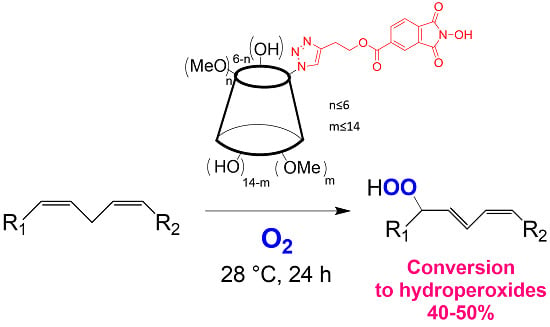
Functionalization of Cyclodextrins with N-Hydroxyphthalimide Moiety: A New Class of Supramolecular Pro-Oxidant Organocatalysts
Abstract
:1. Introduction
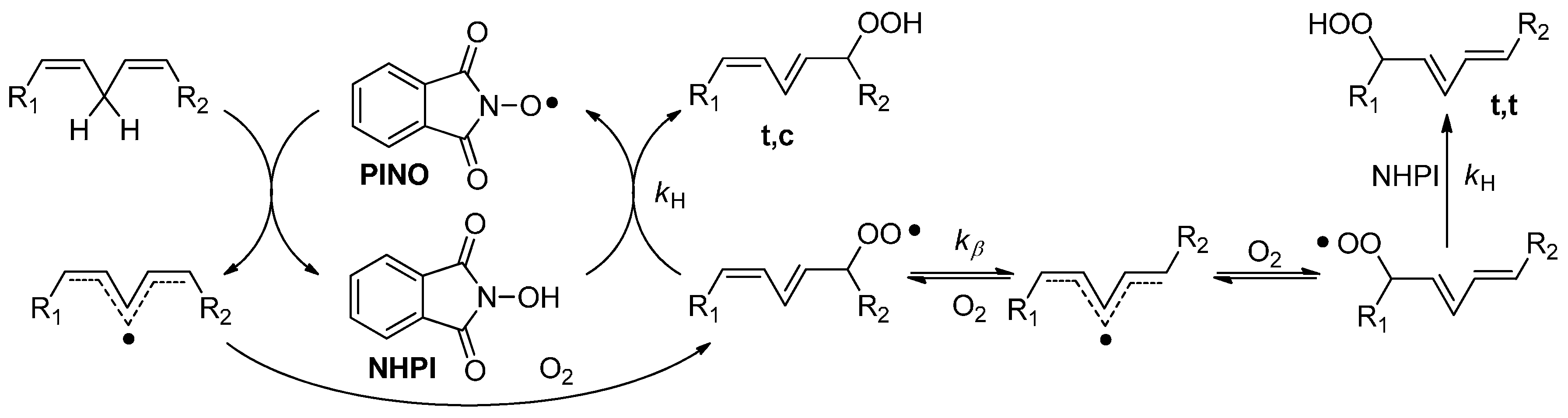
2. Results and Discussion
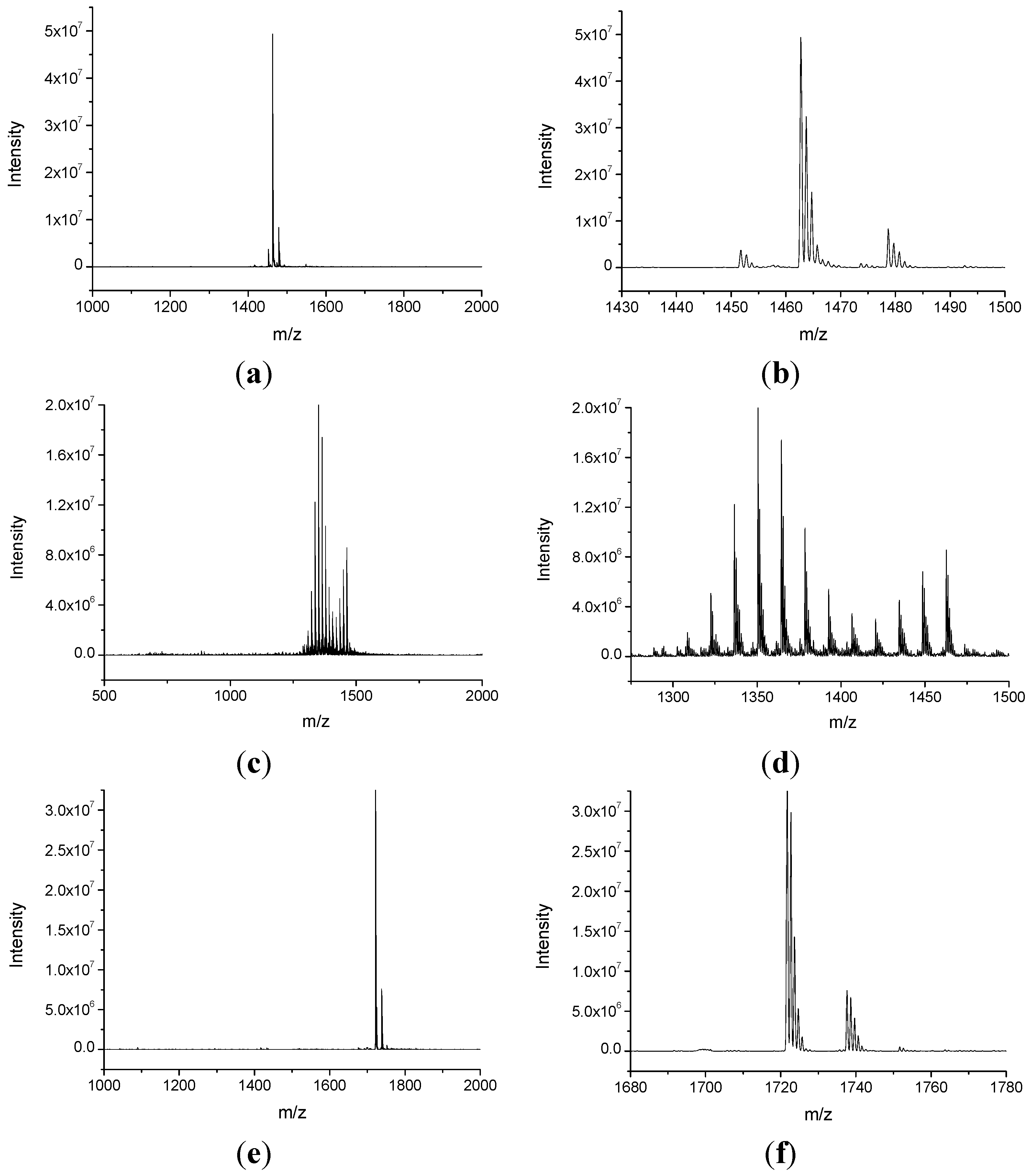
2.1. Synthesis and Characterization of NHPI-Functionalized MeβCD Derivatives
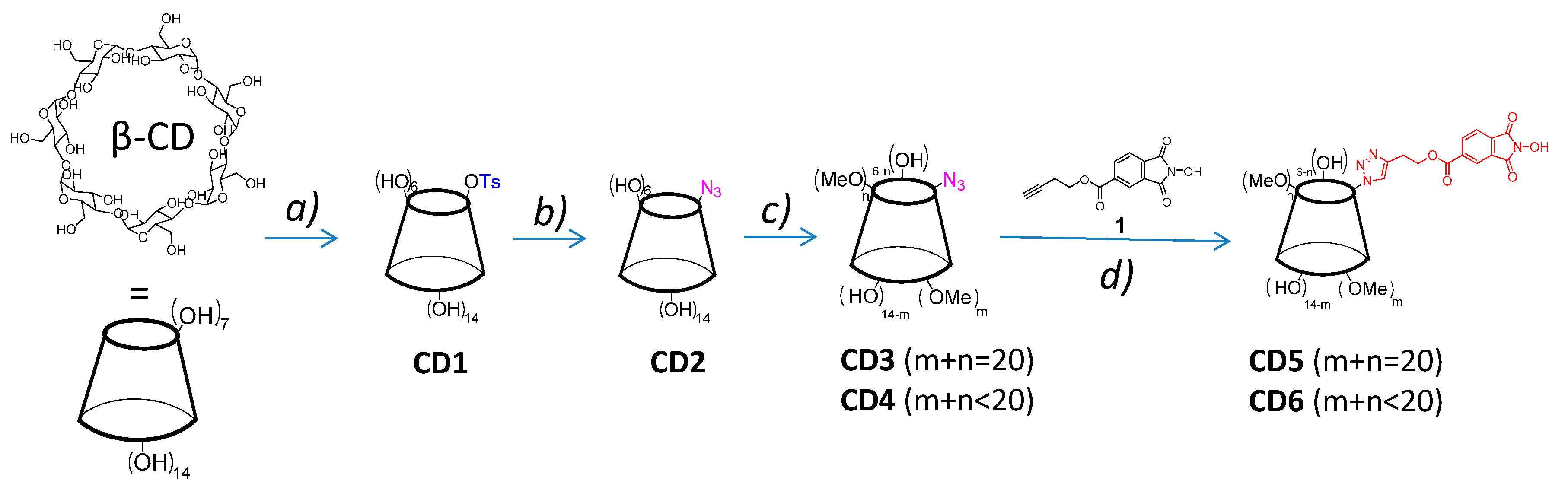
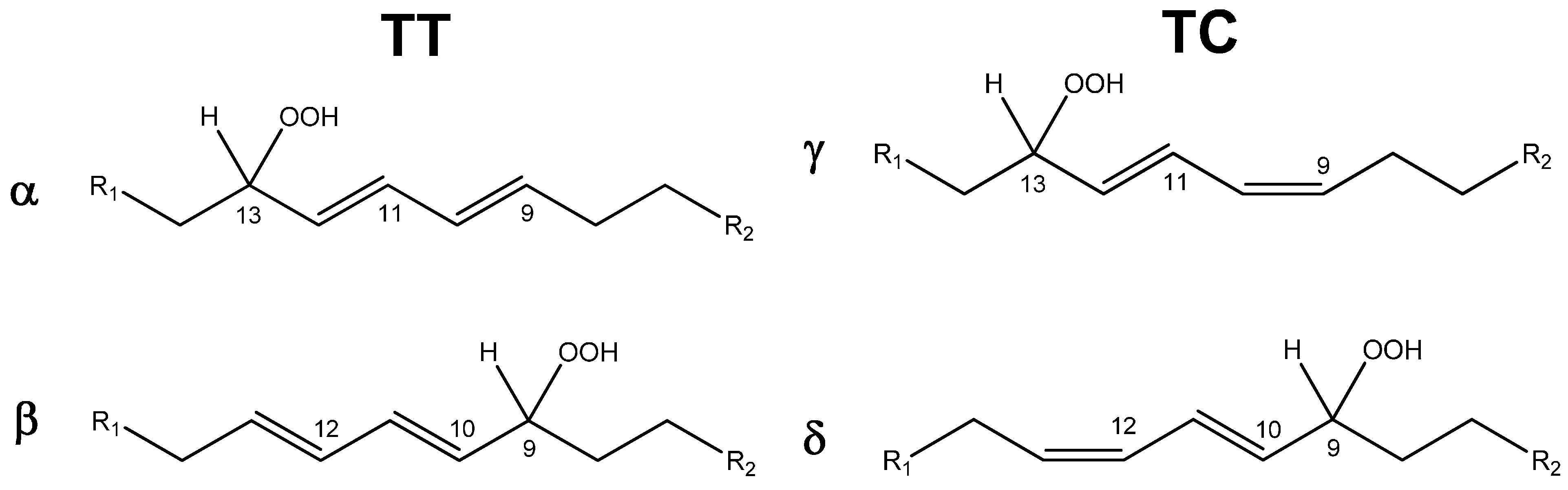
| Entry | Catalyst | Conversion 1 (%) | Selectivity 2 (%) | |
|---|---|---|---|---|
| TC | TT | |||
| 1 | 5.4 | 62 | 38 | |
| 2 | NHPI | 42.7 | 49.5 | 50.5 |
| 3 | CD5 | 54.7 | 50.6 | 49.4 |
| 4 | CD6 | 48.0 | 53.8 | 46.2 |
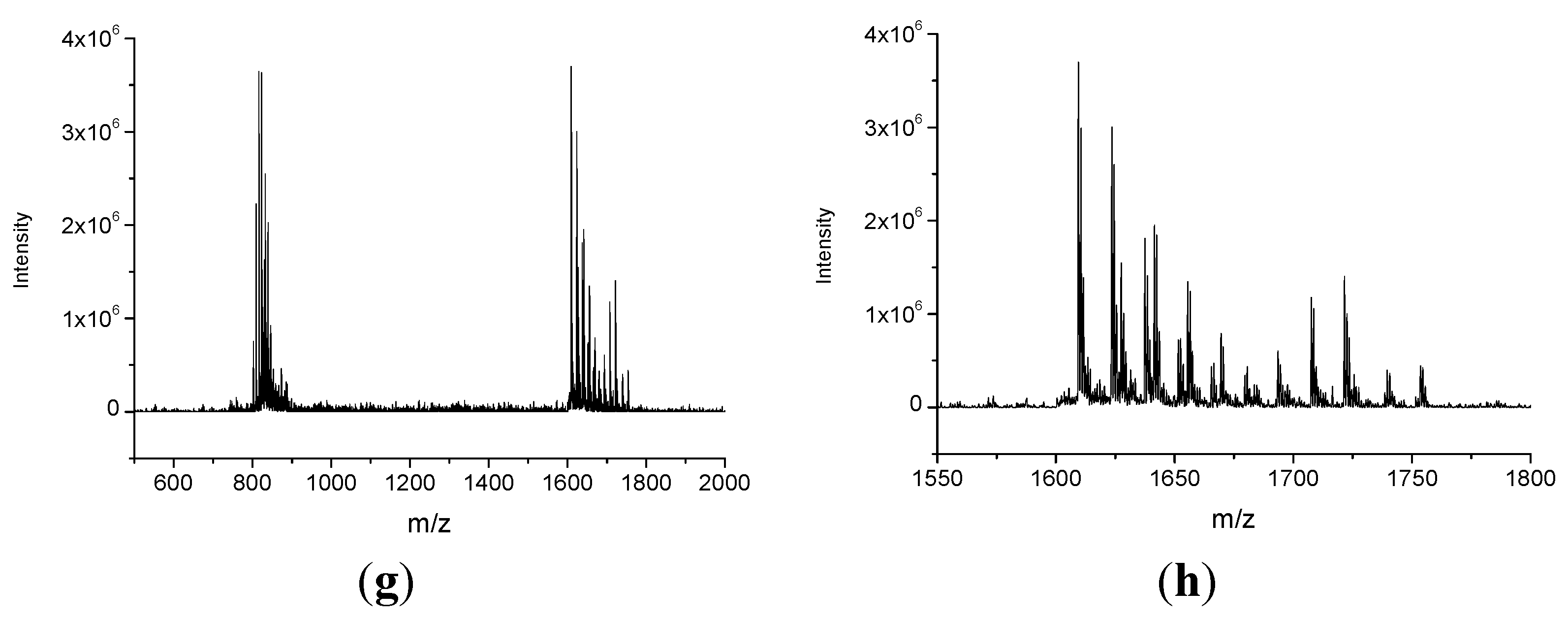
2.2. Catalytic Activity
3. Experimental Section
3.1. General
3.2. Synthesis of the Derivative 1 (But-3-yn-1-yl 2-hydroxy-1,3-dioxoisoindoline-5-carboxylate)
3.3. Synthesis of CD5 Derivative
3.4. Synthesis of CD6 Derivative
3.5. General Procedure for the Methyl Linoleate Aerobic Peroxidation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Verbon, E.H.; Post, J.A.; Boonstra, J. The influence of reactive oxygen species on cell cycle progression in mammalian cells. Gene 2012, 511, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Garber, K. Biochemistry: A radical treatment. Nature 2012, 489, S4–S6. [Google Scholar] [CrossRef] [PubMed]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.M.; Bardeesy, N. Cancer: When antioxidants are bad. Nature 2011, 475, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; Tuveson, D.A. The Promise and Perils of Antioxidants for Cancer Patients. N. Engl. J. Med. 2014, 371, 177–178. [Google Scholar] [PubMed]
- Glasauer, A.; Chandel, N.S. Targeting antioxidants for cancer therapy. Biochem. Pharmacol. 2014, 92, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Raj, L.; Ide, T.; Gurkar, A.U.; Foley, M.; Schenone, M.; Li, X.; Tolliday, N.J.; Golub, T.R.; Carr, S.A.; Shamji, A.F.; et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011, 475, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Kwon, B.; Han, E.; Park, M.; Yang, W.; Cho, W.; Yoo, W.; Khang, G.; Lee, D. Amplification of oxidative stress by a dual stimuli-responsive hybrid drug enhances cancer cell death. Nat. Commun. 2015, 6, 6907. [Google Scholar] [CrossRef] [PubMed]
- Recupero, F.; Punta, C. Free Radical Functionalization of Organic Compounds Catalyzed by N-Hydroxyphthalimide. Chem. Rev. 2007, 107, 3800–3842. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Gentili, P.; Lanzalunga, O. Hydrogen Abstraction and Electron Transfer with Aminoxyl Radicals: Synthetic and Mechanistic Issues. Angew. Chem. Int. Ed. 2008, 47, 4790–4796. [Google Scholar] [CrossRef] [PubMed]
- Coseri, S. Phthalimide-N-oxyl (PINO) radical, a powerful catalytic agent: Its generation and versatility towards various organic substrates. Catal. Rev. 2009, 51, 218–292. [Google Scholar] [CrossRef]
- Melone, L.; Punta, C. Metal-free aerobic oxidations mediated by N-hydroxyphthalimide. A concise review. Beilstein J. Org. Chem. 2013, 9, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Wertz, S.; Studer, A. Nitroxide-catalyzed transition-metal-free aerobic oxidation processes. Green Chem. 2013, 15, 3116–3134. [Google Scholar] [CrossRef]
- Melone, L.; Gambarotti, C.; Prosperini, S.; Pastori, N.; Recupero, F.; Punta, C. Hydroperoxidation of tertiary alkylaromatics catalyzed by N-Hydroxyphthalimide and aldehydes under mild conditions. Adv. Synth. Catal. 2011, 353, 147–154. [Google Scholar] [CrossRef]
- Melone, L.; Prosperini, S.; Gambarotti, C.; Pastori, N.; Recupero, F.; Punta, C. Selective catalytic aerobic oxidation of substituted ethylbenzenes under mild conditions. J. Mol. Catal. A 2012, 355, 155–160. [Google Scholar] [CrossRef]
- Melone, L.; Franchi, P.; Lucarini, M.; Punta, C. Sunlight induced oxidative photoactivation of N-hydroxyphthalimide mediated by naphthalene imides. Adv. Synth. Catal. 2013, 355, 3210–3220. [Google Scholar] [CrossRef]
- Liu, G.; Tang, R.; Wang, Z. Metal-Free Allylic Oxidation with Molecular Oxygen Catalyzed by g-C3N4 and N-Hydroxyphthalimide. Catal. Lett. 2014, 144, 717–722. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, P.; Wang, Y.; Li, H. Metal-free allylic/benzylic oxidation strategies with molecular oxygen: Recent advances and future prospects. Green Chem. 2014, 16, 2344–2374. [Google Scholar] [CrossRef]
- Chen, K.; Jia, L.; Wang, C.; Yao, J.; Chen, Z.; Li, H. Theoretical Design of Multi-Nitroxyl Organocatalysts with Enhanced Reactivity for Aerobic Oxidation. ChemPhysChem 2014, 15, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, K.; Zhang, W.; Yao, J.; Li, H. Efficient metal-free oxidation of ethylbenzene with molecular oxygen utilizing the synergistic combination of NHPI analogues. J. Mol. Catal. A Chem. 2015, 402, 79–82. [Google Scholar] [CrossRef]
- Punta, C.; Rector, C.L.; Porter, N.A. Peroxidation of Polyunsaturated Fatty Acid Methyl Esters Catalyzed by N-Methyl Benzohydroxamic Acid: A New and Convenient Method for Selective Synthesis of Hydroperoxides and Alcohols. Chem. Res. Toxicol. 2005, 18, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Melone, L.; Prosperini, S.; Ercole, G.; Pastori, N.; Punta, C. Is it possible to implement N-hydroxyphthalimide homogeneous catalysis for industrial applications? A case study of cumene aerobic oxidation. J. Chem. Technol. Biotechnol. 2014, 89, 1370–1378. [Google Scholar] [CrossRef]
- Petroselli, M.; Franchi, P.; Lucarini, M.; Punta, C.; Melone, L. Aerobic Oxidation of Alkylaromatics using a Lipophilic N-Hydroxyphthalimide: Overcoming the Industrial Limit of Catalyst Solubility. ChemSusChem 2014, 7, 2695–2703. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, K.; Kariya, R.; Alam, M.; Matsuda, K.; Hattori, S.; Maeda, Y.; Motoyama, K.; Kojima, A.; Arima, H.; Okada, S. The antitumor effects of methyl-β-cyclodextrin against primary effusion lymphoma via the depletion of cholesterol from lipid rafts. Biochem. Biophys. Res. Commun. 2014, 455, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, N.; Malvi, P.; Singh Meena, A.; Vikram Singh, S.; Chaube, B.; Vannuruswamy, G.; Kulkarni, M.J.; Bhat, M.K. Cholesterol depletion by methyl-β-cyclodextrin augments tamoxifen induced cell death by enhancing its uptake in melanoma. Mol. Cancer 2014, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Hryniewicz-Jankowska, A.; Augoff, K.; Biernatowska, A.; Podkalicka, J.; Sikorski, A.F. Membrane rafts as a novel target in cancer therapy. Biochim. Biophys. Acta 2014, 1845, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Cyclodextrins as functional excipients: Methods to enhance complexation efficiency. J. Pharm. Sci. 2012, 101, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, R.J.; Nshime, B.; de LaMarre, M.; de Jong, S.; Scott, P.; Lantz, A.W. Cyclodextrins as complexation and extraction agents for pesticides from contaminated soil. Chemosphere 2013, 91, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Tekpli, X.; Holme, J.A.; Sergent, O.; Lagadic-Gossmann, D. Role for membrane remodeling in cell death: Implication for health and disease. Toxicology 2013, 304, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Gambarotti, C.; Punta, C.; Recupero, F. Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons: Weinheim, Germany, 2005. [Google Scholar]
- Pratt, D.A.; Mills, J.H.; Porter, N.A. Theoretical calculations of carbon-oxygen bond dissociation enthalpies of peroxyl radicals formed in the autoxidation of lipids. J. Am. Chem. Soc. 2003, 125, 5801–5810. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Lucarini, M.; Mugnaini, M.; Pedulli, G.F.; Minisci, F.; Recupero, F.; Fontana, F.; Astolfi, P.; Greci, L. Hydroxylamines as oxidation catalysts: Thermochemical and kinetic studies. J. Org. Chem. 2003, 68, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Pajunen, T.I.; Koskela, H.; Hase, T.; Hopia, A. NMR properties of conjugated linoleic acid (CLA) methyl ester hydroperoxides. Chem. Phys. Lipids 2008, 154, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Brady, B.; Lynam, N.; O’Sullivan, T.; Ahern, C.; Darcy, R. 6A-O-p-Toluenesulfonyl-β-Cyclodextrin. Org. Synth. 2000, 77, 225. [Google Scholar] [CrossRef]
- Muderawan, I.W.; Ong, T.T.; Chia Lee, T.; Young, D.J.; Ching, C.B.; Choon Ng, S. A reliable synthesis of 2- and 6-amino-β-cyclodextrin and permethylated-b-cyclodextrin. Tetrahedron Lett. 2005, 46, 7905–7907. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds CD5 and CD6 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melone, L.; Petroselli, M.; Pastori, N.; Punta, C. Functionalization of Cyclodextrins with N-Hydroxyphthalimide Moiety: A New Class of Supramolecular Pro-Oxidant Organocatalysts. Molecules 2015, 20, 15881-15892. https://doi.org/10.3390/molecules200915881
Melone L, Petroselli M, Pastori N, Punta C. Functionalization of Cyclodextrins with N-Hydroxyphthalimide Moiety: A New Class of Supramolecular Pro-Oxidant Organocatalysts. Molecules. 2015; 20(9):15881-15892. https://doi.org/10.3390/molecules200915881
Chicago/Turabian StyleMelone, Lucio, Manuel Petroselli, Nadia Pastori, and Carlo Punta. 2015. "Functionalization of Cyclodextrins with N-Hydroxyphthalimide Moiety: A New Class of Supramolecular Pro-Oxidant Organocatalysts" Molecules 20, no. 9: 15881-15892. https://doi.org/10.3390/molecules200915881
APA StyleMelone, L., Petroselli, M., Pastori, N., & Punta, C. (2015). Functionalization of Cyclodextrins with N-Hydroxyphthalimide Moiety: A New Class of Supramolecular Pro-Oxidant Organocatalysts. Molecules, 20(9), 15881-15892. https://doi.org/10.3390/molecules200915881