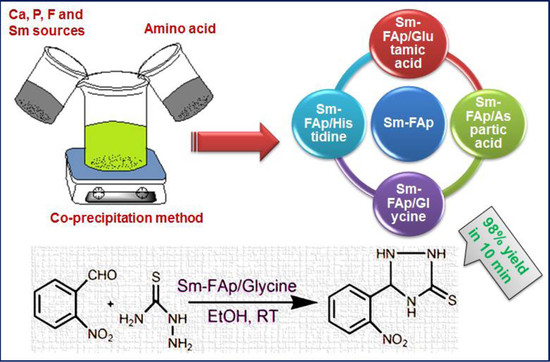
Nanostructured Samarium Doped Fluorapatites and Their Catalytic Activity towards Synthesis of 1,2,4-Triazoles
Abstract
:1. Introduction
2. Results and Discussion
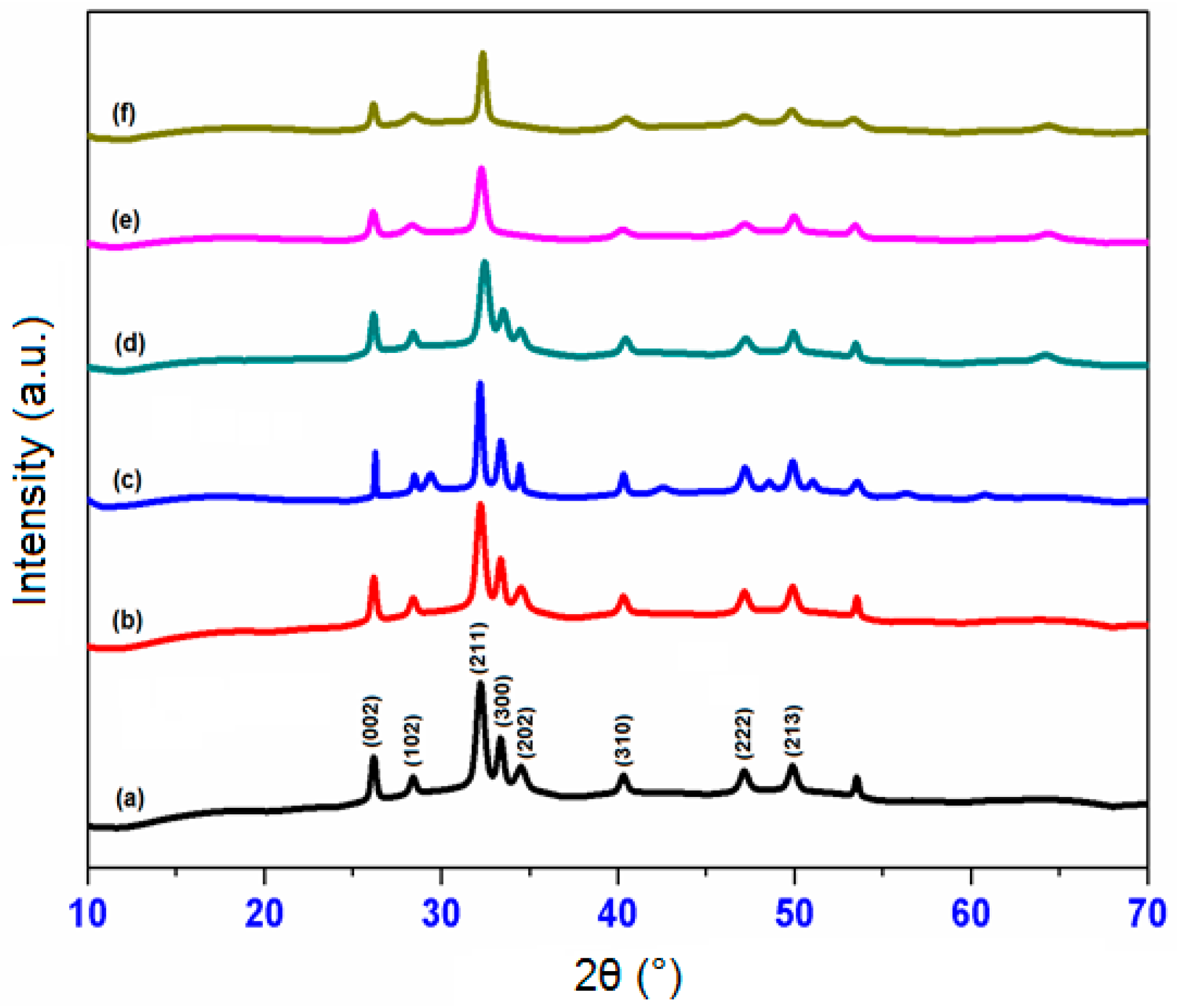
2.1. XRD Analysis
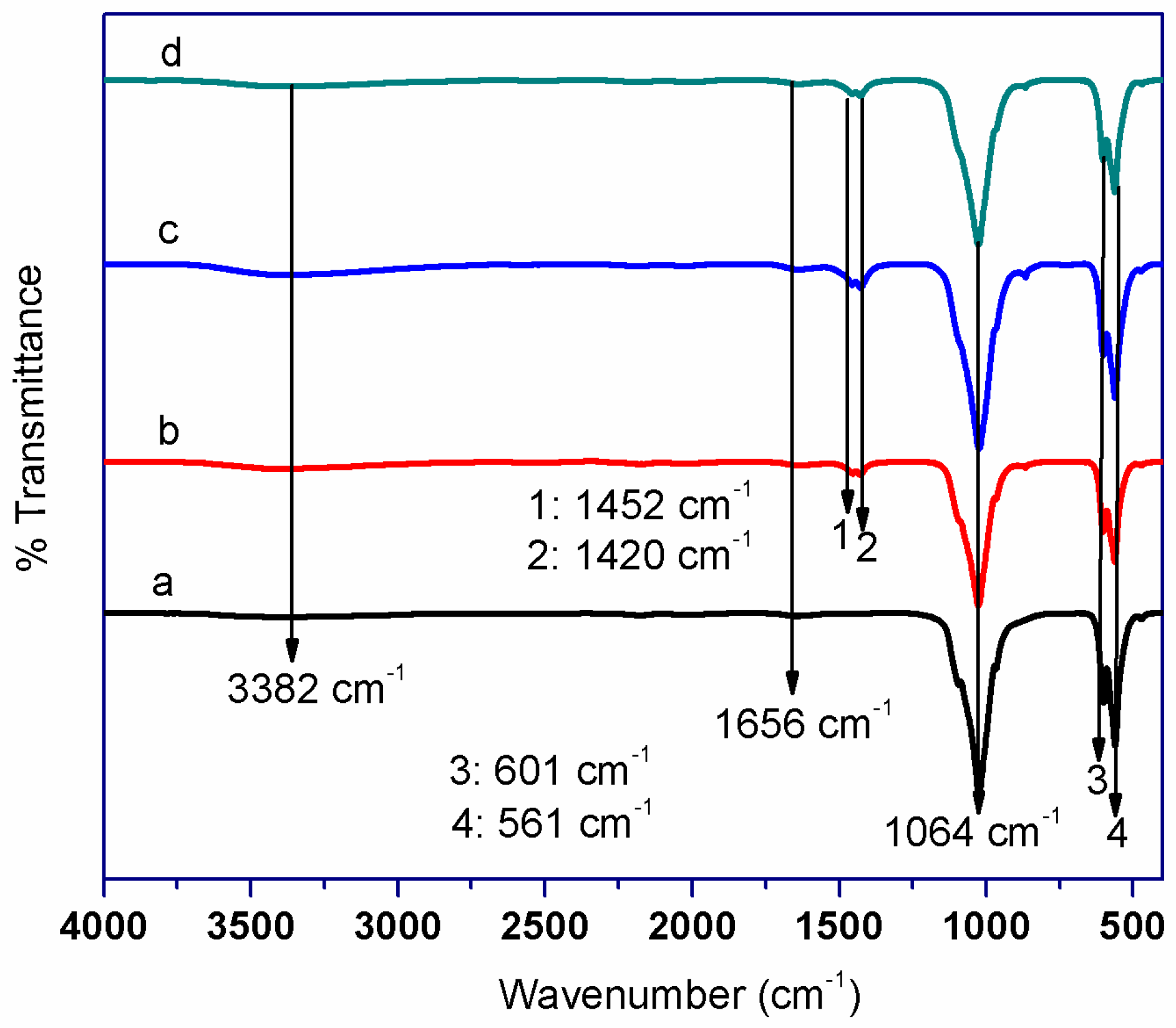
2.2. FT-IR Analysis
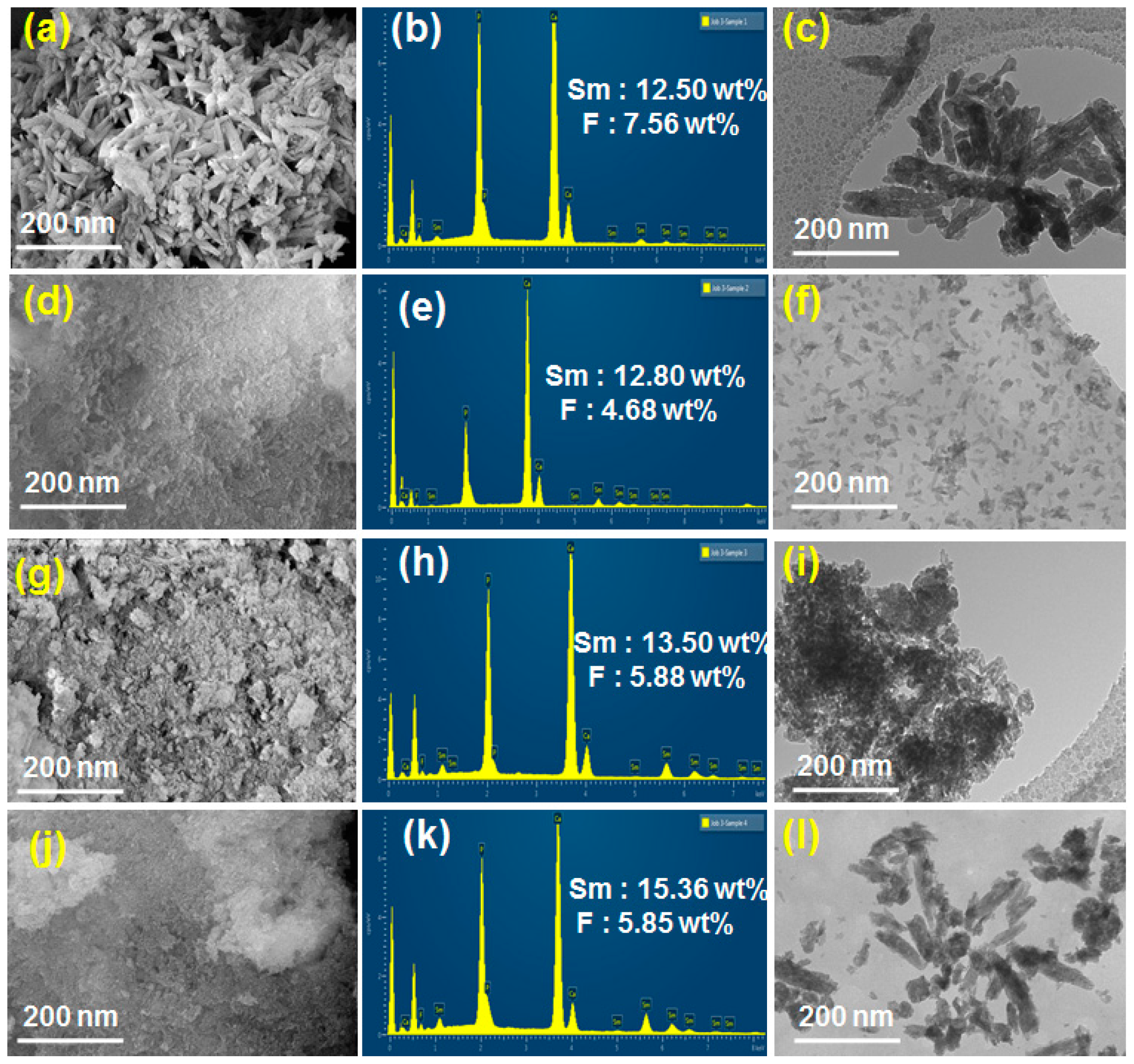
2.3. SEM and TEM Analysis of Sm-FAp and the Influence of Amino Acids
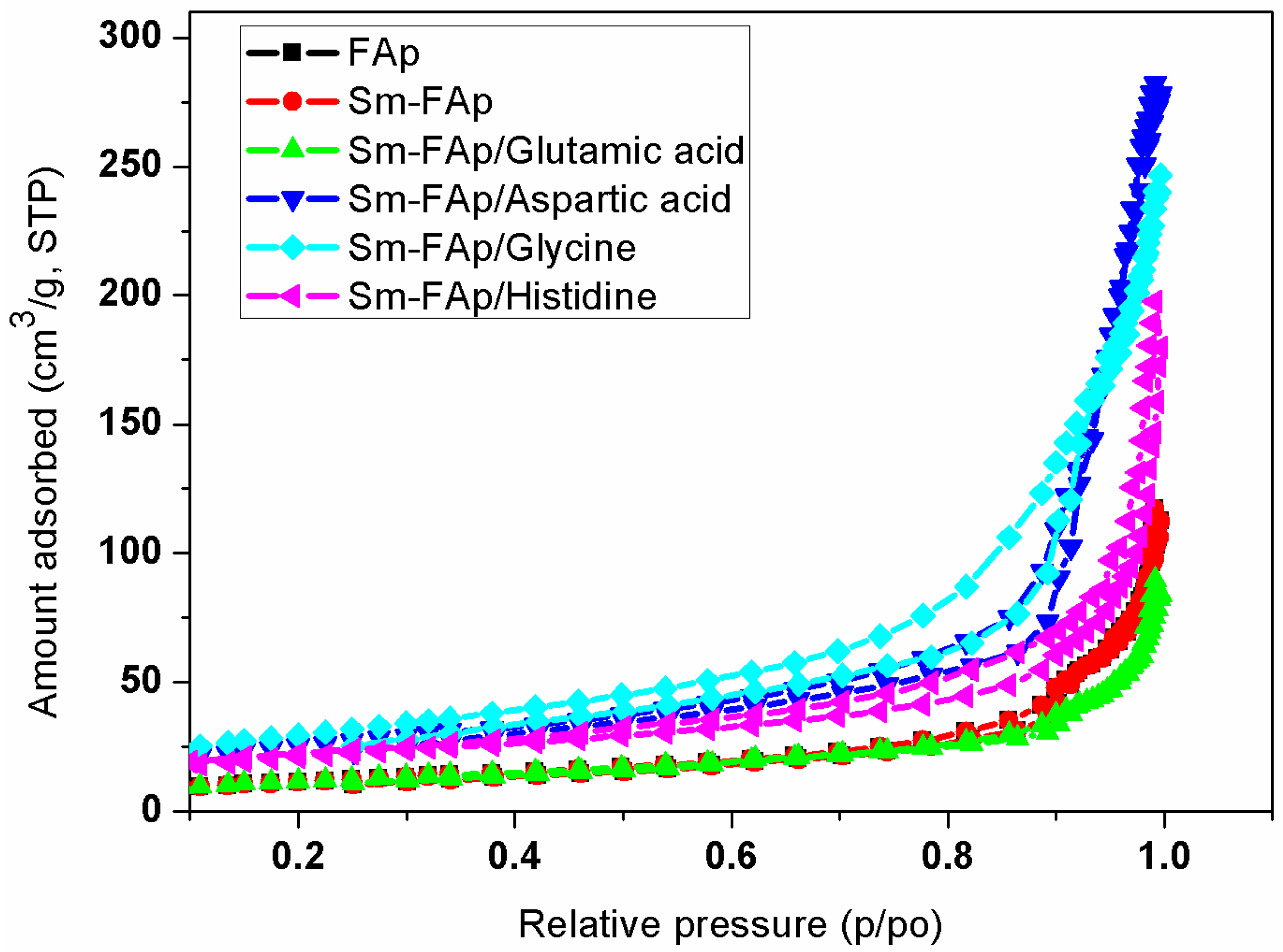
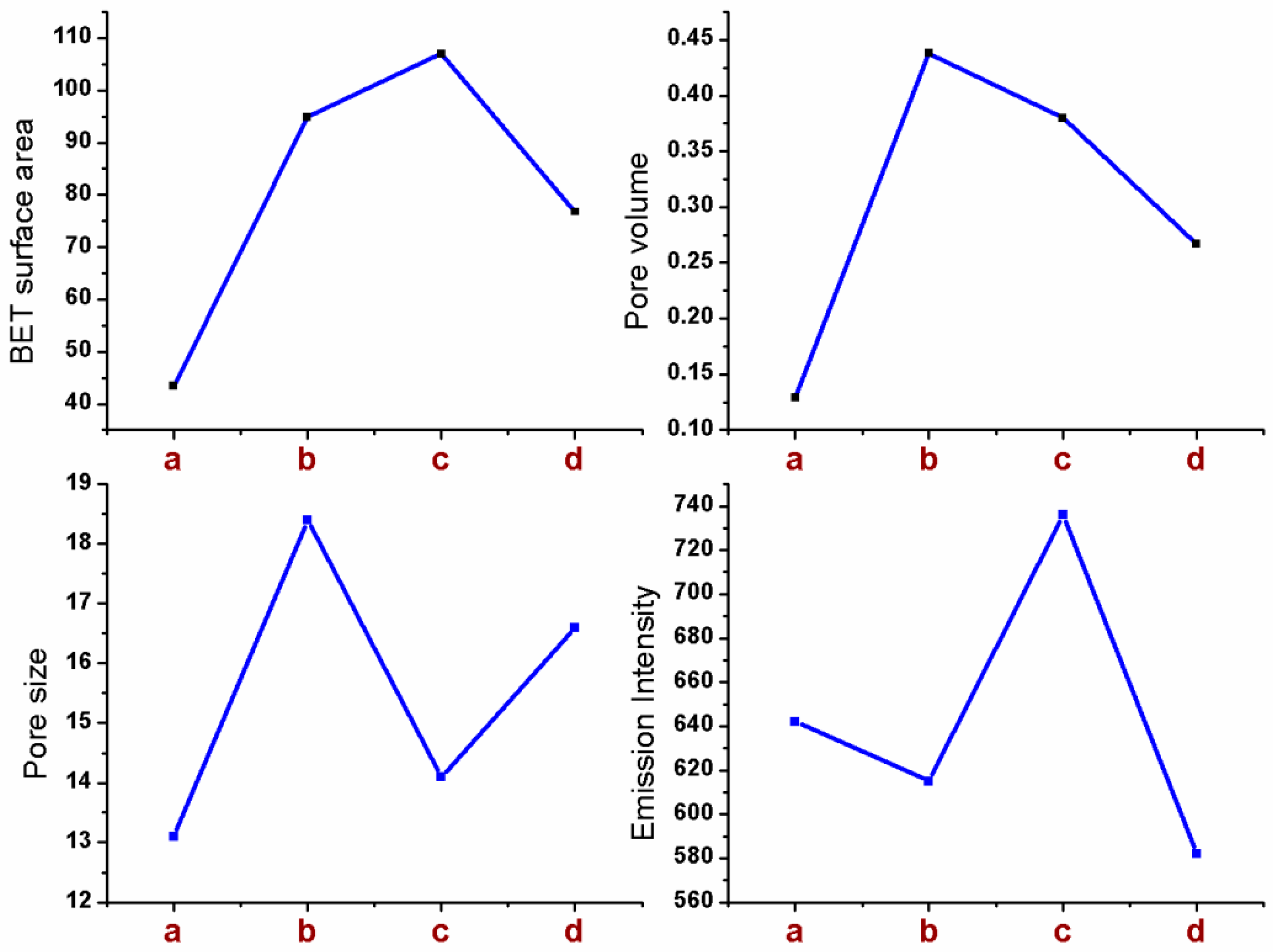
2.4. Textural Properties of Sm-FAp with Influence of Amino Acids
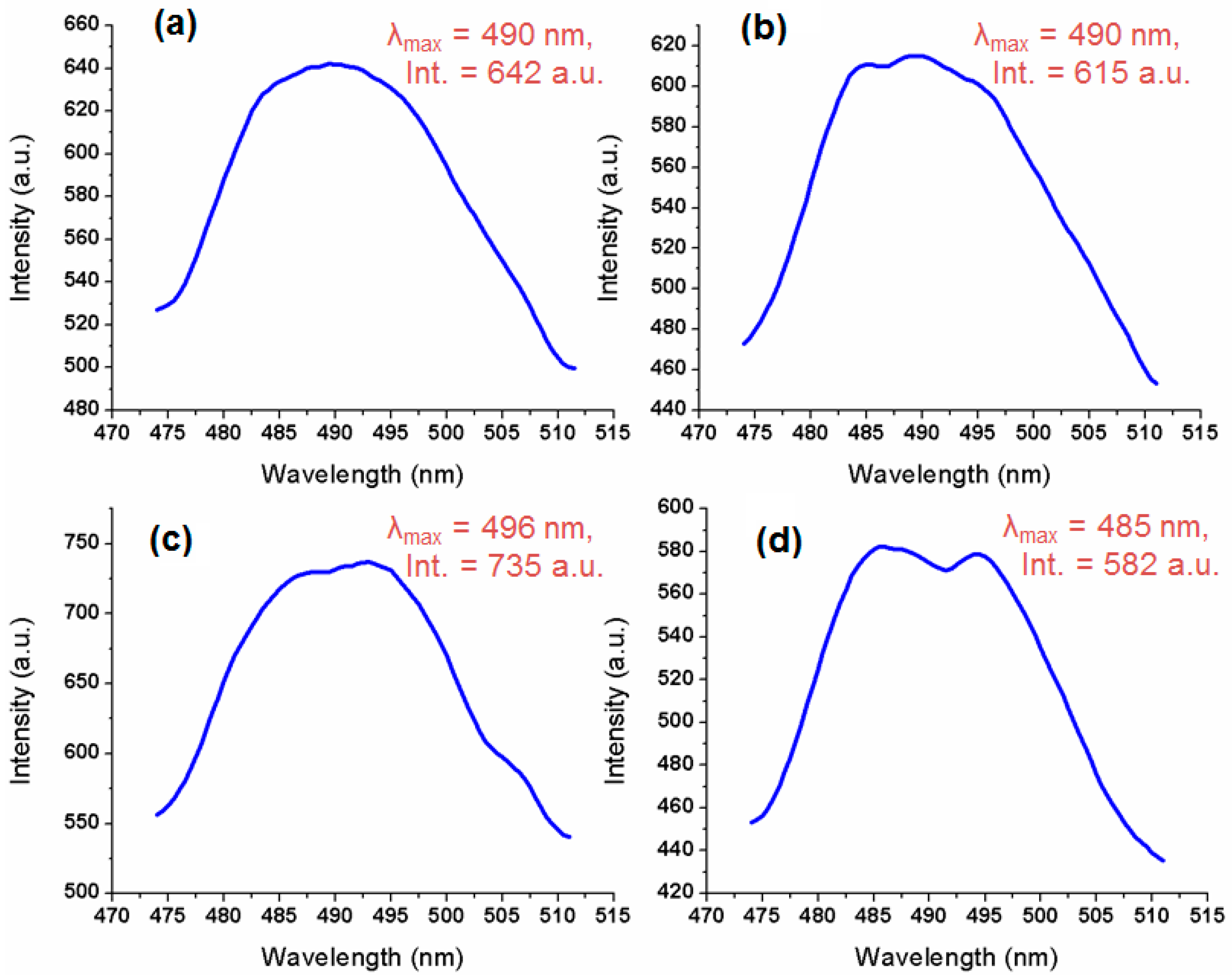
2.5. Fluorescence Activity of Sm-FAp with Amino Acids
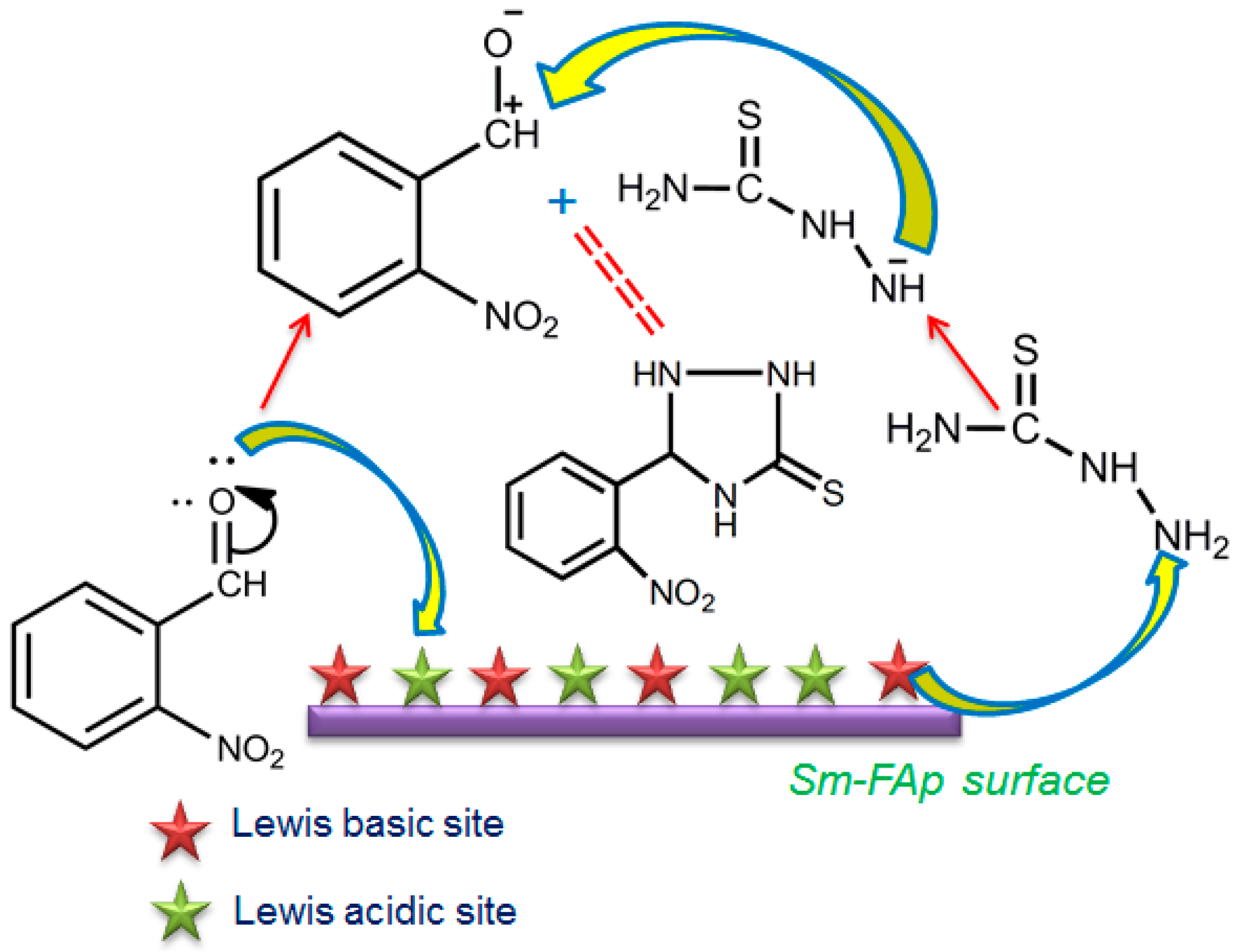
2.6. Catalytic Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of Sm-FAp by Using Amino Acids
3.3. Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gomez, J.M.; Iafisco, M.; Delagado, J.M.; Sarda, S.; Drouet, C. Progress on the preparation of nanocrystalline apatites and surface characterization: Overview of fundamental and applied aspects. Prog. Cryst. Growth Charact. Mater. 2013, 59, 1–46. [Google Scholar] [CrossRef]
- Sharifnabi, A.; Fathi, M.H.; Yekta, B.E.; Hossainalipour, M. The structural and bio-corrosion barrier performance of Mg-substituted fluorapatite coating on 316Lstainlesssteel human body implant. Appl. Surf. Sci. 2014, 288, 331–340. [Google Scholar] [CrossRef]
- Kheradmandfard, M.; Fathi, M.H. Preparation and characterization of Mg-doped fluorapatite nanopowders by sol–gel method. J. Alloy. Compd. 2010, 504, 141–145. [Google Scholar] [CrossRef]
- Stanic, V.; Radosavljevic, A.S.; Radovanovic, V.Z.; Nastasijevic, B.; Cincovic, M.M.; Markovi, J.P.; Budimir, M.D. Synthesis, structural characterisation and antibacterial activity of Ag+-doped fluorapatite nanomaterials prepared by neutralization method. Appl. Surf. Sci. 2015, 337, 72–80. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Hong, S.I.; Mangalaraj, D.; Ponpandian, N.; Chen, P.C. Template free growth of novel hydroxyapatite nanorings: Formation mechanism and their enhanced functional properties. Cryst. Growth Des. 2012, 12, 3565–3574. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, J.; Li, X.; Zhang, X.; Meng, Q.; Yuan, L.; Zhang, J.; Fu, X.; Duan, X.; Chen, H.; et al. Dextran-coated fluorapatite crystals doped with Yb3+/Ho3+ for labeling and tracking chondrogenic differentiation of bone marrow mesenchymal stem cells in vitro and in vivo. Biomaterials 2015, 52, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, P.; Erd, D.C.; Mrose, M.E.; Sabina, A.P.; Smith, D.K. Mineral Powder Diffraction File Data Book; JCPDS International Centre for Diffraction Data: Swarthmore, PA, USA, 1986; Powder Diffraction File Number: 15–876; p. 383. [Google Scholar]
- Comodi, P.; Liu, Y.; Zanazzi, P.F.; Montagnoli, M. Structural and vibrational behaviour of fluorapatite with pressure. Part 1: In situ single-crystal X-ray diffraction investigation. Phys. Chem. Miner. 2001, 28, 219–224. [Google Scholar] [CrossRef]
- Viipsi, K.; Sjorberg, S.; Tonsuaadu, K.; Shchukarev, A. Hydroxy- and fluorapatite as sorbents in Cd(II)–Zn(II) multi-component solutions in the absence/presence of EDTA. J. Hazard. Mater. 2013, 252–253, 91–98. [Google Scholar]
- Peld, M.; Tonsuaadu, K.; Bender, V. Sorption and Desorption of Cd2+ and Zn2+ Ions in Apatite-Aqueous Systems. Environ. Sci. Technol. 2004, 38, 5626–5631. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Washitake, Y.; Nishimura, T.; Takahiro, I.; Keisuke, Y.; Takayoshi, U.; Uemura, S. Calcium-phosphate vanadate apatite (CPVAP)-catalyzed aerobic oxidation of propargylic alcohols with molecular oxygen. Tetrahedron 2004, 60, 9031–9036. [Google Scholar] [CrossRef]
- Saber, A.; Smahi, A.; Solhy, A.; Nazih, R.; Elaabar, B.; Maizi, M.; Sebti, S. Heterogeneous catalysis of Friedel-Crafts alkylation by the fluorapatite alone and doped with metal halides. J. Mol. Catal. A Chem. 2003, 202, 229–237. [Google Scholar] [CrossRef]
- Venugopal, A.; Scurrell, M.S. Hydroxyapatite as a novel support for gold and ruthenium catalysts: Behaviour in the water gas shift reaction. Appl. Catal. A 2003, 245, 137–147. [Google Scholar] [CrossRef]
- Mondelli, C.; Ferri, D.; Baiker, A. Ruthenium at work in Ru-hydroxyapatite during the aerobic oxidation of benzyl alcohol: An in situ ATR-IR spectroscopy study. J. Catal. 2008, 258, 170–176. [Google Scholar] [CrossRef]
- Kaneda, K.; Mizugaki, T. Development of concerto metal catalysts using apatite compounds for green organic syntheses. Energy Environ. Sci. 2009, 2, 655–673. [Google Scholar] [CrossRef]
- Wang, K.; Kennedy, G.J.; Cook, R.A. Hydroxyapatite-supported Rh(CO)2(acac) (acac = acetylacetonate): Structure characterization and catalysis for 1-hexene hydroformylation. J. Mol. Catal. A Chem. 2009, 298, 88–93. [Google Scholar] [CrossRef]
- Gruselle, M. Apatites: A new family of catalysts in organic synthesis. J. Organomet. Chem. 2015, 793, 93–101. [Google Scholar] [CrossRef]
- Mohamed, Z.; Younes, A.; Ahmed, R.; Said, S.; Hamid, D.; Marc, D. Fluorapatite: Efficient catalyst for the Michael addition. Tetrahedron Lett. 2003, 44, 2463–2465. [Google Scholar]
- Hara, T.; Kanai, S.; Mori, K.; Mizugaki, T.; Ebitani, K.; Jitsukawa, K.; Kaneda, K. Highly Efficient C–C Bond-Forming Reactions in Aqueous Media Catalyzed by Monomeric Vanadate Species in an Apatite Framework. J. Org. Chem. 2006, 71, 7455–7462. [Google Scholar] [CrossRef] [PubMed]
- Mason, H.E.; McCubbin, F.M.; Smirnov, A.; Phillips, B.L. Solid-state NMR and IR spectroscopic investigation of the role of structural water and F in carbonate-rich fluorapatite. Am. Mineral. 2009, 94, 507–516. [Google Scholar] [CrossRef]
- Gruselle, M.; Tonis, K.; Rene, T.; Alexandrine, F.; Kadri, K.; Valdek, M.; Rainer, T.; Birgit, M.; Kaia, T. Calcium Hydroxyapatites as Efficient Catalysts for the Michael C–C Bond Formation. ACS Catal. 2011, 1, 1729–1733. [Google Scholar] [CrossRef]
- Yu, H.; Deng, D.G.; Li, Y.Q.; Xu, S.Q.; Li, Y.Y.; Yu, C.P.; Ding, Y.Y.; Lu, H.W.; Yin, H.Y.; Nie, Q.L. Electronic structure and luminescent properties of Ca5(PO4)2(SiO4):Eu2+ green-emitting phosphor for white light emitting diodes. Opt. Commun. 2013, 289, 103–108. [Google Scholar] [CrossRef]
- Shang, M.M.; Li, G.G.; Geng, D.L.; Yang, D.M.; Kang, X.J.; Zhang, Y.; Lian, H.Z.; Lin, J. Blue Emitting Ca8La2(PO4)6O2:Ce3+/Eu2+ Phosphors with High Color Purity and Brightness for White LED: Soft-Chemical Synthesis, Luminescence, and Energy Transfer Properties. J. Phys. Chem. C 2012, 116, 10222–10231. [Google Scholar] [CrossRef]
- Wang, Q.; Ci, Z.P.; Wang, Y.H.; Zhu, G.; Wen, Y.; Shi, Y.R. Crystal structure, photoluminescence properties and energy transfer of Ce3+, Mn2+ co-activated Ca8NaGd(PO4)6F2 phosphor. Mater. Res. Bull. 2013, 48, 1065–1070. [Google Scholar] [CrossRef]
- Michalis, K.; Sonia, A.C.C.; Pedro, B.T.; Josel, F. Redox properties and VOC oxidation activity of Cu catalysts supportedon Ce1−xSmxOδ mixed oxides. J. Hazard. Mater. 2013, 261, 512–521. [Google Scholar]
- Dominguez, R.D.; Alarcon, F.G.; Aguilar, F.M.; Sanchez, A.R.I.; Falcony, C.; Dorantes, R.H.J.; González, V.J.L.; Rivas, L.D.I. Effect on the stabilization of the anatase phase and luminescent properties of samarium-doped TiO2 nanocrystals prepared by microwave irradiation. J. Alloy. Compd. 2016, 687, 121–129. [Google Scholar] [CrossRef]
- Florence, H.; Marie, I.L.; Jean, L.N. A new preparation of samarium dibromide and its use in stoichiometric and catalytic pinacol coupling reactions. Tetrahedron Lett. 2002, 44, 5507–5510. [Google Scholar]
- Hua, Y.W.; Hai, B.W.; Xin, H.L.; Jian, H.L.; Mei, H.Y.; Chuan, J.H.; Wei, Z.W.; Hui, L.W. Samarium-modified vanadium phosphate catalyst for the selective oxidation of n-butane to maleic anhydride. Appl. Surf. Sci. 2015, 351, 243–249. [Google Scholar]
- Jiang, D.; Li, D.; Xie, J.; Zhu, J.; Chen, M.; Lu, X.; Dang, S. Shape-controlled synthesis of F-substituted hydroxyapatite microcrystals in the presence of Na2EDTA and citric acid. J. Colloid Interface Sci. 2010, 350, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Vasiliev, A.N.; Zlotnikov, E.; Khinast, J.G.; Riman, R.E. Chemisorption of silane compounds on hydroxyapatites of various morphologies. Scr. Mater. 2008, 58, 1039–1042. [Google Scholar] [CrossRef]
- Neira, I.S.; Kolenko, Y.V.; Lebedev, O.I.; Tendeloo, G.V.; Gupta, H.S.; Guitian, F.; Yoshimura, M. An Effective Morphology Control of Hydroxyapatite Crystals via Hydrothermal Synthesis. Cryst. Growth Des. 2009, 9, 466–474. [Google Scholar] [CrossRef]
- Zhiqiang, N.; Yadong, Li. Removal and Utilization of Capping Agents in Nanocatalysis. Chem. Mater. 2014, 26, 72–83. [Google Scholar]
- Chen, H.; Sun, K.; Tang, Z.; Law, R.V.; Mansfield, J.F.; Jakubowska, A.C.; Clarkson, B.H. Synthesis of Fluorapatite Nanorods and Nanowires by Direct Precipitation from Solution. Cryst. Growth Des. 2006, 6, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.; Ogden, M.I. Controlling crystal growth with modifiers. CrystEngComm 2010, 12, 1016–1023. [Google Scholar] [CrossRef]
- Hwang, E.T.; Tatavarty, R.; Chung, J.; Gu, M.B. New Functional Amorphous Calcium Phosphate Nanocomposites by Enzyme-Assisted Biomineralization. ACS Appl. Mater. Interfaces 2013, 5, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Fujimoto, Y.; Maekawa, T. Synthesis of gold nanoparticles using various amino acids. J. Colloid Interface Sci. 2015, 447, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Rhim, J.W. Amino acid mediated synthesis of silver nanoparticles andpreparation of antimicrobial agar/silver nanoparticles composite films. Carbohydr. Polym. 2015, 130, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, X.; Zhou, J.; Kong, Q.; Li, C. Single-crystal Au microflakes modulated by amino acids and their sensing and catalytic properties. J. Colloid Interface Sci. 2016, 467, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Hasegawa, T.; Yamada, R.; Ogino, C.; Mizuhata, M.; Kondo, A. Green synthesis of Au, Pd and Au@Pd core–shell nanoparticles via a tryptophan induced supramolecular interface. RSC Adv. 2013, 3, 18367–18372. [Google Scholar] [CrossRef]
- Zhang, H.G.; Zhu, Q.S.; Wang, Y. Morphologically Controlled Synthesis of Hydroxyapatite with Partial Substitution of Fluorine. Chem. Mater. 2005, 17, 5824–5830. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Mirzaei, Y. An efficient one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles at room temperature by green synthesized Cu NPs using Otostegia persica leaf extract. J. Colloid Interface Sci. 2016, 468, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jiang, L.; Shen, C.; Xu, W.; Zhang, P. A highly efficient synthesis of N-glycosyl-1,2,3-triazoles using a recyclable cellulose-copper(0) catalyst in water. Catal. Commun. 2016, 79, 11–16. [Google Scholar] [CrossRef]
- Iniyavan, P.; Balaji, G.L.; Sarveswari, S.; Vijayakumar, V. CuO nanoparticles: Synthesis and application as an efficient reusable catalyst for the preparation of xanthene substituted 1,2,3-triazoles via click chemistry. Tetrahedron Lett. 2015, 56, 5002–5009. [Google Scholar] [CrossRef]
- Sahu, J.K.; Ganguly, S.; Kaushik, A. Triazoles: A valuable insight into recent developments and biological activities. Chin. J. Nat. Med. 2013, 11, 456–465. [Google Scholar] [CrossRef]
- Kumar, P.; Joshi, C.; Srivastava, A.K.; Gupta, P.; Boukherroub, R.; Jain, S.L. Visible Light Assisted Photocatalytic [3 + 2] Azide–Alkyne “Click” Reaction for the Synthesis of 1,4-Substituted 1,2,3-Triazoles Using a Novel Bimetallic Ru-Mn Complex. ACS Sustain. Chem. Eng. 2016, 4, 69–75. [Google Scholar] [CrossRef]
- Turker, L. Azo-bridged triazoles: Green energetic materials. Def. Tech. 2016, 12, 1–15. [Google Scholar] [CrossRef]
- Singh, S.; Jonnalagadda, S.B. Synthesis and characterization of thermally stable metal substituted hydroxyapatites for the selective oxidation of light paraffins. Bull. Chem. Soc. Ethiop. 2013, 27, 57–68. [Google Scholar]
- Pillai, M.K.; Singh, S.; Jonnalagadda, S.B. Solvent-free Knoevenagel condensation over Cobalt hydroxyapatite. Synth. Commun. 2010, 41, 3710–3715. [Google Scholar] [CrossRef]
- Singh, S.; Jonnalagadda, S.B. Selective oxidation of n-pentane over V2O5 supported on hydroxyapatite. Catal. Lett. 2008, 120, 200–206. [Google Scholar]
- Pillai, M.K.; Singh, S.; Jonnalagadda, S.B. Solvent-free Knoevenagel condensation over iridium and platinum hydroxyapatites. Kinet. Catal. 2011, 52, 536–539. [Google Scholar] [CrossRef]
- Gangu, K.K.; Dadhich, A.S.; Mukkamala, S.B. Hydrothermal synthesis, crystal structure and luminescence property of a three dimensional Sm(III) coordination polymer with 2,5-pyridinedicarboxylic acid. J. Chem. Sci. 2015, 127, 2225–2230. [Google Scholar] [CrossRef]
- Palazzo, B.; Dominic, W.; Michele, L.; Elisabetta, F.; Luca, B.; Gianmario, M.; Claudia, L.B.; Giuseppe, C.; Norberto, R. Amino acid synergetic effect on structure, morphology and surface properties of biomimetic apatite nanocrystals. Acta Biomater. 2009, 5, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Frindell, K.L.; Bartl, M.H.; Popitsch, A.; Stucky, G.D. Sensitized Luminescence of Trivalent Europium by Three-Dimensionally Arranged Anatase Nanocrystals in Mesostructured Titania Thin Films. Angew. Chem. Int. Ed. 2002, 41, 959–962. [Google Scholar] [CrossRef]
- Cuimiao, Z.; Shanshan, H.; Dongmei, Y.; Xiaojiao, K.; Mengmeng, S.; Chong, P.; Jun, L. Tunable luminescence in Ce3+, Mn2+-codoped calcium fluorapatite through combining emissions and modulation of excitation: A novel strategy to white light emission. J. Mater. Chem. 2010, 20, 6674–6680. [Google Scholar]
- Zaitoun, M.A.; Momani, K.; Jaradat, Q.; Qurashi, I.M. Synthesis and luminescence properties of encapsulated sol–gel glass samarium complexes. Spectrochim. Acta A 2013, 115, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, V.B.; Basavapoornima, C.H.; Jayasankar, C.K. Spectroscopic and fluorescence properties of Sm3+-doped zinc fluorophosphate glasses. J. Rare Earths 2014, 32, 918–926. [Google Scholar] [CrossRef]
- Venkatramu, V.; Babu, P.; Jayasankar, C.K.; Troster, T.; Sievers, W.; Wortmann, G. Optical spectroscopy of Sm3+ ions in phosphate and fluorophosphate glasses. Opt. Mater. 2007, 29, 1429–1439. [Google Scholar] [CrossRef]
- Pramod, K.S.; Jilavi, M.H.; Nass, R.; Schmidt, H. Tailoring the particle size from μm→nm scale by using a surface modifier and their size effect on the fluorescence properties of europium doped yttria. J. Lumin. 1999, 82, 187–193. [Google Scholar]
- Kim, Y.; Kang, S. Effect of particle size on photoluminescence emission intensity in ZnO. Acta Mater. 2011, 59, 3024–3031. [Google Scholar] [CrossRef]
- Rathinam, R.; Lalitha, A. PEG-assisted two-component approach for the facile synthesis of 5-aryl-1,2,4-triazolidine-3-thiones under catalyst-free conditions. RSC Adv. 2015, 5, 51188–51192. [Google Scholar]
- Jayavant, D.P.; Pore, D.M. [C16MPy]AlCl3Br: An efficient novel ionic liquid for synthesis of novel 1,2,4-triazolidine-3-thiones in water. RSC Adv. 2014, 4, 14314–14319. [Google Scholar]
- Mane, M.M.; Pore, D.M. A novel one pot multi-component strategy for facile synthesis of 5-aryl-[1,2,4]triazolidine-3-thiones. Tetrahedron Lett. 2014, 55, 6601–6604. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.

| Sample | BET-Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | Average Crystallite Size from XRD (nm) | Acidity (mmol NH3/g) |
|---|---|---|---|---|---|
| FAp/without amino acid | 32 ± 1.3 | 0.13 ± 0.02 | 11.5 ± 0.2 | 17.52 ± 0.4 | 205 ± 6.7 |
| Sm-FAp/without amino acid | 39 ± 3.1 | 0.21 ± 0.02 | 12.6 ± 0.3 | 15.58 ± 0.4 | 271 ± 6.2 |
| Sm-FAp/Glutamic acid | 43 ± 1.1 | 0.22 ± 0.02 | 13.1 ± 0.2 | 9.8 ± 0.3 | 349 ± 12.6 |
| Sm-FAp/Aspartic acid | 94 ± 1.5 | 0.44 ± 0.03 | 18.4 ± 0.3 | 6.2 ± 0.4 | 412 ± 11.4 |
| Sm-FAp/Glycine | 106 ± 1.3 | 0.38 ± 0.01 | 14.1 ± 0.3 | 5.7 ± 0.4 | 607 ± 14.4 |
| Sm-FAp/Histidine | 76 ± 1.3 | 0.27 ± 0.01 | 16.6 ± 0.3 | 10.6 ± 0.4 | 375 ± 11.5 |
| Catalyst | Time (min) | Yield (%) |
|---|---|---|
| Without catalyst | 120 | 60 |
| Un-doped FAp (without amino acid) | 35 | 80 |
| Sm-FAp/Glutamic acid | 15 | 94 |
| Sm-FAp/Aspartic acid | 10 | 95 |
| Sm-FAp/Glycine | 10 | 98 |
| Sm-FAp/Histidine | 20 | 93 |
| Entry | Aldehyde | Product | Yield % | Time (min) |
|---|---|---|---|---|
| 1 | 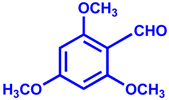 | 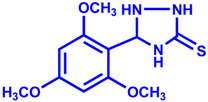 | 92 | 15 |
| 2 | 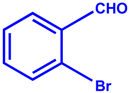 | 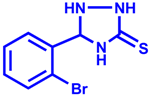 | 93 | 20 |
| 3 | 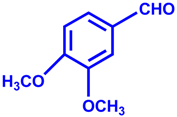 | 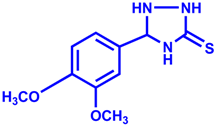 | 96 | 15 |
| 4 |  | 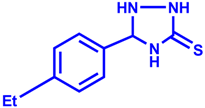 | 94 | 10 |
| Sample | Ca(NO3)3·4H2O (mmol) | Sm(NO3)3·6H2O | Na3PO4·12 H2O (mmol) | NaF (mmol) | Amino Acid (2.0 mmol) |
|---|---|---|---|---|---|
| a | 2.5 | 0.074 g | 1.5 | 0.5 | Glutamic acid (0.330 g) |
| b | 2.5 | 0.074 g | 1.5 | 0.5 | Aspartic acid (0.266 g) |
| c | 2.5 | 0.074 g | 1.5 | 0.5 | Glycine (0.150 g) |
| d | 2.5 | 0.074 g | 1.5 | 0.5 | Histidine (0.310 g) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangu, K.K.; Maddila, S.; Maddila, S.N.; Jonnalagadda, S.B. Nanostructured Samarium Doped Fluorapatites and Their Catalytic Activity towards Synthesis of 1,2,4-Triazoles. Molecules 2016, 21, 1281. https://doi.org/10.3390/molecules21101281
Gangu KK, Maddila S, Maddila SN, Jonnalagadda SB. Nanostructured Samarium Doped Fluorapatites and Their Catalytic Activity towards Synthesis of 1,2,4-Triazoles. Molecules. 2016; 21(10):1281. https://doi.org/10.3390/molecules21101281
Chicago/Turabian StyleGangu, Kranthi Kumar, Suresh Maddila, Surya Narayana Maddila, and Sreekantha B. Jonnalagadda. 2016. "Nanostructured Samarium Doped Fluorapatites and Their Catalytic Activity towards Synthesis of 1,2,4-Triazoles" Molecules 21, no. 10: 1281. https://doi.org/10.3390/molecules21101281
APA StyleGangu, K. K., Maddila, S., Maddila, S. N., & Jonnalagadda, S. B. (2016). Nanostructured Samarium Doped Fluorapatites and Their Catalytic Activity towards Synthesis of 1,2,4-Triazoles. Molecules, 21(10), 1281. https://doi.org/10.3390/molecules21101281