Synthesis of Biscoumarin and Dihydropyran Derivatives and Evaluation of Their Antibacterial Activity
Abstract
:1. Introduction
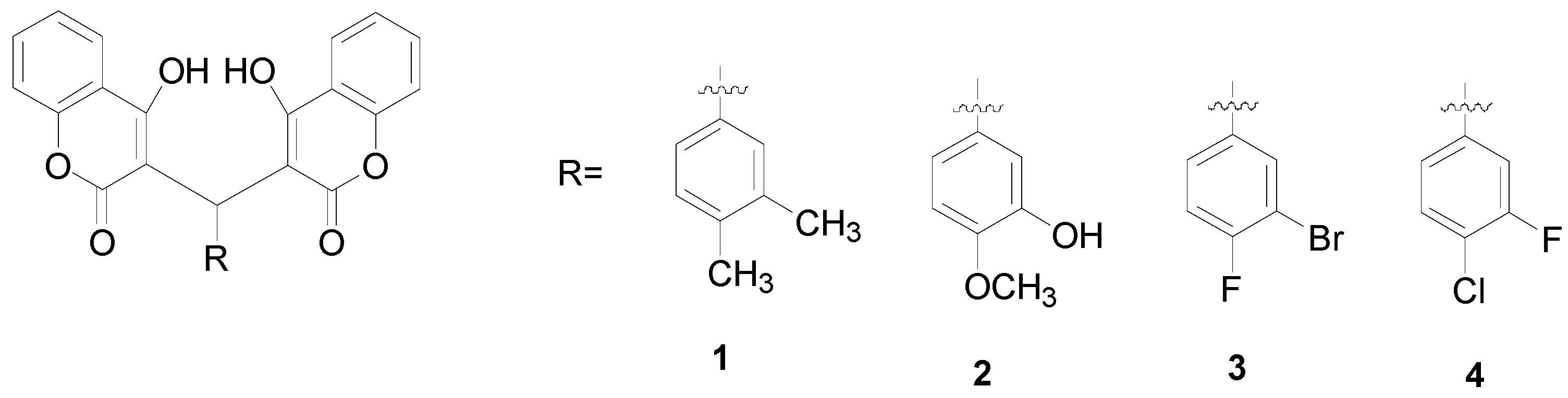
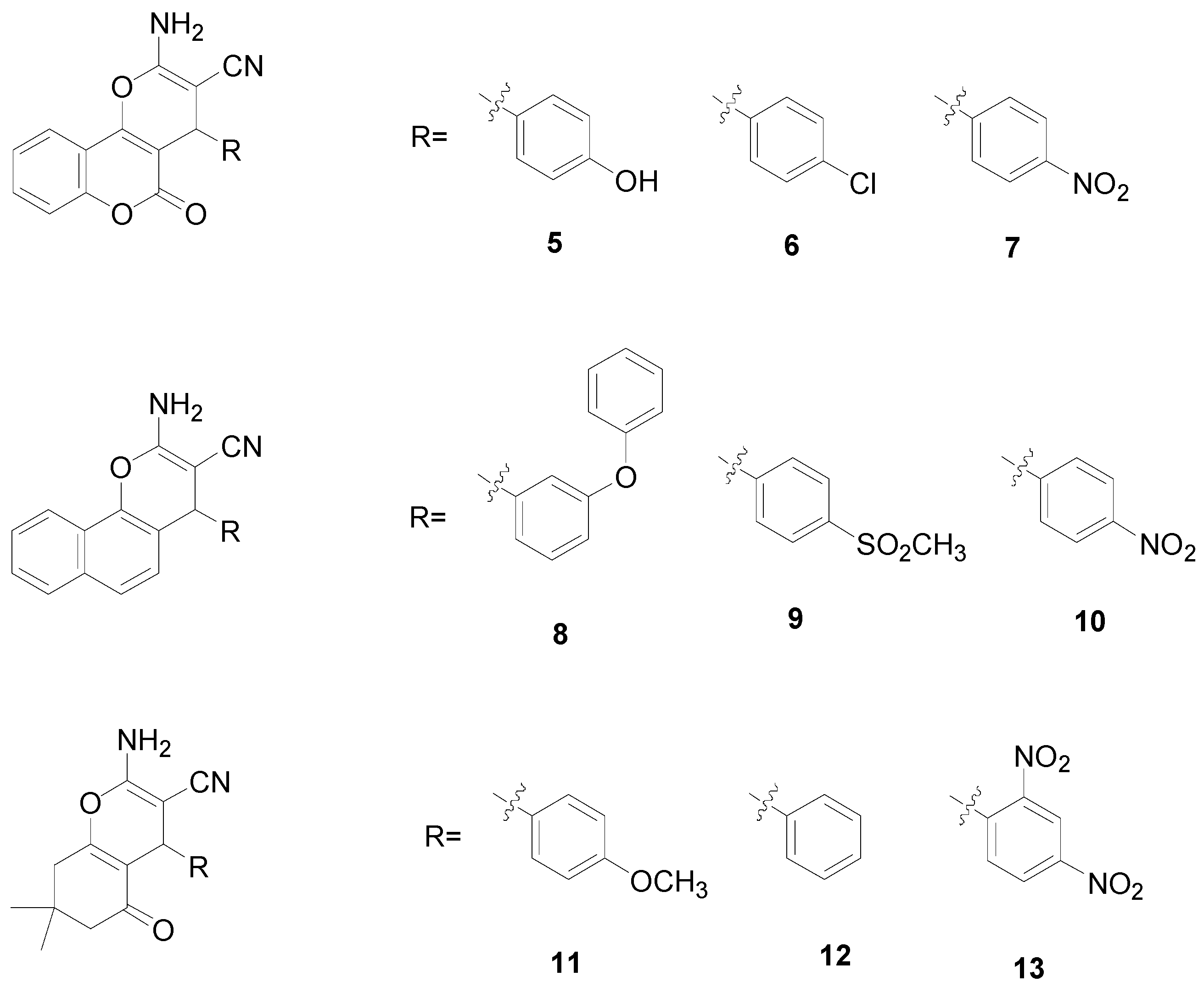
2. Results and Discussion
2.1. Molecular Structure
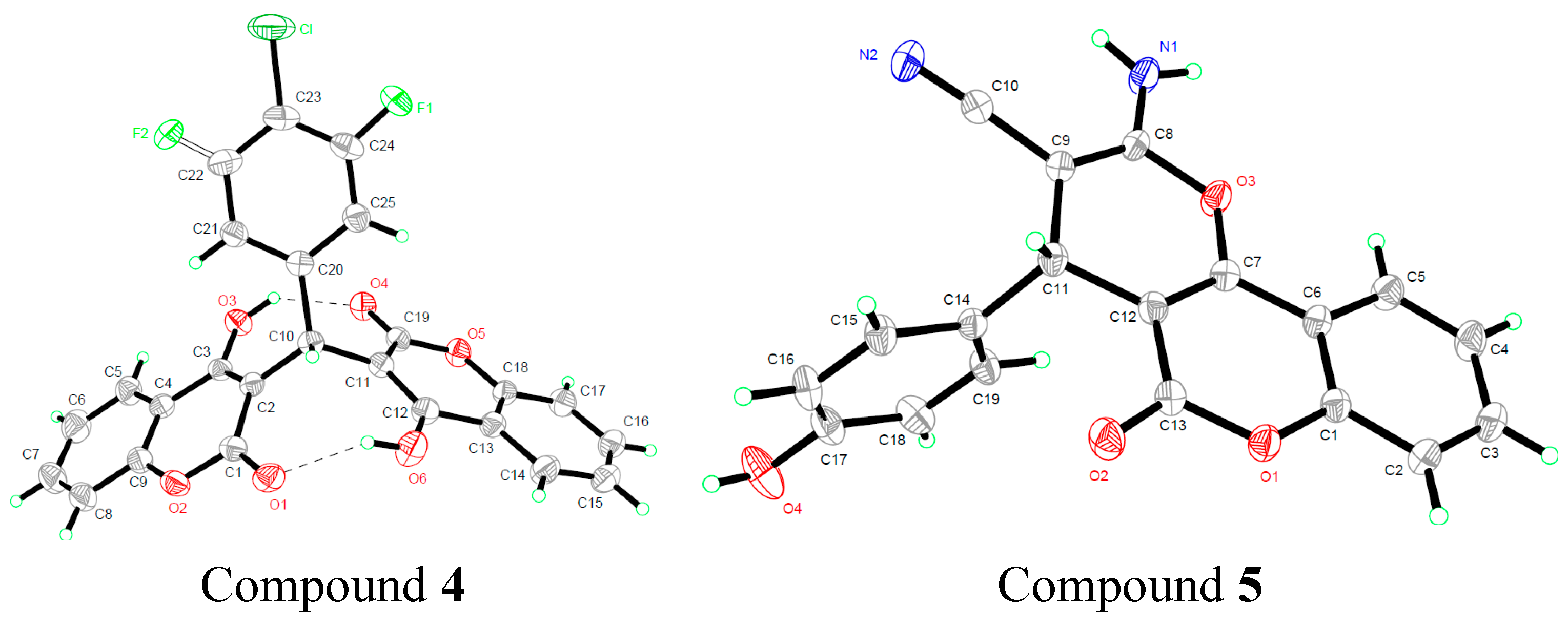

2.2. Quantum Chemical Calculations
2.2.1. Geometric Parameters of Compounds 1–4
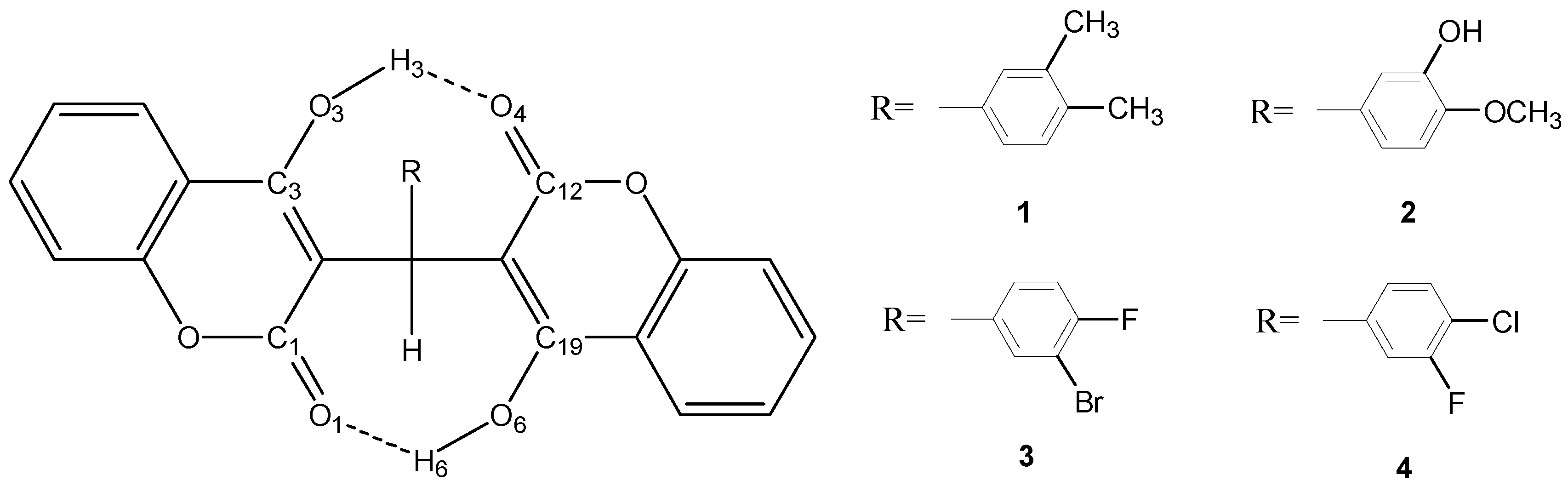
| X-ray | 6-31G* | 6-31+G** | 6-311G* | |
|---|---|---|---|---|
| R(C1—O1) | 1.224 | 1.234 | 1.236 | 1.226 |
| R(C1—O2) | 1.35 | 1.372 | 1.37 | 1.371 |
| R(C19—O4) | 1.221 | 1.23 | 1.233 | 1.223 |
| R(C19—O5) | 1.362 | 1.375 | 1.372 | 1.374 |
| R(C2—C10) | 1.508 | 1.526 | 1.526 | 1.526 |
| R(C10—C11) | 1.516 | 1.525 | 1.524 | 1.524 |
| R(C10—C20) | 1.536 | 1.539 | 1.539 | 1.537 |
| A(C1—O2—C9) | 121.11 | 121.67 | 121.7 | 121.7 |
| A(O1—C1—O2) | 116.05 | 115.7 | 115.9 | 115.95 |
| A(C18—O5—C19) | 121.34 | 121.9 | 121.89 | 121.92 |
| A(O4—C19—O5) | 114.64 | 115.67 | 115.85 | 115.96 |
| A(C2—C10—C20) | 114.84 | 115.8 | 115.94 | 115.88 |
| A(C2—C10—C11) | 113.67 | 112.59 | 112.77 | 112.68 |
| A(C11—C10—C20) | 113.79 | 114.51 | 114.61 | 114.57 |
| D(C1—C2—C10—C20) | 137.5 | 133.73 | 133.05 | 133.63 |
| D(C1—C2—C10—C11) | 89.01 | 91.83 | 92.01 | 91.65 |
| D(C12—C11—C10—C20) | 129.87 | 131.31 | 130.59 | 130.23 |
| D(C12—C11—C10—C2) | 96.14 | 93.64 | 93.85 | 94.44 |
2.2.2. Estimation of the Single and Total HB Energies in Compounds 1–4
| System | Total Electronic Energies a | E(O6—H6···O1) | E(O3—H3···O4) | E(Total HB) |
|---|---|---|---|---|
| 1ab | −1452.593097 | −119.118935 | ||
| 1a | −1491.908094 | −52.4391115 | ||
| 1b | −1491.888121 | −67.1051545 | ||
| 2ab | −1603.031177 | −122.1776425 | ||
| 2a | −1603.011081 | −52.762048 | ||
| 2b | −1603.004738 | −69.4155945 | ||
| 3ab | −4083.681309 | −116.1757495 | ||
| 3a | −4083.66182 | −51.1683695 | ||
| 3b | −4083.656549 | −65.00738 | ||
| 4ab | −1972.171758 | −116.8373755 | ||
| 4a | −1972.15218 | −51.402039 | ||
| 4b | −1972.146835 | −65.4353365 |
2.3. Minimal Inhibitory Concentration (MIC) Assay
| Drugs | MIC (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| S. aureas | MRSA | Mu50 | LAC | S. epidermidis | MRSE | E. Coli | |
| Compound 1 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| Compound 2 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| Compound 3 | 16 | 16 | 8 | 8 | 8 | 8 | >256 |
| Compound 4 | 8 | 8 | 8 | 8 | 8 | 8 | >256 |
| Compound 5 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| Compound 6 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| Compound 7 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| Compound 8 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| Compound 9 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| Compound 10 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| Compound 11 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| Compound 12 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| Compound 13 | >256 | >256 | >256 | >256 | >256 | >256 | >256 |
| Levofloxacin | <0.125 (S) | 4 (R) | 4 (R) | 8 (R) | 0.125 (S) | 0.125 (S) | 0.25 (S) |
| Ceftazidime | 8 (S) | >256 (R) | 256 (R) | 64 (R) | 1 (S) | 256 (R) | 0.25 (S) |
| Ceftriaxone | 2 (S) | >256 (R) | 256 (R) | 32 (R) | 1 (S) | 256 (S) | 0.25 (S) |
| Gentamicin | 0.12 (S) | 64 (R) | 32 (R) | 0.25 (S) | 0.25 (S) | 32 (R) | 2 (S) |
| Piperacillin | 2 (S) | >128 (R) | >128 (R) | 8 (R) | 2 (S) | >128 (R) | 2 (S) |
| Vancomycin | 0.25 (S) | 8 (I) | 8 (I) | 0.5 (S) | 0.25 (S) | 0.5 (S) | >256 (R) |
2.4. In Vitro Toxicity Measurement
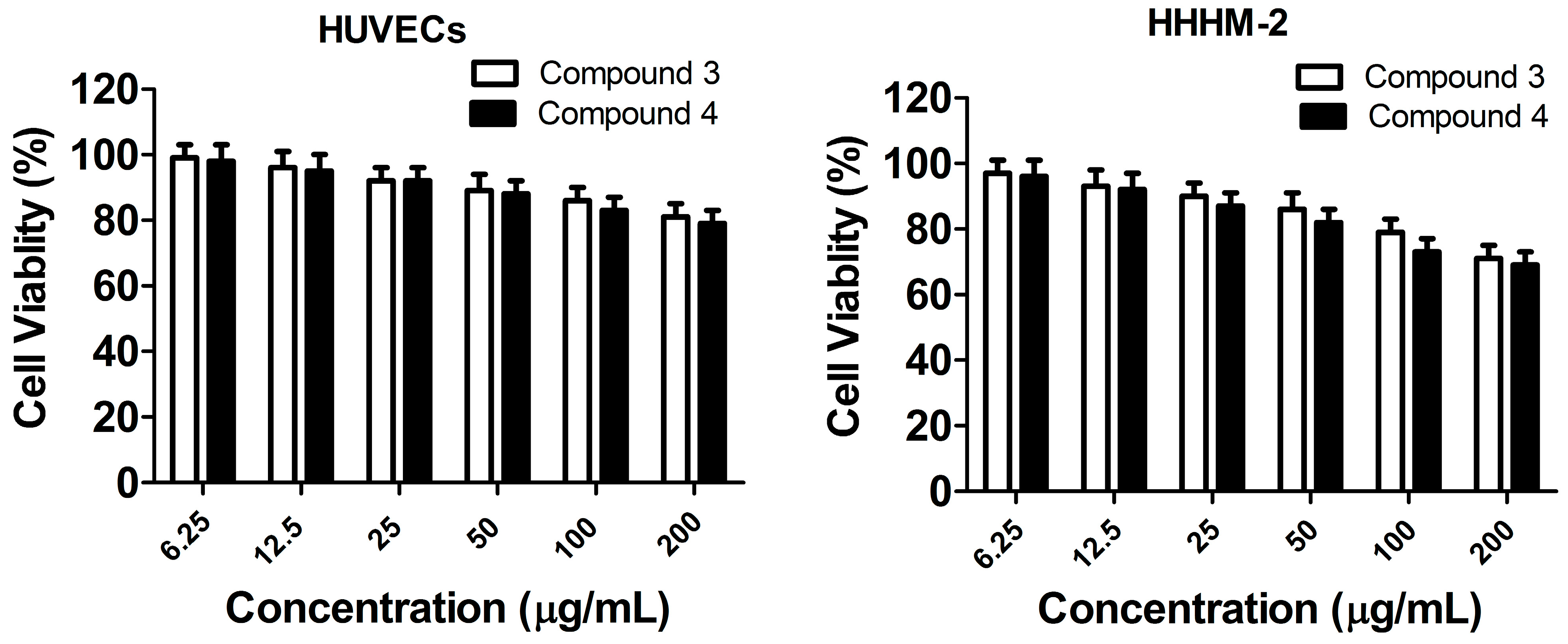
2.5. Discussion
3. Experimental Section
3.1. Apparatus and Materials
3.2. Synthesis and Characterization of Compounds 1–13
3.3. X-ray Crystallography
| Compound 4 | Compound 5 | Compound 8 | Compound 12 | |
|---|---|---|---|---|
| Formula | C25H14ClFO6 | C19H12N2O4 | C26H18N2O2 | C18H18N2O2 |
| Mr | 464.0463 | 332.0797 | 390.1368 | 294.1368 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c | P21/c | P21/c |
| a/Å | 10.1874(7) | 8.8632(6) | 11.908(3) | 11.2938(5) |
| b/Å | 10.1667(6) | 13.1743(8) | 15.4731(17) | 9.4591(5) |
| c/Å | 20.0790(12) | 13.0565(6) | 11.9343(18) | 14.9132(7) |
| α/° | 90 | 90 | 90 | 90 |
| β/° | 96.571(7) | 94.484(6) | 116.15(2) | 99.304(4) |
| γ/° | 90 | 90 | 90 | 90 |
| V/Å3 | 2066.0(2) | 1519.89(15) | 1973.9(6) | 1572.21(13) |
| Z | 4 | 4 | 4 | 4 |
| Dcalc/g·cm−3 | 1.491 | 1.452 | 1.314 | 1.244 |
| μ(Mo Kα)/mm−1 | 0.236 | 0.104 | 0.084 | 0.082 |
| θ range/° | 2.70 to 25.00 | 2.78 to 25.00 | 2.63 to 25.00 | 2.56 to 25.00 |
| Reflections collected | 7346 | 5980 | 4969 | 6337 |
| No. unique data [R(int)] | 3621 [0.0256] | 2670 [0.0263] | 3099 [0.0186] | 2775 [0.0280] |
| No. data with I ≥ 2σ(I) | 2293 | 1991 | 2029 | 2109 |
| R1 | 0.0501 | 0.0453 | 0.0542 | 0.0465 |
| ωR2(all data) | 0.1704 | 0.1559 | 0.1561 | 0.1627 |
| CCDC | 1032644 | 1032645 | 1032646 | 1032647 |
3.4. Quantum Chemical Calculations
3.5. Minimal Inhibitory Concentration (MIC) Assay
3.6. Cytotoxicity Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dukic, V.M.; Lauderdale, D.S.; Wilder, J.; Daum, R.S.; David, M.Z. Epidemics of Community-Associated Methicillin-Resistant Staphylococcus aureus in the United States: A Meta-Analysis. PLoS ONE 2013, 8, e52722. [Google Scholar] [CrossRef] [PubMed]
- Sandora, T.J.; Goldmann, D.A. Preventing Lethal Hospital Outbreaks of Antibiotic-Resistant Bacteria. N. Engl. J. Med. 2012, 367, 2168–2170. [Google Scholar] [CrossRef] [PubMed]
- Frisina, P.G.; Kutlik, A.M.; Ann, M.; Barrett, A.M. Left-Sided Brain Injury Associated With More Hospital-Acquired Infections During Inpatient Rehabilitation Original Research Article. Arch. Phys. Med. Rehab. 2013, 94, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Climo, M.W.; Yokoe, D.S.; Warren, D.K.; Perl, T.M.; Bolon, M.; Herwaldt, L.A.; Weinstein, R.A.; Sepkowitz, K.A.; Jernigan, J.A.; Sanogo, K.; et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N. Engl. J. Med. 2013, 368, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Natural and Synthetic Coumarin Derivatives with Anti-Inflammatory/Antioxidant Activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.S.; Mallireddigari, M.R.; Cosenza, S.; Gumireddy, K.; Bell, S.C.; Reddy, E.P.; Reddy, M.V.R. Synthesis of new coumarin 3-(N-aryl) sulfonamides and their anticancer activity. Bioorg. Med. Chem. Lett. 2004, 14, 4093–4097. [Google Scholar] [CrossRef] [PubMed]
- Sardari, S.; Mori, Y.; Horita, K.; Micetich, R.G.; Nishibe, S.; Daneshtalab, M. Synthesis and antifungal activity of coumarins and angular furanocoumarins. Bioorg. Med. Chem. 1999, 7, 1933–1940. [Google Scholar] [CrossRef]
- Leal, L.K.A.M.; Ferreira, A.A.G.; Bezerra, G.A.; Matos, F.J.A.; Viana, G.S.B. Antinociceptive, anti-inflammatory and bronchodilator activities of Brazilian medicinal plants containing coumarin: A comparative study. J. Ethnopharmacol. 2000, 70, 151–159. [Google Scholar] [CrossRef]
- Kontogiorgis, C.A.; Hadjipavlou-Litina, D.J. Synthesis and Antiinflammatory Activity of Coumarin Derivatives. J. Med. Chem. 2005, 48, 6400–6408. [Google Scholar] [CrossRef] [PubMed]
- Manolov, I.; Maichle-Moessmer, C.; Danchev, N. Synthesis, structure, toxicological and pharmacological investigations of 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2006, 41, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Ojala, T.; Remes, S.; Haansuu, P.; Vuorela, H.; Hiltunen, R.; Haahtela, K.; Vuorela, P. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J. Ethnopharmacol. 2000, 73, 299–305. [Google Scholar] [CrossRef]
- Trendafilova, N.; Bauer, G.; Mihaylov, T. DFT and AIM studies of intramolecular hydrogen bonds in dicoumarols. Chem. Phys. 2004, 302, 95–104. [Google Scholar] [CrossRef]
- Mihaylov, T.; Georgieva, I.; Bauer, G.; Kostova, I.; Manolov, I.; Trendafilova, N. Theoretical study of the substituent effect on the intramolecular hydrogen bonds in di(4-hydroxycoumarin) derivatives. Int. J. Quantum Chem. 2006, 106, 1304–1315. [Google Scholar] [CrossRef]
- Schiøtt, B.; Iversen, B.B.; Madsen, G.K.H.; Bruice, T.C. Characterization of the short Strong hydrogen bond in benzoylacetone by ab initio calculations and accurate diffraction experiments. Implications for the electronic nature of low-barrier hydrogen bonds in enzymatic reactions. J. Am. Chem. Soc. 1998, 120, 12117–12124. [Google Scholar]
- Khan, K.M.; Iqbal, S.; Lodhi, M.A.; Maharvi, G.M.; Zia-Ullah; Choudhary, M.I.; Atta-ur-Rahman; Perveen, S. Biscoumarin: New class of urease inhibitors; economical synthesis and activity. Bioorg. Med. Chem. 2004, 12, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.T.; Lal, M.; Ali, S.; Khan, Md.M. One-pot three-component reaction for the synthesis of pyran annulated heterocyclic compounds using DMAP as a catalyst. Tetrahedron Lett. 2011, 52, 5327–5332. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97, Program for Solution Crystal Structure and Refinement. University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Nineteenth Informational Supplement M100-S19; Wayne, PA, USA, 2009. [Google Scholar]
- Sample Availability: Samples of the compounds 1–13 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Lv, C.-W.; Li, X.-J.; Qu, D.; Hou, Z.; Jia, M.; Luo, X.-X.; Li, X.; Li, M.-K. Synthesis of Biscoumarin and Dihydropyran Derivatives and Evaluation of Their Antibacterial Activity. Molecules 2015, 20, 17469-17482. https://doi.org/10.3390/molecules200917469
Li J, Lv C-W, Li X-J, Qu D, Hou Z, Jia M, Luo X-X, Li X, Li M-K. Synthesis of Biscoumarin and Dihydropyran Derivatives and Evaluation of Their Antibacterial Activity. Molecules. 2015; 20(9):17469-17482. https://doi.org/10.3390/molecules200917469
Chicago/Turabian StyleLi, Jing, Chang-Wei Lv, Xiao-Jun Li, Di Qu, Zheng Hou, Min Jia, Xiao-Xing Luo, Xia Li, and Ming-Kai Li. 2015. "Synthesis of Biscoumarin and Dihydropyran Derivatives and Evaluation of Their Antibacterial Activity" Molecules 20, no. 9: 17469-17482. https://doi.org/10.3390/molecules200917469
APA StyleLi, J., Lv, C.-W., Li, X.-J., Qu, D., Hou, Z., Jia, M., Luo, X.-X., Li, X., & Li, M.-K. (2015). Synthesis of Biscoumarin and Dihydropyran Derivatives and Evaluation of Their Antibacterial Activity. Molecules, 20(9), 17469-17482. https://doi.org/10.3390/molecules200917469






