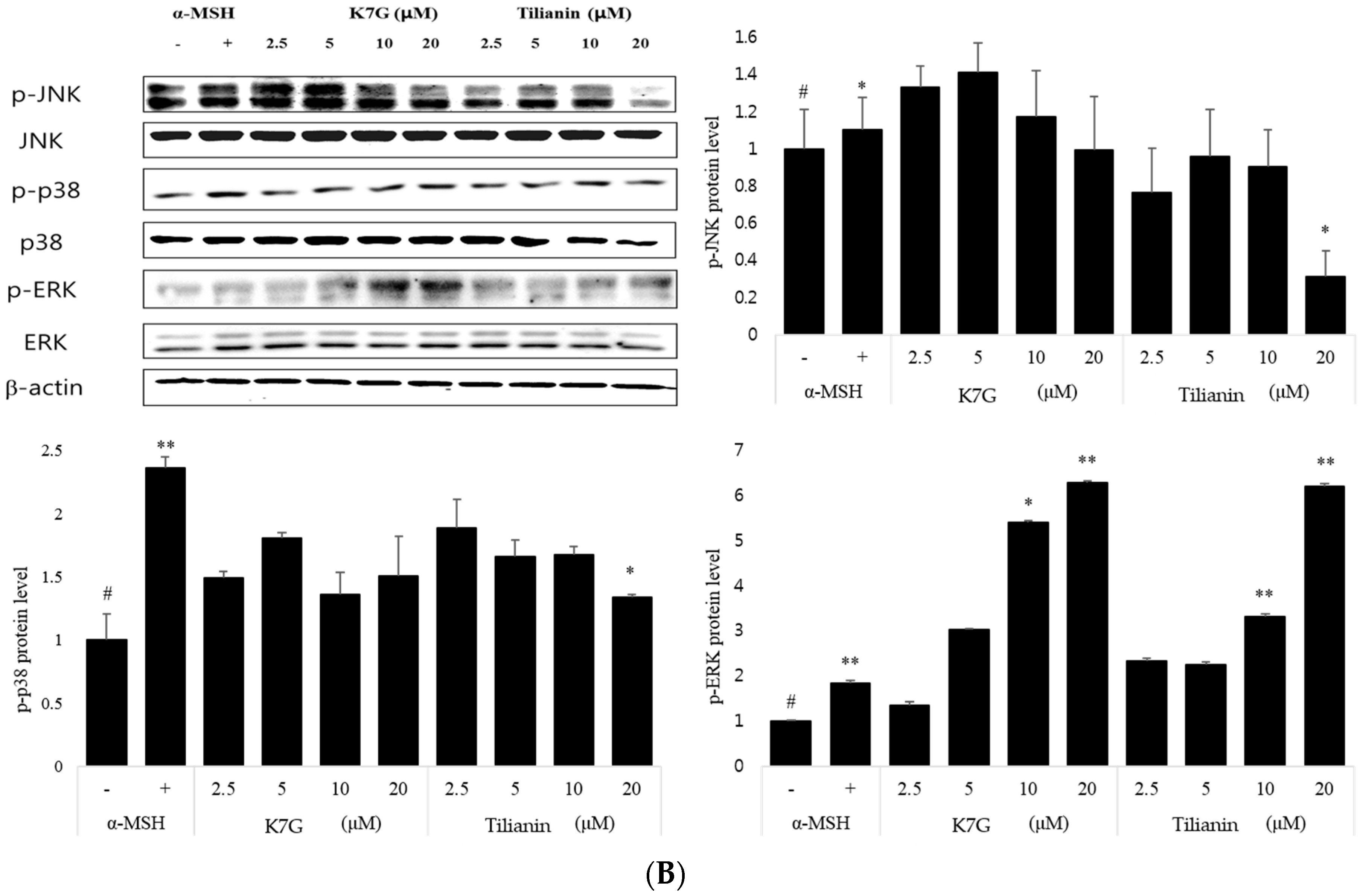
2.4. Effect on the MAP Kinase Signaling Pathway
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Plant Material
3.3. Extraction and Isolation from the Leaves of C. japonica
3.4. Cell Culture
3.5. Cytotoxicity Assay
3.6. Determination of the Extracellular Melanin Content
3.7. Western Blot Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- An, S.M.; Kim, H.J.; Kim, J.E.; Boo, Y.C. Flavonoids, taxifolin and luteolin attenuate cellular melanogenesis despite increasing tyrosinase protein levels. Phytother. Res. 2008, 22, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Hamdani, M. Plants used to treat skin diseases. Pharmacogn. Rev. 2014, 8, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Park, Y.J.; Park, J.G. Peutz-jeghers syndrome: A new understanding. J. Korean Med. Sci. 1999, 14, 2–7. [Google Scholar] [CrossRef] [PubMed]
- d’Ischia, M.; Wakamatsu, K.; Cicoira, F.; Mauro, E.D.; Garcia-Borron, J.C.; Commo, S.; Galvan, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigm. Cell Melanoma Res. 2015, 28, 520–544. [Google Scholar] [CrossRef] [PubMed]
- Seong, Z.K.; Kim, H.S.; Won, Y.M.; Kim, J.L.; Song, H.H.; Kim, D.Y.; Oh, S.R.; Cho, H.W.; Cho, J.H.; Lee, H.K. Phenylacylphenol derivatives with anti-melanogenic activity from stewartia pseudocamellia. Arch. Pharm. Res. 2016, 39, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.M.; Prudente, A.S.; Horinouchi, C.D.S.; Cechinel-Filho, V.; Fávero, G.M.; Cabrini, D.A.; FleithOtuki, M. Inhibitory effect of GB-2a (I3-naringenin-II8-eriodictyol) on melanogenesis. J. Ethnopharmacol. 2015, 174, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Tsao, Y.T.; Huang, Y.F.; Kuo, C.Y.; Lin, Y.C.; Chiang, W.C.; Wang, W.K.; Hsu, C.W.; Lee, C.H. Hinokitiol inhibits melanogenesis via AKT/mTOR signaling in B16F10 mouse melanoma cells. Int. J. Mol. Sci. 2016, 17, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Lee, J.; Huh, S.; Lee, J.; Kim, Y.S.; Kim, G.; Park, D. Phloridzin-induced melanogenesis is mediated by the camp signaling pathway. Food Chem. Toxicol. 2009, 47, 2436–2440. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.Y.; Lin, C.C.; Wang, H.Y.; Shih, Y.; Chou, S.T. The melanogenesis alteration effects of achillea millefolium l. Essential oil and linalyl acetate: Involvement of oxidative stress and the JNK and ERK signaling pathways in melanoma cells. PLoS ONE 2014, 9, e95186. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Hwang, E.S.; Lee, J.E.; Kim, S.Y.; Kwon, S.B.; Park, K.C. Sphingosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent mitf degradation. J. Cell Sci. 2003, 116, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Choi, B.R.; Lee, E.K.; Kim, S.H.; Yi, H.Y.; Park, H.R.; Song, C.H.; Lee, Y.J.; Ku, S.K. Inhibitory effect of dried pomegranatee concentration powder on melanogenesis in B16F10 melanoma cells; involvement of P38 and PKA signaling pathways. Int. J. Mol. Sci. 2015, 16, 24219–24242. [Google Scholar] [CrossRef] [PubMed]
- Nga, L.T.; Lin, L.T.; Chen, C.L.; Chen, H.W.; Wud, S.J.; Lin, C.C. Anti-melanogenic effects of -tocotrienol are associated with tyrosinase-related proteins and MAPK signaling pathway in B16 melanoma cells. Phytomedicine 2014, 21, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Yang, C.H.; Chiou, Y.L. Citrus flavanone naringenin enhances melanogenesis through the activation of WNT/B-catenin signalling in mouse melanoma cells. Phytomedicine 2011, 18, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Bang, T.S.; Lee, J.W.; Lew, J.H.; Eom, S.H.; Lee, K.; Choi, H.Y. Protective effect of the methanol extract from Cryptotaenia japonica hassk. Against lipopolysaccharide-induced inflammation in vitro and in vivo. BMC Complement. Altern. Med. 2012, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Lua, J.; Qian, W.; Xua, L.; Huang, G.; Cong, W.; Wang, Z.; Deng, X.; Wang, D.; Guan, S. Phytochemical composition and toxicity of an antioxidant extract from Pimpinella brachycarpa (Kom.) Nakai. Environ. Toxicol. Pharmacol. 2012, 34, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.G.; Jeong, S.H.; Choi, C.M. Classification of the edible plants on the market in busan. J. Life Sci. 2003, 13, 764–774. [Google Scholar]
- Lee, M.S. Volatile flavor components of Artemisia selengensis and Cryptoraenia japonica. Korean J. Food Sci. Technol. 1987, 19, 279–284. [Google Scholar]
- Choo, M.H.; Lee, J.J.; Lee, M.Y. Effect of Pimpinella brachycarpa ethanol extract on chronically ethanol-induced liver damage in rats. J. Life Sci. 2007, 17, 1406–1413. [Google Scholar] [CrossRef]
- Lin, J.H.; Lin, Y.T.; Huang, Y.J.; Wen, K.C.; Chen, R.M.; Ueng, T.H.; Liao, C.H. Isolation and cytotoxocoty of flavonoids from daphne genkwae flos. J. Food Drug Anal. 2001, 9, 6–11. [Google Scholar]
- Ozgen, U.; Sevindik, H.; Kazaz, C.; Yigit, D.; Kandemir, A.; Secen, H.; Calis, I. A new sulfated α-ionone glycoside from Sonchus erzincanicus matthews. Molecules 2010, 15, 2593–2599. [Google Scholar] [CrossRef] [PubMed]
- Blade, A.M.; Pieters, L.A.; Claeys, M.; Traore, M.S.; Balde, M.A.; Biallo, M.S.T.; Blade, E.S.; Diane, S.; Vlietinck, A.J. Quinic acid esters from Pavetta owariensis var. Owariensis (rubiaceae). J. Plant Sci. 2015, 3, 20–23. [Google Scholar]
- Sikorska, M.; Matlawska, I. Kaempferol, isorhamnetin and their glycosides in the flowers of Asclepias syriaca L. Acta Pol. Pharm. 2001, 58, 269–272. [Google Scholar] [PubMed]
- Chang, S.W.; Kim, K.H.; Lee, I.K.; Choi, S.U.; Ryu, S.Y.; Lee, K.R. Phytochemical constituents of Bistorta manshuriensis. Nat. Prod. Sci. 2009, 15, 234–240. [Google Scholar]
- Wu, S.; Sun, A.; Liu, R. Separation and purification of baicalin and wogonoside from the chinese medicinal plant scutellaria baicalensis georgi by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1066, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; He, C.; Awuti, G.; Zeng, C.; Xing, J.; Huang, W. Simultaneous determination of six active compounds in tixin badiranjibuya granules, a traditional chinese medicine, by RP-HPLC-UV method. J. Anal. Methods Chem. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S. Tumor Models in Cancer Research; Humana Press: New York, NY, USA, 2002; pp. 3–19. [Google Scholar]
- Overwijk, W.W.; Restifo, N.P. B16 as a mouse model for human melanoma. Curr. Protoc. Immunol. 2001, 20, 1–32. [Google Scholar] [PubMed]
- Baek, S.H.; Lee, S.H. Sesamon decreases melanin biosynthesis in melanocyte cells and zebrafish: Possible involvement of MITF via the intracellular cAMP and p38/JNK signalling pathways. Exp. Dermatol. 2015, 24, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Lee, J.H.; Kim, M.H.; Lee, J.A.; Kim, Y.B.; Jung, E.; Kim, Y.S.; Lee, J.; Park, D. Hordenine, a single compound produced during barley germination, inhibits melanogenesis in human melanocytes. Food Chem. 2013, 141, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Englaro, W.; Bertolotto, C.; Busca, R.; Brunet, A.; Pages, G.; Ortonne, J.P.; Ballott, R. Inhibition of the mitogen-activated protein kinase pathway triggers B16 melanoma cell differentiation. J. Biol. Chem. 1998, 273, 9966–9970. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.H.; Chiang, Y.C.; Tsai, M.H.; Liang, C.J.; Hsu, L.F.; Li, S.Y.; Wang, M.C.; Yen, F.L.; Lee, C.W. Eupafolin, a skin whitening flavonoid isolated from Phyla nodiflora, downregulated melanogenesis: Role of MAPK and AKT pathways. J. Ethnopharmacol. 2014, 151, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.O.; Lee, S.A.; Kim, S.S.; Jang, Y.S.; Chun, J.C.; Lee, J.C. Acteoside inhibits melanogenesis in B16F10 cells through ERK activation and tyrosinase down-regulation. J. Pharm. Pharmacol. 2011, 63, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.


| Sample | Cont. (μM) | Melanin Content (%) | Cell Viability | Sample | Cont. (μM) | Melanin Content (%) | Cell Viability |
|---|---|---|---|---|---|---|---|
| Vanillic acid | 5 | 98.03 | 97.5 | Tilianin | 5 | 108.55 | 105.3 |
| 10 | 104.27 | 98.6 | 10 | 86.01 | 101.2 | ||
| 20 | 103.02 | 99.3 | 20 | 32.64 | 102.5 | ||
| Chlorogenic acid | 5 | 119.69 | 97.2 | Apigetrin | 5 | 130.83 | 78.8 |
| 10 | 108.55 | 97.5 | 10 | 157.25 | 95.6 | ||
| 20 | 60.1 | 101.6 | 20 | 182.12 | 97.7 | ||
| 3-O-Feruloylqunic acid | 5 | 93.47 | 92.2 | Wogoniside | 5 | 96.03 | 105.3 |
| 10 | 107.79 | 93.5 | 10 | 112.2 | 97.9 | ||
| 20 | 108.79 | 97.7 | 20 | 106.05 | 94.5 | ||
| Kaempferol-7-O-β-d-glucuronide | 5 | 104.87 | 105.3 | Naringenin | 5 | 117.2 | 96.6 |
| 10 | 66.67 | 97.9 | 10 | 106.5 | 99.8 | ||
| 20 | 23.08 | 94.5 | 20 | 98.5 | 95.6 | ||
| Acacetin-7-O-β-d-glucuronide | 5 | 198.46 | 96.6 | Genistein | 5 | 121.2 | 64.5 |
| 10 | 250.26 | 99.8 | 10 | 108.3 | 95.4 | ||
| 20 | 24.62 | 95.6 | 20 | 115.6 | 95.3 | ||
| Acacetin | 5 | 106.2 | 90.4 | β-sitosterol | 5 | 105.65 | 64.5 |
| 10 | 115.3 | 78.5 | 10 | 98.23 | 95.6 | ||
| 20 | 109.3 | 90.6 | 20 | 101.57 | 97.2 | ||
| Diosmetin | 5 | 111.02 | 93.8 | Arbutin | 200 | 29.14 | 93.8 |
| 10 | 106.08 | 99.8 | a-MSH | + | 100 | ||
| 20 | 103.58 | 95.6 | - | 100 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, Z.-K.; Lee, S.-Y.; Poudel, A.; Oh, S.-R.; Lee, H.-K. Constituents of Cryptotaenia japonica Inhibit Melanogenesis via CREB- and MAPK-Associated Signaling Pathways in Murine B16 Melanoma Cells. Molecules 2016, 21, 1296. https://doi.org/10.3390/molecules21101296
Seong Z-K, Lee S-Y, Poudel A, Oh S-R, Lee H-K. Constituents of Cryptotaenia japonica Inhibit Melanogenesis via CREB- and MAPK-Associated Signaling Pathways in Murine B16 Melanoma Cells. Molecules. 2016; 21(10):1296. https://doi.org/10.3390/molecules21101296
Chicago/Turabian StyleSeong, Zuh-Kyung, Sung-Yoon Lee, Amrit Poudel, Sei-Ryang Oh, and Hyeong-Kyu Lee. 2016. "Constituents of Cryptotaenia japonica Inhibit Melanogenesis via CREB- and MAPK-Associated Signaling Pathways in Murine B16 Melanoma Cells" Molecules 21, no. 10: 1296. https://doi.org/10.3390/molecules21101296
APA StyleSeong, Z.-K., Lee, S.-Y., Poudel, A., Oh, S.-R., & Lee, H.-K. (2016). Constituents of Cryptotaenia japonica Inhibit Melanogenesis via CREB- and MAPK-Associated Signaling Pathways in Murine B16 Melanoma Cells. Molecules, 21(10), 1296. https://doi.org/10.3390/molecules21101296






