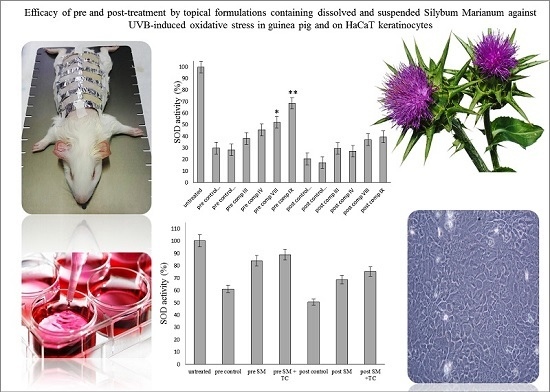
Efficacy of Pre- and Post-Treatment by Topical Formulations Containing Dissolved and Suspended Silybum marianum against UVB-Induced Oxidative Stress in Guinea Pig and on HaCaT Keratinocytes
Abstract
:1. Introduction
2. Results
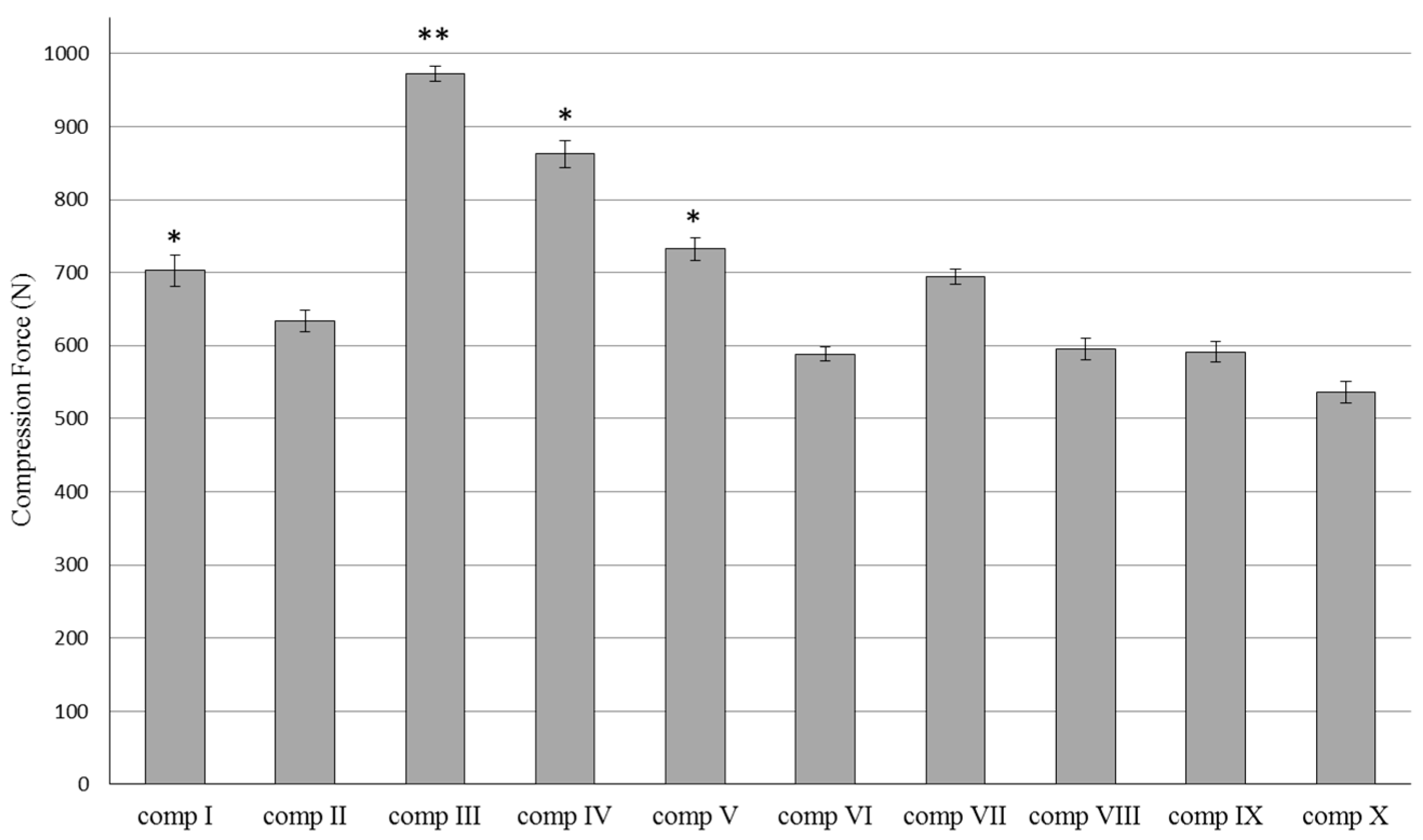
2.1. Texture Analyzer Studies
2.2. In Vitro Release Studies of Silymarin
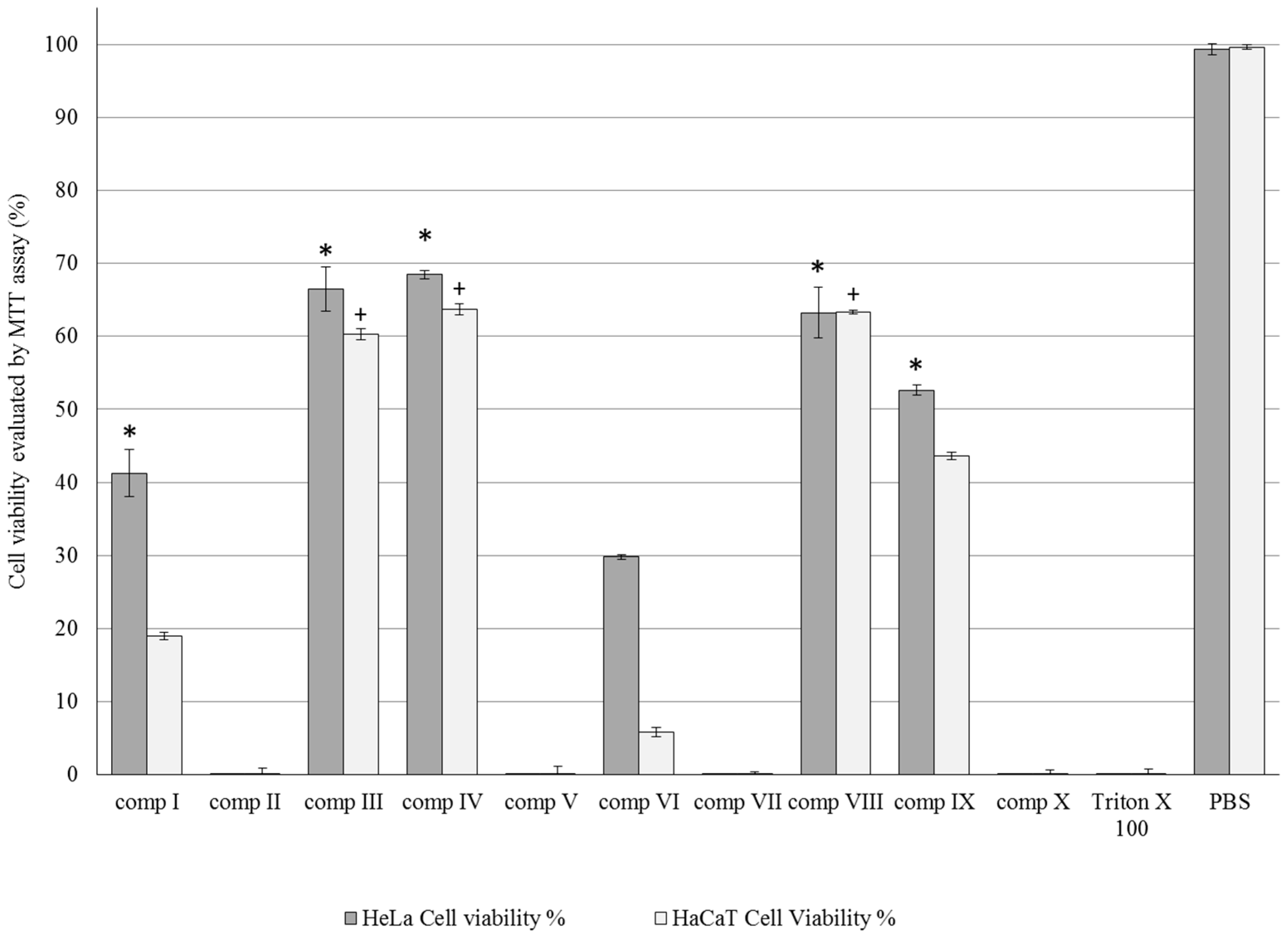
2.3. MTT Cytotoxicity Tests
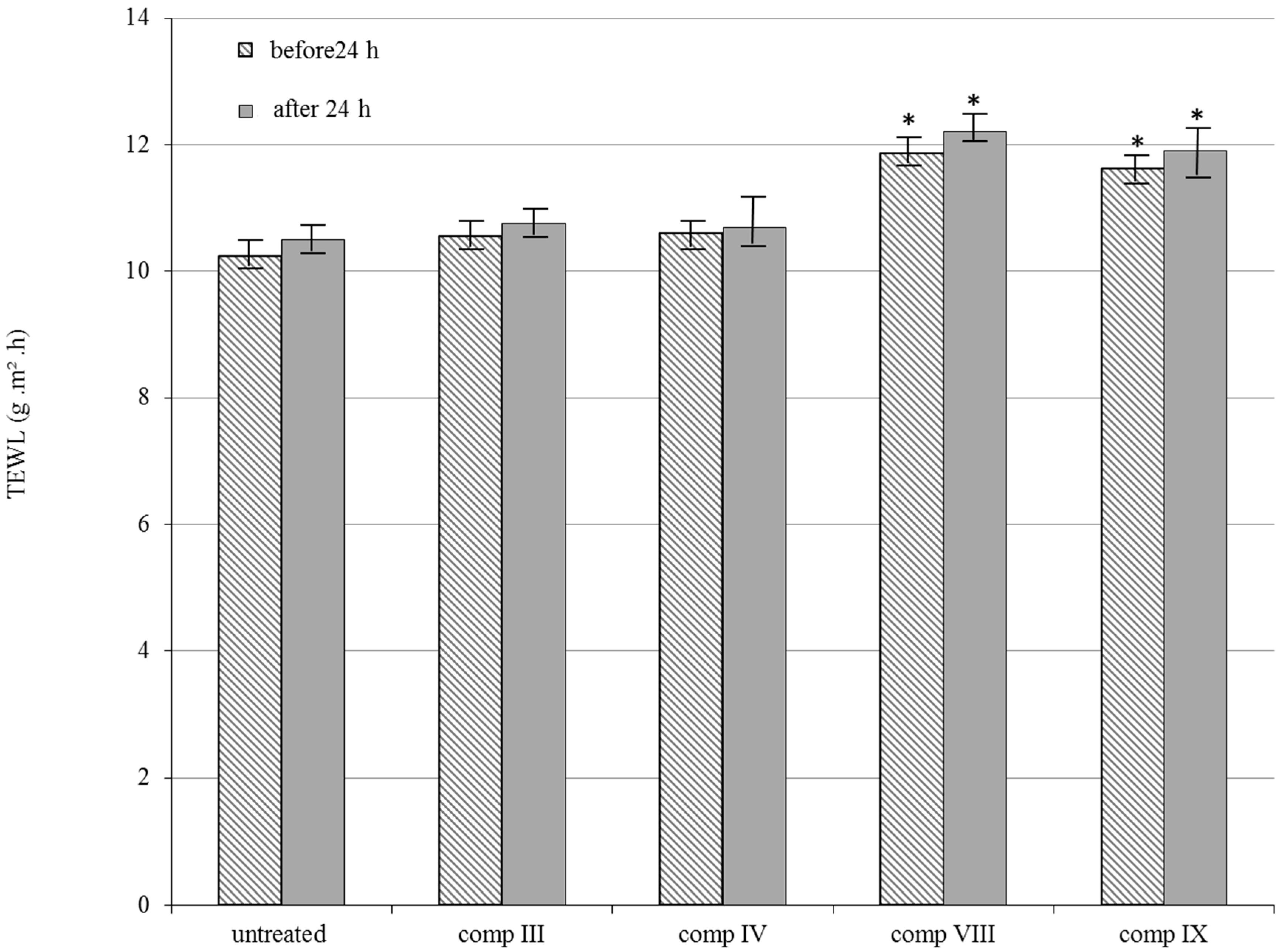
2.4. In Vivo TEWL Measurements
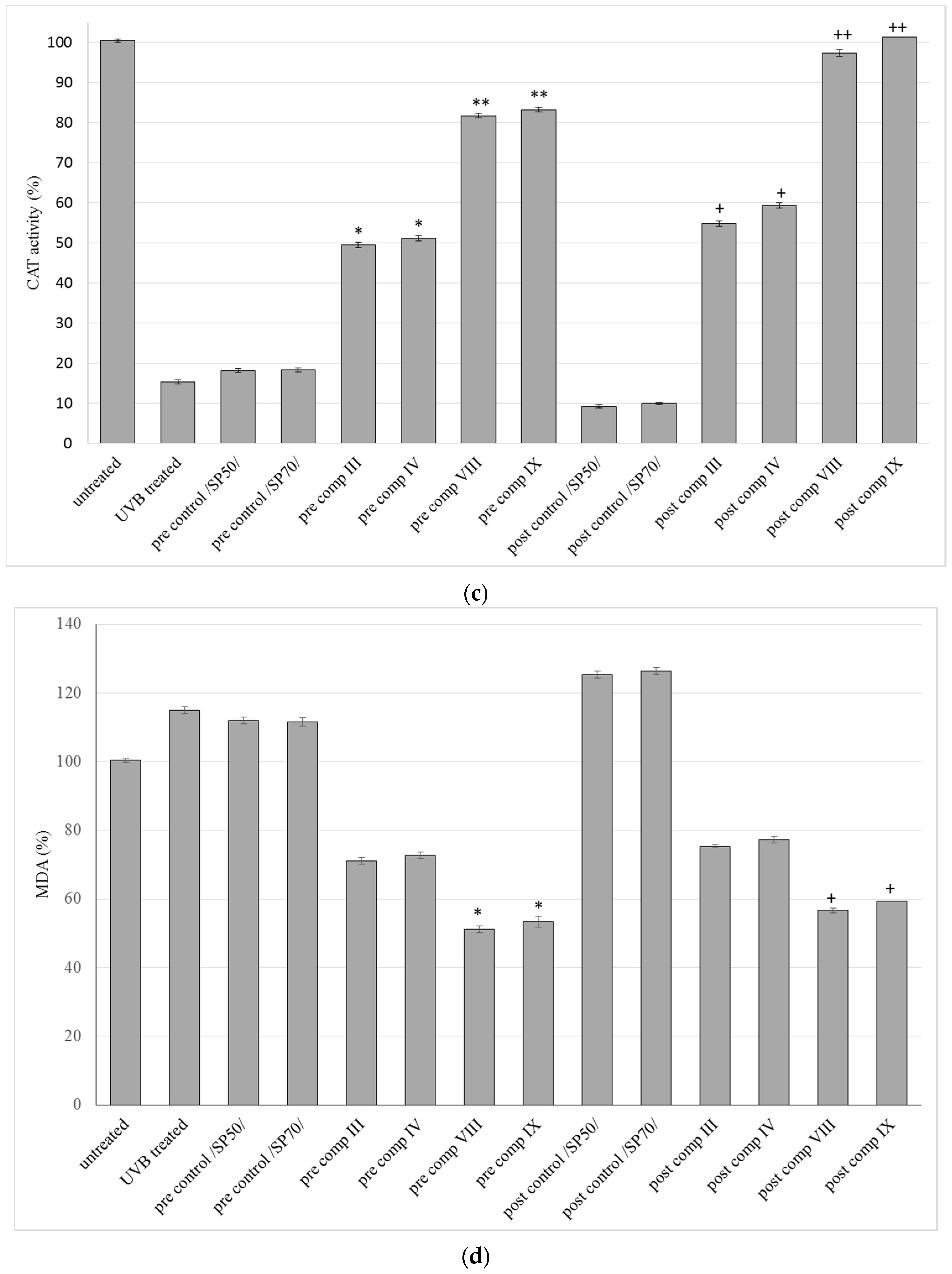
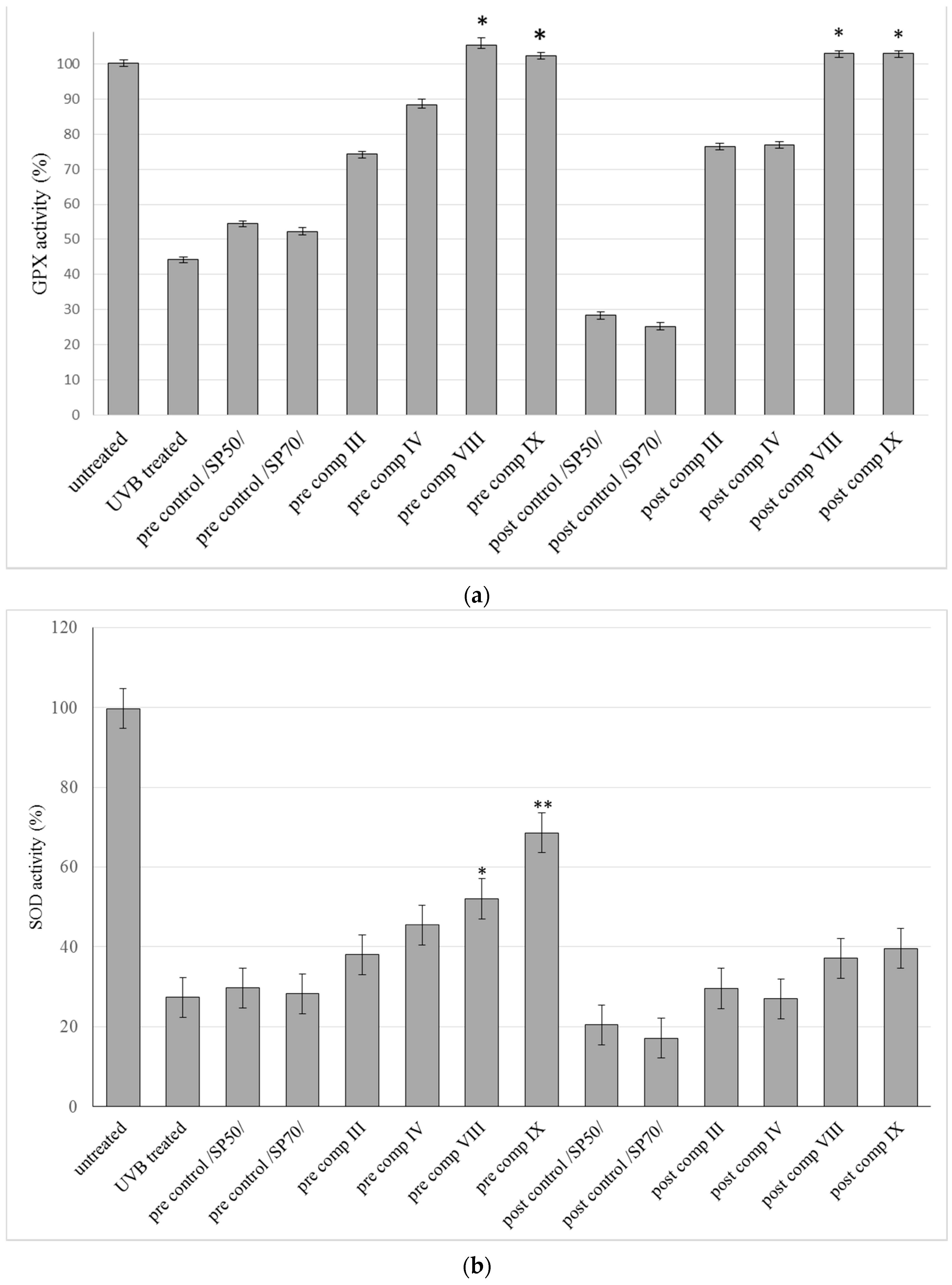
2.5. In Vitro Antioxidant Activity on HaCaT Cell Line
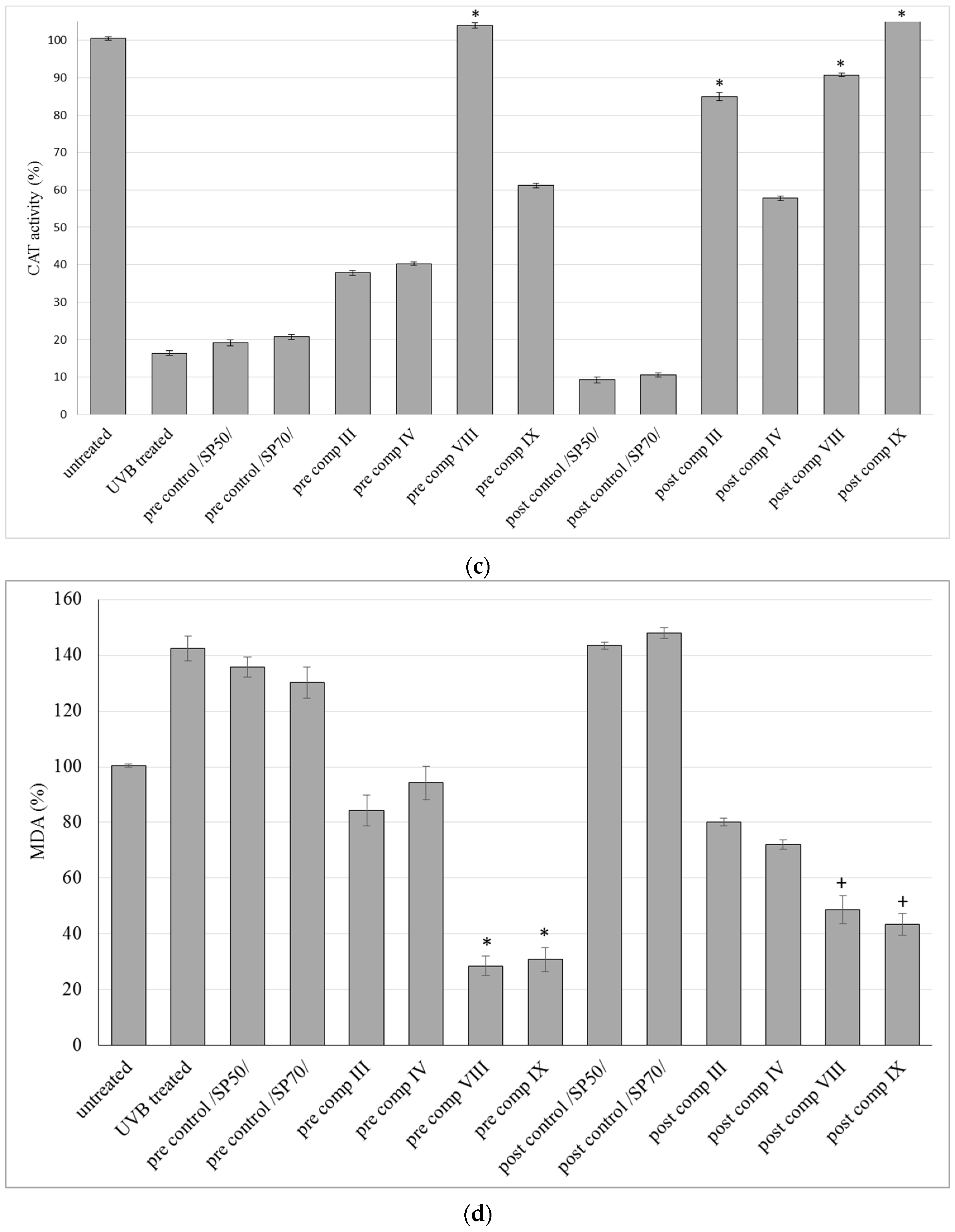
2.6. Antioxidant Activity on Guinea Pig Model
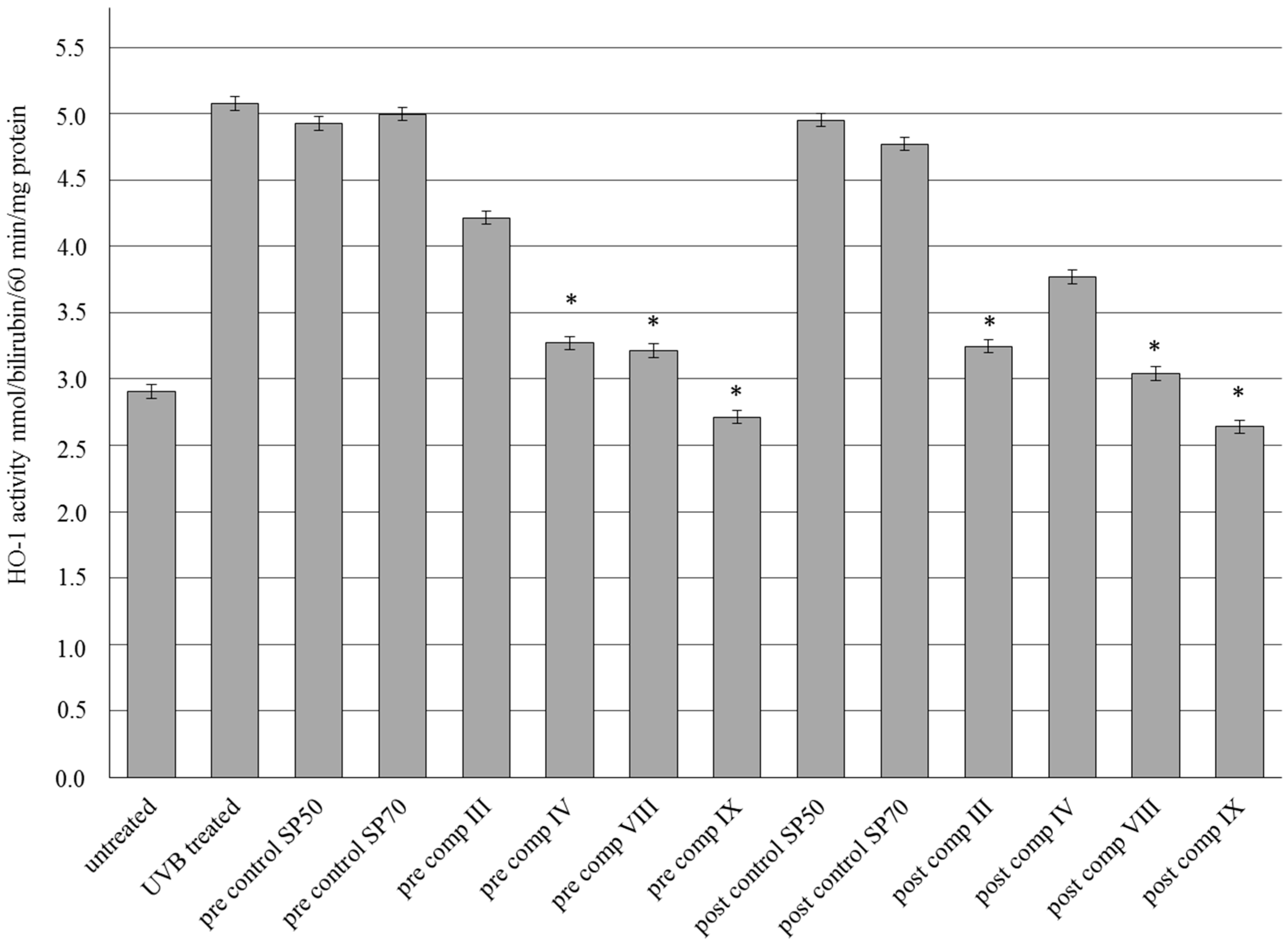
2.7. HO-1 Enzyme Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Formulation of Topical Ointments
4.3. Texture Analyzer Studies
4.4. In Vitro Release Studies
4.5. Cell Cultures
4.6. MTT-Assay
4.7. In Vivo Skin Irritation Test
4.8. UV-B Irradiation on HaCaT Cells, Pre-Treatment and Post-Treatment with Suspended and Dissolved Silymarin in TC
4.9. Experimental Animals
4.10. UV-B Irradiation, Pre-Treatment and Post-Treatment with Silymarin Cream Formulations, in Vivo
4.11. Antioxidant Activity
4.11.1. Superoxide Dismutase Activity
4.11.2. Catalase Activity
4.11.3. Glutathione Peroxidase Activity
4.11.4. Lipid Peroxidation (MDA) Test
4.12. Measurement of HO-1 Enzyme Activity
4.13. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rasul, A.; Akhtar, N.; Khan, B.A.; Mahmood, T.; Zaman, S.; Atif, A.; Haji, M.; Khan, S.; Parveen, S. Assessment of anti erythmic and skin whitening effects of milk thistle extract. Afr. J. Pharm. Pharmacol. 2011, 5, 2306–2309. [Google Scholar]
- Biedermann, D.; Vavrikova, E.; Cvak, L.; Kren, V. Chemistry of silybin. Nat. Prod. Rep. 2014, 31, 1138–1157. [Google Scholar] [CrossRef] [PubMed]
- Kren, V.; Walterova, D. Silybin and silymarin—New effects and applications. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Chech Repub. 2005, 149, 29–41. [Google Scholar] [CrossRef]
- Kuki, Á.; Nagy, L.; Deák, G.; Nagy, M.; Zsuga, M.; Kéki, S. Identification of Silymarin Constituents: An Improved HPLC-MS Method. Chromatographia 2012, 75, 175–180. [Google Scholar] [CrossRef]
- Frascini, F.; Demartini, G.; Esposti, D. Pharmacology of silymarin. Clin. Drug Investig. 2002, 22, 51–65. [Google Scholar] [CrossRef]
- Gazák, R.; Walterová, D.; Kren, V. Silybin and Silymarin—New and Emerging Applications in Medicine. Curr. Med. Chem. 2007, 14, 315–338. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K. Silymarin and skin cancer prevention: Antiinflammatory, antioxidant and immunomodulatory effects (Review). Int. J. Oncol. 2005, 26, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, A.; Psotová, J.; Walterová, D. Natural phenolics in the prevention of UV-Induced skin damage. A review. Biomed. Pap. 2003, 147, 137–145. [Google Scholar] [CrossRef]
- Narayanapillai, S.; Agarwal, C.; Tilley, C.; Agarwal, R. Silibinin is a potent sensitizer of UVA radiation-induced oxidative stress and apoptosis in human keratinocyte HaCaT cells. Photochem. Photobiol. 2012, 88, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Yoshihisa, Y.; Rehman, M.U.; Shimizu, T. Astaxanthin, a xanthophyll carotenoid, inhibits ulltraviolet-induced apoptosis in keratinocytes. Exp. Dermatol. 2014, 23, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Fernando, P.M.D.J.; Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Hewage, S.R.K.M.; Chae, S.W.; Hyun, J.W. Rosmarinic Acid Attenuates Cell Damage against UVB Radiation-Induced Oxidative Stress via Enhancing Antioxidant Effects in Human HaCaT Cells. Biomol. Ther. 2016, 24, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, A.; Zdarilová, A.; Walterová, D.; Vostálová, J. Flavonolignans from Silybum marianum moderate UVA-induced oxidative damage to HaCaT keratinocytes. J. Dermatol. Sci. 2007, 48, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Katiyar, S.K. Chemoprevention of photocarcinogenesis by selected dietary botanicals. Photochem. Photobiol. Sci. 2006, 5, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Mantena, S.K.; Meeran, S.M. Silymarin protects epidermal keratinocytes from ultraviolet radiation-induced apoptosis and DNA damage by nucleotide excision repair mechanism. PLoS ONE 2011, 6, e21410. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Takahashi, T.; Nakahira, K.; Uehara, K.; Shimizu, H.; Matsumi, M.; Morita, K.; Hirakawa, M.; Akagi, R.; Sassa, S. Protective role of heme oxygenase-1 in the intestinal tissue injury in an experimental model of sepsis. Crit. Care Med. 2003, 31, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Liu, G.; Zhomg, J.L. UVA-induced protection of skin through the induction of heme oxygenase-1. BioSci. Trends 2011, 5, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.; Pinnel, M.D. Cutaneous photodamage, oxidative stress and topical antioxidant protection. J. Am. Acad. Deramtol. 2003, 48, 1–19. [Google Scholar]
- Vaid, M.; Katiyar, S.K. Molecular mechanisms of inhibition of photocarcinogenesis by silymarin, a phytochemical from milk thistle (Silybum marianum L. Gaertn.) (Review). Int. J. Oncol. 2010, 36, 1053–1060. [Google Scholar] [PubMed]
- Couteau, C.; Cheignon, C.; Paparis, E.; Coiffard, L.J. Silymarin, a molecule of interest for topic photoprotection. Nat. Prod. Res. 2012, 26, 2211–2214. [Google Scholar] [CrossRef] [PubMed]
- Campanini, M.Z.; Pinho-Ribeiro, F.A.; Ivan, A.L.M.; Ferriera, V.S.; Vilela, F.M.P.; Vicentini, F.T.; Martinez, R.M.; Zarpelon, A.C.; Fonseca, M.J.V.; Faria, T.J.; et al. Efficacy of topical formulations containing Pimenta pseudocaryophyllus extracts against UVB-induced oxidative stress and inflammation in hairless mice. J. Photoch. Photobiol. B 2013, 127, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.F.; Lin, Y.K.; Zhang, L.W.; Chang, C.H.; Fang, J.Y. Topical delivery of silymarin constituents via the skin route. Acta Pharm. Sin. 2010, 31, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F. Silymarin as a natural antioxidant: An overview of the current evidence and perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Kohli, K.; Ali, M. Reassessing Bioavailability of Silymarin. Altern. Med. Rev. 2011, 16, 239–249. [Google Scholar] [PubMed]
- Szűts, A.; Szabó-Révész, P. Sucrose esters as natural surfactants in drug delivery systems—A mini-review. Int. J. Pharm. 2012, 433, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nino, M.; Calabrò, G.; Santoianni, P. Topical delivery of active principles: The field of dermatological research. Dermatol. Online J. 2010, 16, 4. [Google Scholar] [PubMed]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliver. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Ujhelyi, Z.; Fenyvesi, F.; Váradi, J.; Fehér, P.; Kiss, T.; Veszelka, S.; Deli, M.A.; Vecsernyés, M.; Bácskay, I. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur. J. Pharm. Sci. 2012, 47, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Physical chemical analysis of percutaneous absorption process from creams and ointments. J. Soc. Cosmet. Chem. 1960, 11, 85–97. [Google Scholar]
- Higuchi, T. Rate release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.P.; Wu, J.X.; Fan, Y.; Adamson, E.D. UV activates growth factor receptors via reactive oxygen intermediates. J. Cell Biol. 1996, 133, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Peus, D.; Vasa, R.A.; Meves, A.; Pott, M.; Beyerle, A.; Squillace, K.; Pittelkow, M.R. H2O2 is an important mediator of UVB-induced EGF-receptor phosphorylation in cultured keratinocytes. J. Investig. Dermatol. 1998, 110, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Peus, D.; Vasa, R.A.; Beyerle, A.; Meves, A.; Krautmacher, C.; Pittelkow, M.R. UVB activates ERK1/2 and p38 signaling pathways via reactive oxygen species in cultured keratinocytes. J. Investig. Dermatol. 1999, 112, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Afaq, F.; Azizuddin, K.; Mukhtar, H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (−)-epigallocatechin-3-gallate. Toxicol. Appl. Pharmacol. 2001, 176, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K. Tretament of silymarin, a plant flavonoid, prevents ultraviolet light-induced immune suppression and in oxidative stress in mouse skin. Int. J. Oncol. 2002, 21, 1213–1222. [Google Scholar] [PubMed]
- Woo, J.S.; Kim, T.S.; Park, J.H.; Chi, S.C. Formulation and biopharmaceutical evaluation of silymarin using SMEDDS. Arch. Pharm. Res. 2007, 30, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Faucci, M.T.; Bramanti, G.; Corti, P. Evaluation of transcutol asa clonazepa, transdermal permeation enhancer from hydrophilic gel formulations. Eur. J. Pharm. Sci. 2000, 9, 365–372. [Google Scholar] [CrossRef]
- Csóka, I.; Csányi, E.; Zapantis, G.; Nagy, E.; Fehér-Kiss, A.; Horváth, G.; Blazsó, G.; Eros, I. In vitro and in vivo percutaneous absorption of topical dosage forms: Case studies. Int. J. Pharm. 2005, 291, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tamburic, S.; Craig, D.Q.M.; Vuleta, G.; Milic, J. An investigation into the use of thermorheology and texture analysis in the evaluation of W/O creams stabilized with a silicone emulsifier. J. Pharm. Dev. Technol. 1996, 1, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Ujhelyi, Z.; Kalantari, A.; Vecsernyés, M.; Róka, E.; Fenyvesi, F.; Póka, R.; Kozma, B.; Bácskay, I. The enhanced inhibitory effect of different antitumor agents in self-microemulsifying drug delivery systems on human cervical cancer HeLa cells. Molecules 2015, 20, 13226–13239. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Adamczyk, J.; Palak, J.; Stoklosa, S.; Kubis, B.; Pastuszek, P.; Slota, E.; Wnuk, M. Curcumin-mediated decrease in the expression of nucleolar organizer regions in cervical cancer (HeLa) cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 1, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Seop, S.Y.; Hyun, M.Y.; Yoo, K.H.; Kim, B.J.; Kim, M.N.; Cho, J.W. Safety evaluation of topical valproate application. Toxicol. Res. 2013, 29, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Konsoula, R.; Barile, F.A. Correlation of in vitro cytotoxicity with paracellular permeability in Caco-2 cells. Toxicol. In Vitro 2005, 19, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.T.; Frank, J.K.; Fricker, G.; Brandl, M. Biopharmaceutical classification of poorly soluble drugs with respect to “enabling formulations”. Eur. J. Pharm. Sci. 2013, 50, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Panapisal, V.; Charoensri, S.; Tantituvanont, A. Formulation of Microemulsion Systems for Dermal Delivery of Silymarin. AAPS PharmSciTech 2012, 13, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Sailou, C.; Kitazawa, M.; McLaughin, L.; Yang, J.P.; Lodge, J.K.; Tetsuka, T.; Iwasaki, K.; Cillard, J.; Okamoto, T.; Packer, L. Antioxidants modulate acute solar ultraviolet radiation-induced NF-kappa-B activation in a human keratinocyte cell line. Free Radic. Biol. Med. 1999, 26, 174–183. [Google Scholar] [CrossRef]
- Tyrell, R.M. Solar ultraviolet a radiation: An oxidizing skin carcinogen that activates heme oxygenase-1. Antioxid. Redox Signal. 2004, 6, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to Heme Oxygenase-1 and its Anti-Inflammatory Therapeutic Potential. Biochem. Pharmacol. 2010, 80, 1895. [Google Scholar] [CrossRef] [PubMed]
- Kahol, A.; Singh, K.; Tandon, S.; Kumar, S. Process for Isolation of Hepatoprotective Agent Silymarin from Seeds of the Plant Silybum marianum. Indian Patent 06309678, 23 October 2001. [Google Scholar]
- Liebenberg, W.; Engelbrecht, E.; Wessels, A.; Devarakonda, B.; Yang, W.; Villiers, M. A comparative study of the release of active ingredients from semisolids cosmeceuticals measures with Franz, Enhancer or Flow-through cell diffusion apparatus. J. Food Drug Anal. 2004, 12, 19–28. [Google Scholar]
- Guy, R.H.; Hadgraft, J. On the determination of drug release rates from topical dosage forms. Int. J. Pharm. 1990, 60, R1–R3. [Google Scholar] [CrossRef]
- Sharrer, T. HeLa Herself. Scientist 2006, 20, 22–23. [Google Scholar]
- Wilson, V.G. Growth and differentiation of HaCat keratinocytes. Methods Mol. Biol. 2014, 1195, 33–41. [Google Scholar] [PubMed]
- Palamakula, A.; Khan, M.A. Evaluation of cytotoxicity of oils used in coenzyme Q10 Self-emulsifying Drug Delivery Systems (SEDDS). Int. J. Pharm. 2004, 273, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, N.; Garrigue, J.S.; Razafindratsita, A.; Lambert, G.; Benita, S. Excipient effects on in vitro cytotoxicity of a novel paclitaxel self-emulsifying drug delivery system. J. Pharm. Sci. 2003, 92, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Bak, I.; Czompa, A.; Csepányi, E.; Juhasz, B.; Kalantari, H.; Najm, K.; Aghel, K.; Varga, B.; Haines, D.D.; Tosaki, A. Evaluation of systematic and dermal toxicity and dermal photoprotection by sour cherry kernels. Phytother. Res. 2011, 25, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Tenhunen, R.; Marver, H.S.; Schmid, R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. USA 1968, 61, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.


| Composition | Emulgents | Cetostearyl Alcohol (4.6 g) Stearic Acid (10 g) Propylene Glycol (5 g) IPM (5 g) Nipagin M (1 g) Purified water (ad 100 g) | ||||||
|---|---|---|---|---|---|---|---|---|
| SM (5 g) | TC (14.2 g) | P60 (3 g) | CRC (3 g) | SP 50 (3 g) | SP 70 (3 g) | PS 750 (3 g) | ||
| I | + | + | + | |||||
| II | + | + | + | |||||
| III | + | + | + | |||||
| IV | + | + | + | |||||
| V | + | + | + | |||||
| VI | + | + | + | + | ||||
| VII | + | + | + | + | ||||
| VIII | + | + | + | + | ||||
| IX | + | + | + | + | ||||
| X | + | + | + | + | ||||
| Composition | Release Rate | Diffusion Coefficient |
|---|---|---|
| k·102 (µg/cm2·min½) ± S.D. | D 105 (cm2/min) ± S.D. | |
| I | 0.075 ± 0.12 | 0.21 ± 0.02 |
| II | 1.30 ± 0.32 | 0.73 ± 0.05 |
| III | 1.41 ± 0.33 | 1.36 ± 0.23 |
| IV | 2.01 ± 0.23 | 1.57 ± 0.25 |
| V | 4.24 ± 0.14 | 0.71 ± 0.23 |
| VI | 2.00 ± 0.31 | 0.93 ± 0.25 |
| VII | 5.94 ± 0.45 | 24.1 ± 0.24 * |
| VIII | 4.68 ± 0.26 | 47.23 ± 0.42 * |
| IX | 4.71 ± 0.13 | 30.22 ± 0.20 * |
| X | 3.99 ± 0.35 | 18.00 ± 0.13 * |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fehér, P.; Ujhelyi, Z.; Váradi, J.; Fenyvesi, F.; Róka, E.; Juhász, B.; Varga, B.; Bombicz, M.; Priksz, D.; Bácskay, I.; et al. Efficacy of Pre- and Post-Treatment by Topical Formulations Containing Dissolved and Suspended Silybum marianum against UVB-Induced Oxidative Stress in Guinea Pig and on HaCaT Keratinocytes. Molecules 2016, 21, 1269. https://doi.org/10.3390/molecules21101269
Fehér P, Ujhelyi Z, Váradi J, Fenyvesi F, Róka E, Juhász B, Varga B, Bombicz M, Priksz D, Bácskay I, et al. Efficacy of Pre- and Post-Treatment by Topical Formulations Containing Dissolved and Suspended Silybum marianum against UVB-Induced Oxidative Stress in Guinea Pig and on HaCaT Keratinocytes. Molecules. 2016; 21(10):1269. https://doi.org/10.3390/molecules21101269
Chicago/Turabian StyleFehér, Pálma, Zoltán Ujhelyi, Judit Váradi, Ferenc Fenyvesi, Eszter Róka, Béla Juhász, Balázs Varga, Mariann Bombicz, Dániel Priksz, Ildikó Bácskay, and et al. 2016. "Efficacy of Pre- and Post-Treatment by Topical Formulations Containing Dissolved and Suspended Silybum marianum against UVB-Induced Oxidative Stress in Guinea Pig and on HaCaT Keratinocytes" Molecules 21, no. 10: 1269. https://doi.org/10.3390/molecules21101269
APA StyleFehér, P., Ujhelyi, Z., Váradi, J., Fenyvesi, F., Róka, E., Juhász, B., Varga, B., Bombicz, M., Priksz, D., Bácskay, I., & Vecsernyés, M. (2016). Efficacy of Pre- and Post-Treatment by Topical Formulations Containing Dissolved and Suspended Silybum marianum against UVB-Induced Oxidative Stress in Guinea Pig and on HaCaT Keratinocytes. Molecules, 21(10), 1269. https://doi.org/10.3390/molecules21101269