Synthesis and Pharmacological Evaluation of Novel Benzenesulfonamide Derivatives as Potential Anticonvulsant Agents
Abstract
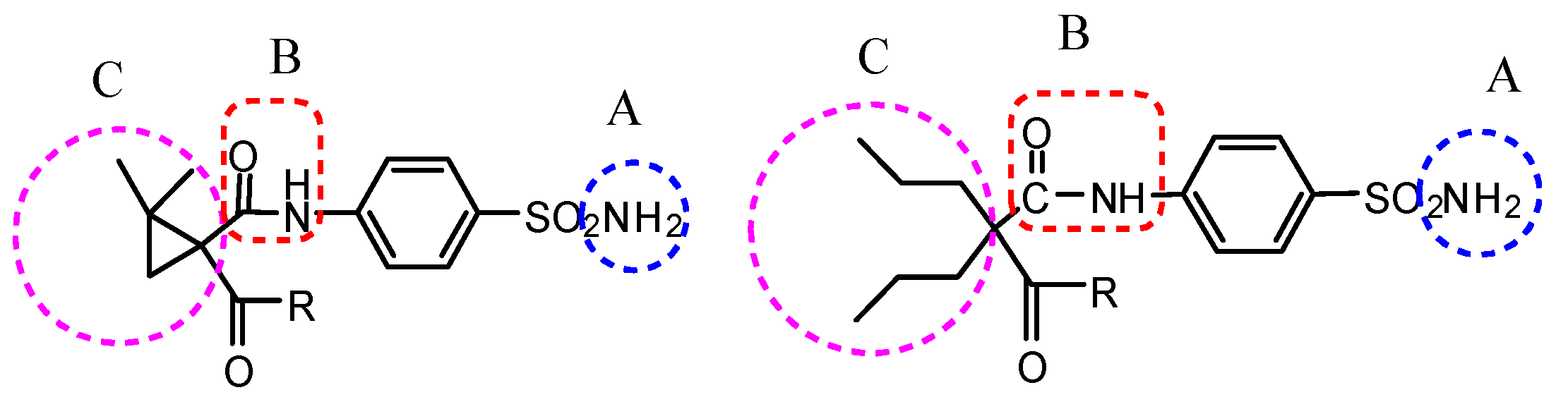
:1. Introduction
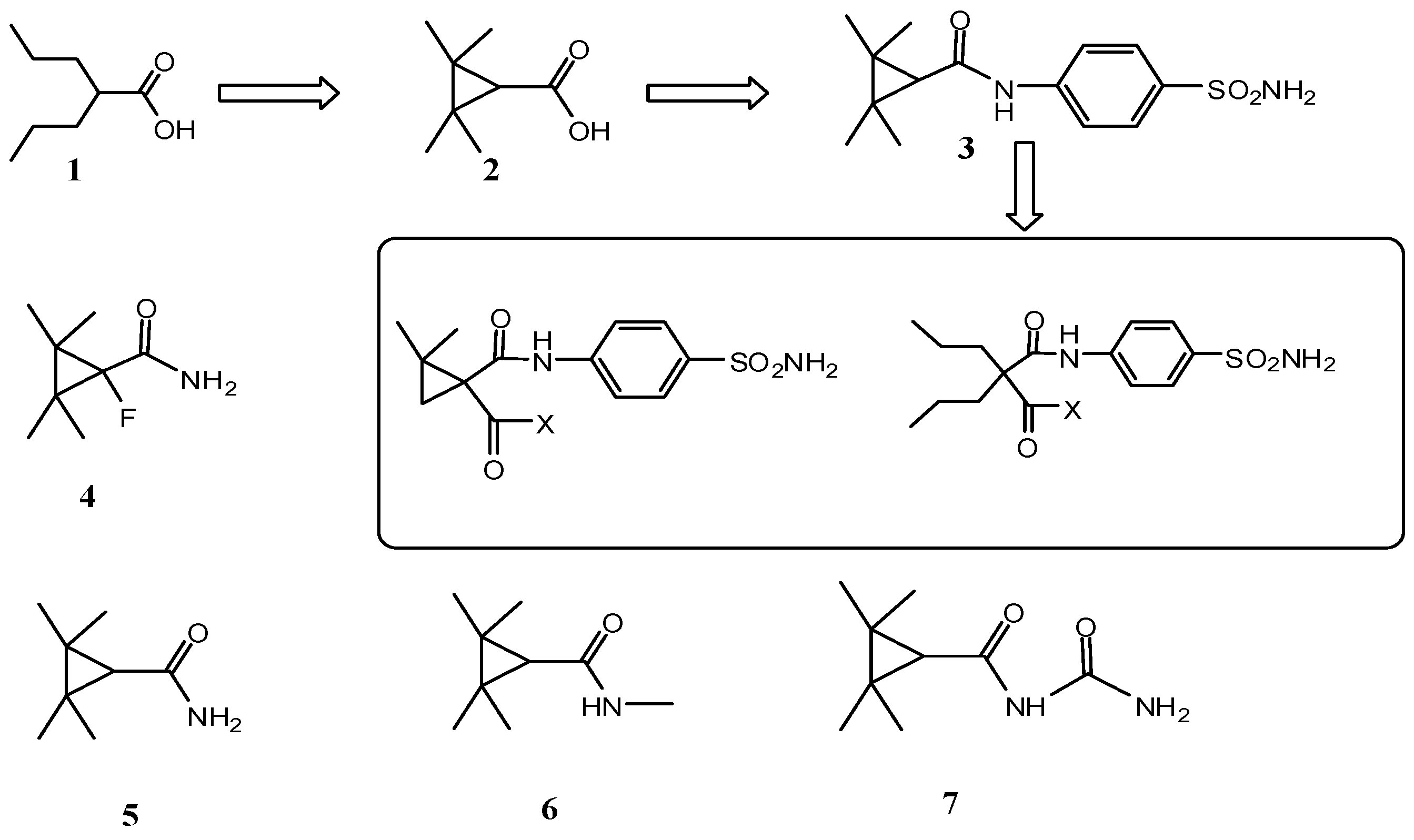
2. Results and Discussion
2.1. Chemistry
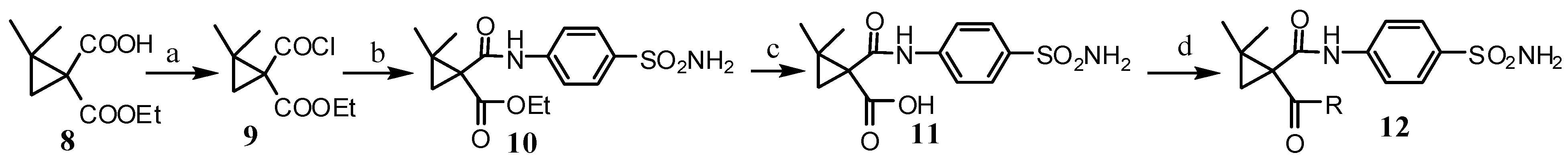
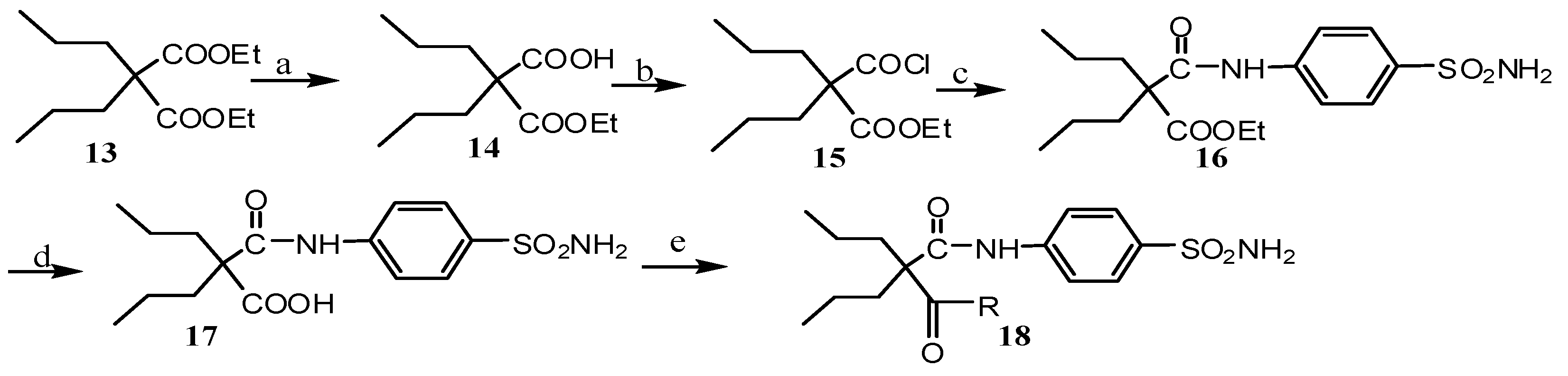
2.2. Pharmacological Evaluation
| Compounds | Intraperitioneal Injection into Mice a | ClogP b | ||||||
|---|---|---|---|---|---|---|---|---|
| MES c | scPTZ d | Neurotoxocity e | ||||||
| No. | R | 0.5 h | 4 h | 0.5 h | 4 h | 0.5 h | 4 h | |
| 10 | -OEt | 100 | - f | 300 | - | 300 | - | 1.633 |
| 11 | -OH | 100 | - | - | - | 300 | - | 0.865 |
| 12a | -NHC(CH3)3 | 100 | - | - | - | - | - | 1.484 |
| 12b | -N(CH2CH3)2 | 100 | 300 | - | - | 300 | - | 1.735 |
| 12c | -NH2 | 30 | 100 | 30 | 100 | 300 | - | −0.019 |
| 12d | -NHCH3 | 30 | 300 | 100 | 300 | - | - | 0.247 |
| 12e |  | 100 | - | - | - | - | - | 1.305 |
| 12f | 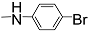 | 300 | - | - | - | 300 | - | 3.223 |
| 12g |  | 300 | 300 | - | - | - | - | 0.797 |
| 12h | 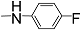 | 100 | 300 | 300 | - | - | - | 2.503 |
| 12i | 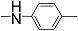 | 100 | - | - | - | - | - | 2.604 |
| 12j | 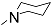 | 100 | - | - | - | - | - | 1.591 |
| 12k |  | 100 | - | 300 | - | 300 | - | 1.834 |
| 12l | 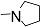 | - | - | - | - | - | - | 1.032 |
| 12m | 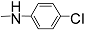 | 300 | - | - | - | - | - | 3.071 |
| 12n | 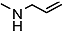 | 100 | 300 | - | - | - | - | 1.021 |
| 16 | -OEt | 300 | - | - | - | 300 | - | 2.815 |
| 17 | -OH | 100 | - | 300 | - | 300 | - | 2.046 |
| 18a | 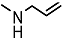 | 100 | 300 | - | - | - | - | 2.203 |
| 18b | -NH2 | 30 | 30 | - | - | 300 | - | 1.163 |
| 18c | 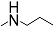 | 100 | 100 | - | - | - | - | 2.487 |
| Phenytoin g | 30 | 30 | - | - | 100 | 100 | ||
| Ethosuximide h | - | - | 100 | 300 | - | - | ||
| Compound | ED50 a | TD50 b | PI c | ||
|---|---|---|---|---|---|
| MES | scPTZ | MES | scPTZ | ||
| 12c | 24.47 (20.05–29.83) d | 22.50 (16.25–31.14) | 499.2 (455.3–547.4) | 20.4 | 22.19 |
| 12d | 25.25 (18.14–35.14) | ND e | >500 | >19.80 | ND e |
| 18b | 16.36 (14.17–18.89) | ND e | 406.7 (337.7–489.7) | 24.85 | ND e |
| phenytoin f | 9.5 (8.1–10.4) | >300 | 65.5 | 6.9 | <0.22 |
| valproate f | 272 | 149 | 426 | 1.6 | 2.9 |
| Compound | Dose a | Administration Route | |
|---|---|---|---|
| i.g b | i.p c | ||
| 12c | 2000 | 0/5 d | 4/5 |
| 500 | ND e | 1/5 | |
| 50 | ND | 0/5 | |
| 12d | 2000 | 0/5 | 5/5 |
| 500 | ND | 1/5 | |
| 50 | ND | 0/5 | |
| 18b | 2000 | 0/5 | 5/5 |
| 500 | ND | 2/5 | |
| 50 | ND | 0/5 | |
| Compound | LD50 a |
|---|---|
| 12c | 762.7 (656.8–885.6) |
| 12d | 922.0 (601.1–1414) |
| 18b | 638.0 (475.0–857.0) |
| phenytoin | 100 (94.3–101.2) |
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.2.1. Ethyl 1-(Chlorocarbonyl)-2,2dimethylcyclopropanecarboxylate 10
3.2.2. 2,2-Dimethyl-1-(4-sulfamoylphenylcarbamoyl)cyclopropanecarboxylic Acid 11
3.2.3. General Produce for the Synthesis of Compounds 12a–n
3.2.4. Ethyl 2-Propyl-2-(4-sulfamoylphenylcarbamoyl)pentanoate 16
3.2.5. 2-Propyl-2-(4-sulfamoylphenylcarbamoyl)pentanoic Acid 17
3.2.6. General Produce for the Synthesis of Compounds 18a–c
3.3. Pharmacology
3.3.1. Preparation of the Compounds for Testing
3.3.2. MES Test
3.3.3. scPTZ Test
3.3.4. Neurotoxicity Screening
3.3.5. Calculation of ClogP
3.3.6. Quntification Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gitto, R.; Caruso, R.; Pagano, B.; de Luca, L.; Citraro, R. Novel potent anticonvulsant agent containing a tetrahydroisoquinoline skeleton. J. Med. Chem. 2006, 49, 5618–5622. [Google Scholar] [CrossRef] [PubMed]
- Shank, R.P.; Gardocki, J.F.; Streeter, A.J. An overview of the preclinical aspects of topiramate: Pharmacology, pharmacokinetics, and mechanism of action. Epilepsia 2000, 41, 3–9. [Google Scholar] [CrossRef]
- Castel-Branco, M.M.; Alves, G.L.; Figueiredo, I.V. The maximal electroshock seizure (MES) model in the preclinical assessment of potential new antiepileptic drugs. Method Find. Exp. Clin. Pharmacol. 2009, 31, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.Q.; Song, M.X.; Zheng, Y.; Quan, Z.S. Design, synthesis and evaluation of the antide-pressant and anticonvulsant activities of triazole-containing quinolinones. Eur. J. Med. Chem. 2014, 73, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Zhang, L.; Wei, C.X.; Piao, H.R.; Quan, Z.S. Design, synthesis of 8-alkoxy-5,6-dihydro-[1,2,4]triazino[4,3-a]quinolin-1-ones with anticonvulsant activity. Eur. J. Med. Chem. 2009, 44, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.B.; Jin, P.; Li, F.N.; Quan, Z.S. Synthesis and anticonvulsant activity of novel purine derivatives. Eur. J. Med. Chem. 2014, 84, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Challal, S.; Buenafe, O.E.; Queiroz, E.F.; Maljevic, S.; Crawford, A.D. Zebrafish bioassay-guided micro-fractionation identifies anticonvulsant steroid glycosides from the Philippine medicinal plant solanum torvum. ACS Chem. Neurosci. 2014, 5, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- White, H.S. Clinical significance of animal seizure models and mechanism of action studies of Potential antiepileptic drugs. Epilepsia 1997, 38, 9–17. [Google Scholar] [CrossRef]
- White, H.S. Preclinical development of antiepileptic drugs: past, present, and future directions. Epilepsia 2003, 44, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Merritt, H.H.; Putnam, T.J. A new series of anticonvulsant drugs tested by experiments on animals. Arch. Neurol. Psychiatry 1938, 39, 1003–1015. [Google Scholar] [CrossRef]
- Nau, H.; Hauck, R.S.; Ehlers, K. Valproic acid-induced neural tube defects in mouse and human: Aspects of chirality, alternative drug development, pharmacokinetics and possible mechanisms. Pharmacol. Toxicol. 1991, 69, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Battino, D.; Andermann, E.; Wada, K. Congenital malformations due to antiepileptic drugs. Epilepsy Res. 1999, 33, 145–158. [Google Scholar] [CrossRef]
- Chang, T.K.; Abbott, F.S. Oxidative stress as a mechanism of valproic acid-associated hepatotoxicity. Drug Metab. Rev. 2006, 38, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Bojic, U.; Elmazar, M.M.A.; Hauck, R.S.; Nau, H. Further branching of valproate-related carboxylic acids reduces the teratogenic activity, but not the anticonvulsant effect. Chem. Res. Toxicol. 1996, 9, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; Shear, N.H.; Jacobson-Brown, P.M. CYP2E1 mediated modulation of valproic acid-induced hepatocytotoxicity. Clin. Bio. Chem. 2001, 34, 211–218. [Google Scholar] [CrossRef]
- Bojic, U.; Ehlers, K.; Ellerbeck, U.; Bacon, C.L.; Nau, H. Studies on the teratogen pharmaco-phore of valproic acid analogues: Evidence of interactions at a hydrophobic centre. Eur. J. Pharmacol. 1998, 354, 289–299. [Google Scholar] [CrossRef]
- Gravemann, U.; Volland, J.; Nau, H. Hydroxamic acid and fluorinated derivatives of valproic acid: Anticonvulsant activity. Neurotoxicol. Teratol. 2008, 30, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Eyal, S.; Meir, B.; Boris, Y. Tetramethylcyclopropyl analogue of a leading antiepileptic drug, valproic acid. Synthesis and evaluation of anticonvulsant activity of its amide derivatives. J. Med. Chem. 2004, 47, 4316–4326. [Google Scholar]
- Shimshoni, J.A.; Bialer, M.; Yagen, B. Synthesis and anticonvulsant activity of aromatic tetramethylcyclopropane-carboxamide derivatives. Bioorg. Med. Chem. 2008, 16, 6297–6305. [Google Scholar] [CrossRef] [PubMed]
- Neta, P.; Meir, B.; Bogdan, W.; Richard, H.F.; Boris, Y. α-Fluoro-2,2,3,3-tetra methylcyclo-propanecarboxamide, a novel potent anticonvulsant derivative of a cyclic analogue of valproic acid. J. Med. Chem. 2009, 52, 2233–2242. [Google Scholar]
- He, X.; Qiu, G.; Yang, J.; Xiao, Y. Synthesis and anticonvulsant activity of new 6-methyl-1-substituted-4,6-diazaspiro[2.4]heptane-5,7-diones. Eur. J. Med. Chem. 2010, 45, 3818–3830. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhong, M.; Zhang, T.; Yang, J. Synthesis and anticonvulsant activity of 1-(8-(benzyloxy) quinolin-2-yl)-6-substituted-4,6-diazaspiro[2,4]heptane-5,7-diones. Eur. J. Med. Chem. 2012, 48, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Pan, Y.; Xu, Z.; Li, R. Synthesis and potential anticonvulsant activity of new N-3-substituted 5,5-cyclopropanespirohydantoins. Eur. J. Med. Chem. 2009, 44, 296–302. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhong, M.; Zhang, T.; Wu, W. Synthesis and anticonvulsant activity of ethyl 1-(2-arylhydrazine carboxa-mido)-2,2-dimethylcyclopropanecarboxylate derivatives. Eur. J. Med. Chem. 2012, 54, 542–548. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhong, M.; Yang, J.; Wu, Z. Synthesis and anticonvulsant activity of 1-(2-(8-(benzyloxy)quinolin-2-yl)-1-butyrylcyclopropyl)-3-substituted urea derivatives. Chem. Biol. Drug Des. 2012, 79, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Zhang, Y.; He, X. Synthesis and anticonvulsant activity of ethyl 2,2-dimethyl-1-(2-substitute-dhydrazinecarboxamido) cyclopropanecarboxylate derivatives. Chem. Biol. Drug Des. 2014, 84, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Harish, K.P.; Mohana, K.N.; Mallesha, L. Synthesis of novel 1-[5-(4-methoxy-phenyl)-[1,3,4]oxadiazol-2-yl]-piperazine derivatives and evaluation of their in vivo anticonvulsant activity. Eur. J. Med. Chem. 2013, 65, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Swinyard, E.A. Laboratory evaluation of antiepileptic drugs. Epilepsia 1969, 10, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Dunham, N.W.; Miya, T.A. A note on a simple apparatus for detecting neurological deficit in rat and mice. J. Am. Pharm. Assoc. Sci. 1957, 46, 208–209. [Google Scholar] [CrossRef]
- Dimmock, J.R.; Pandey, S.N.; Quail, J.W. Evaluation of the semicarbazones, thiosemicarbazones and biscarbohydrazones of some aryl alicyclic ketones for anticonvulsant and other biological properties. Eur. J. Med. Chem. 1995, 30, 303–314. [Google Scholar] [CrossRef]
- Rajak, H.; Deshmukh, R.; Aggarwal, N. Synthesis of novel 2,5-disubstituted 1,3,4-thiadiazoles for their potential anticonvulsant activity: Pharmacophoric model studies. Arch. Pharm. 2009, 342, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Fraser, H.F.; Wikler, A.; Essig, C.F. Degree of physical dependence induced by secobarbital or pentobarbital. J. Am. Med. Assoc. 1958, 166, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Ucar, H.; Kim, V.D.; Cacciaguerra, S.; Spampinato, S. Synthesis and anticonvulsant activity of 2 (3H)-benzoxazolone and 2 (3H)-benzothiazolone derivatives. J. Med. Chem. 1998, 41, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; El-Gnidi, B.A.; Alkskas, I.A. Combating oxidative stress in epilepsy: design, synthesis, quantum chemical studies and anticonvulsant evaluation of 1-(substituted benzylidene/ethylidene)-4-(naphthalen-1-yl)semicarbazides. Eur. J. Med. Chem. 2010, 45, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Swinyard, E.A.; Brown, W.C.; Goodman, L.S. Comparative assays of antiepileptic drugs in mice and rats. J. Pharmacol. Exp. Ther. 1952, 106, 319–330. [Google Scholar] [PubMed]
- Vamecq, J.; Lambert, D.; Poupaert, J.H.; Masereel, B.; Stables, J.P. Anticonvulsant activity and interactions with neuronal voltage-dependent sodium channel of analogues of ameltolide. J. Med. Chem. 1998, 41, 3307–3313. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, J.; Zeng, X.-D.; Hu, X.-M.; Zhou, X.; Hong, X. Synthesis and Pharmacological Evaluation of Novel Benzenesulfonamide Derivatives as Potential Anticonvulsant Agents. Molecules 2015, 20, 17585-17600. https://doi.org/10.3390/molecules200917585
Wang Z, Li J, Zeng X-D, Hu X-M, Zhou X, Hong X. Synthesis and Pharmacological Evaluation of Novel Benzenesulfonamide Derivatives as Potential Anticonvulsant Agents. Molecules. 2015; 20(9):17585-17600. https://doi.org/10.3390/molecules200917585
Chicago/Turabian StyleWang, Zhiming, Jinping Li, Xiao-Dong Zeng, Xian-Ming Hu, Xiaoju Zhou, and Xuechuan Hong. 2015. "Synthesis and Pharmacological Evaluation of Novel Benzenesulfonamide Derivatives as Potential Anticonvulsant Agents" Molecules 20, no. 9: 17585-17600. https://doi.org/10.3390/molecules200917585
APA StyleWang, Z., Li, J., Zeng, X.-D., Hu, X.-M., Zhou, X., & Hong, X. (2015). Synthesis and Pharmacological Evaluation of Novel Benzenesulfonamide Derivatives as Potential Anticonvulsant Agents. Molecules, 20(9), 17585-17600. https://doi.org/10.3390/molecules200917585






