Identification and Antioxidant Properties of Phenolic Compounds during Production of Bread from Purple Wheat Grains
Abstract
:1. Introduction
2. Results and Discussion
2.1. Free, Bound, and Total Phenolic Content Using the Folin-Ciocalteau Method
| Ferulic Acid Equivalent | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Free Phenolic Content (mg FAE/100 g) | Bound Phenolic Content (mg FAE/100 g) | Total Phenolic Content (mg FAE/100 g) | |||||||
| Öelands hvede A | Indigo A | Konini A | Öelands hvede B | Indigo B | Konini B | Öelands hvede | Indigo | Konini | |
| Flour | 113.23 ± 1.97 kl | 105.44 ± 0.82 kl | 111.26 ± 0.49 kl | 125.36 ± 5.26 efg | 121.82 ± 3.95 efg | 123.68 ± 5.53 efg | 238.59 ± 7.24 | 227.26 ± 4.77 | 234.94 ± 6.02 |
| Mixing | 129.05 ± 1.64 ghi | 125.76 ± 4.93 ij | 128.70 ± 0.82 ghi | 138.57 ± 2.37 bc | 135.96 ± 2.37 bcd | 137.08 ± 1.32 bcd | 267.62 ± 4.01 | 261.72 ± 7.30 | 265.78 ± 2.14 |
| 30 min fermenting | 136.14 ± 4.77 efg | 128 ± 2.47 hi | 135.67 ± 1.48 efgh | 129.64 ± 2.37 cde | 125.73 ± 5.79 efg | 127.96 ± 2.63 def | 265.78 ± 7.14 | 253.73 ± 8.26 | 263.63 ± 4.11 |
| 65min fermenting | 142.3 ± 0.66 e | 130.21 ± 4.28 fghi | 137.53 ± 6.74 ef | 141.73 ± 5.26 b | 138.20 ± 5.53 bc | 140.61 ± 4.74 b | 284.03 ± 5.92 | 268.41 ± 9.80 | 278.14 ± 11.48 |
| Bread loaf | 185.91 ± 3.78 c | 176.72 ± 0.99 d | 183.53 ± 2.96 cd | 158.10 ± 5.79 a | 154.75 ± 5.79 a | 156.43 ± 3.95 a | 344.01 ± 9.57 | 331.47 ± 6.78 | 339.96 ± 6.91 |
| Bread crust | 228.81 ± 5.92 a | 219.16 ± 6.08 b | 229.86 ± 1.81 a | 122.57 ± 5.00 efg | 116.80 ± 3.16 g | 119.59 ± 6.05 gf | 351.38 ± 10.92 | 335.96 ± 9.24 | 349.45 ± 7.86 |
| Bread crumb | 123.81 ± 6.08 ij | 114.28 ± 3.78 k | 119.05 ± 5.26 jk | 163.13 ± 0.26 a | 159.22 ± 4.74 a | 161.64 ± 8.16 a | 286.94 ± 6.35 | 273.50 ± 8.52 | 280.69 ± 13.42 |
| Gallic Acid Equivalent | |||||||||
| Free Phenolic Content (mg GAE/100 g) | Bound Phenolic Content (mg GAE/100 g) | Total Phenolic Content (mg GAE/100 g) | |||||||
| Öelands hvede C | Indigo C | Konini C | Öelands hvede D | Indigo D | Konini D | Öelands hvede | Indigo | Konini | |
| Flour | 83.15 ± 1.37 kl | 77.74 ± 0.57 kl | 81.77 ± 0.34 kl | 94.32 ± 3.65 efg | 91.87 ± 2.74 efg | 93.16 ± 3.83 efg | 177.47 ± 5.02 | 169.61 ± 3.31 | 174.94 ± 4.17 |
| Mixing | 94.11 ± 1.14 ghi | 91.85 ± 1.14 ij | 93.87 ± 0.57 efgh | 103.48 ± 1.64 bc | 101.68 ± 1.64 bcd | 102.45 ± 0.91 bcd | 197.60 ± 2.78 | 193.53 ± 5.06 | 196.32 ± 1.48 |
| 30 min fermenting | 99.03 ± 3.31 efg | 93.39 ± 1.71 hi | 98.71 ± 1.03 efgh | 97.29 ± 1.64 cde | 94.58 ± 4.01 efg | 96.13 ± 1.82 def | 196.32 ± 4.95 | 187.97 ± 5.73 | 194.84 ± 2.85 |
| 65 min fermenting | 103.31 ± 0.46 e | 94.92 ± 0.46 fghi | 100.00 ± 4.68 ef | 105.68 ± 3.65 b | 103.23 ± 3.83 bc | 104.90 ± 3.28 b | 208.98 ± 4.11 | 198.15 ± 6.80 | 204.90 ± 7.96 |
| Bread loaf | 133.55 ± 2.62 c | 127.18 ± 2.62 d | 131.21 ± 2.05 cd | 117.03 ± 4.01 a | 114.71 ±4.01 a | 115.87 ± 2.74 a | 250.58 ± 6.64 | 241.89 ± 4.70 | 247.08 ± 4.79 |
| Bread crust | 163.31 ± 4.11 a | 156.61 ± 4.11 b | 164.03 ± 1.25 a | 92.39 ± 3.47 efg | 88.39 ± 2.19 g | 90.32 ± 4.20 fg | 255.69 ± 7.57 | 245.00 ± 6.41 | 254.35 ± 5.45 |
| Bread crumb | 90.48 ± 4.22 ij | 83.87 ± 4.22 k | 87.18 ± 3.65 jk | 120.52 ± 0.18 a | 117.81 ± 3.28 a | 119.48 ± 5.66 a | 211.00 ± 4.40 | 201.68 ± 5.91 | 206.66 ± 9.31 |
2.2. Antioxidant Activities (AOAs) of Soluble and Insoluble Phenolic Compounds
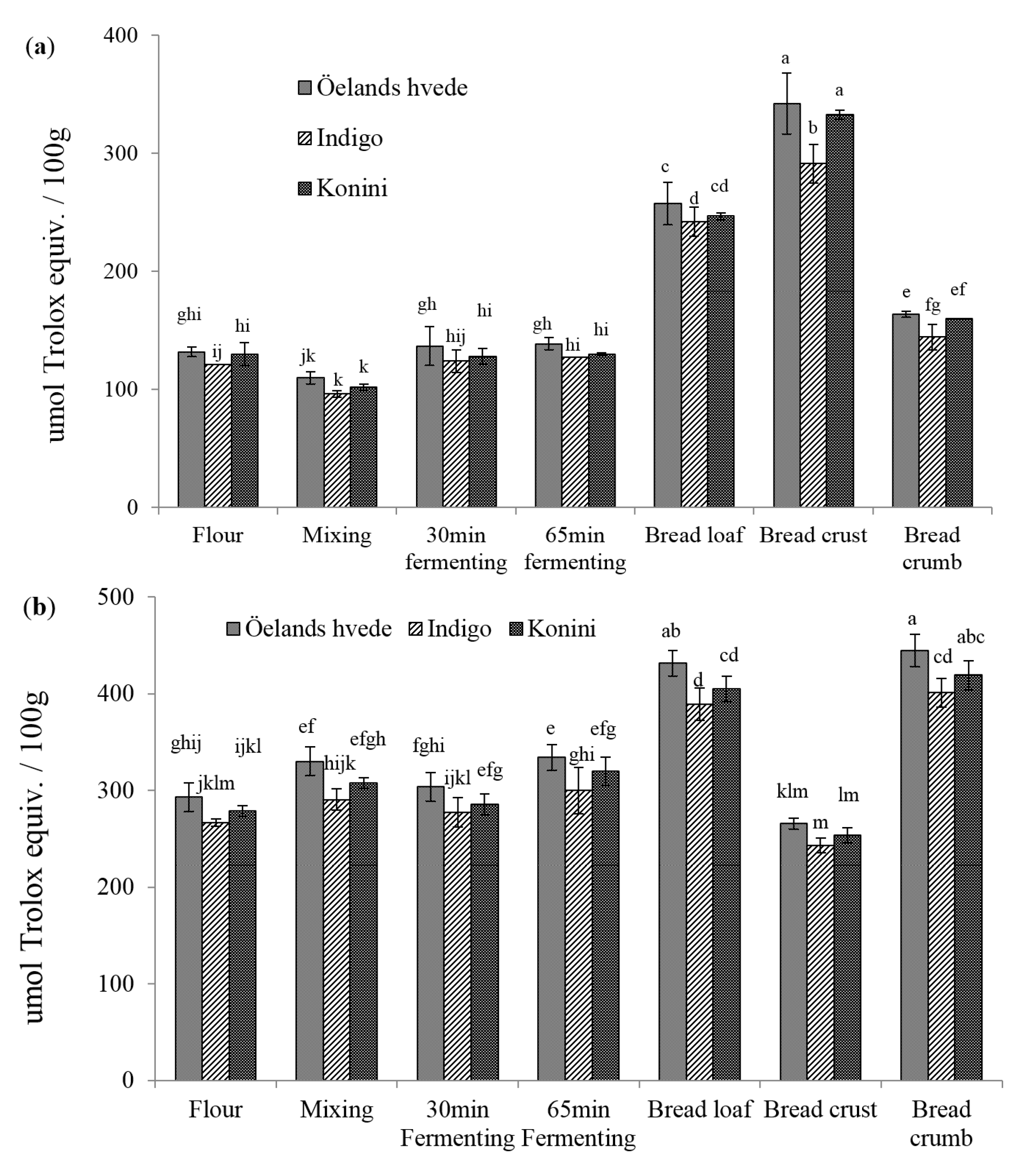
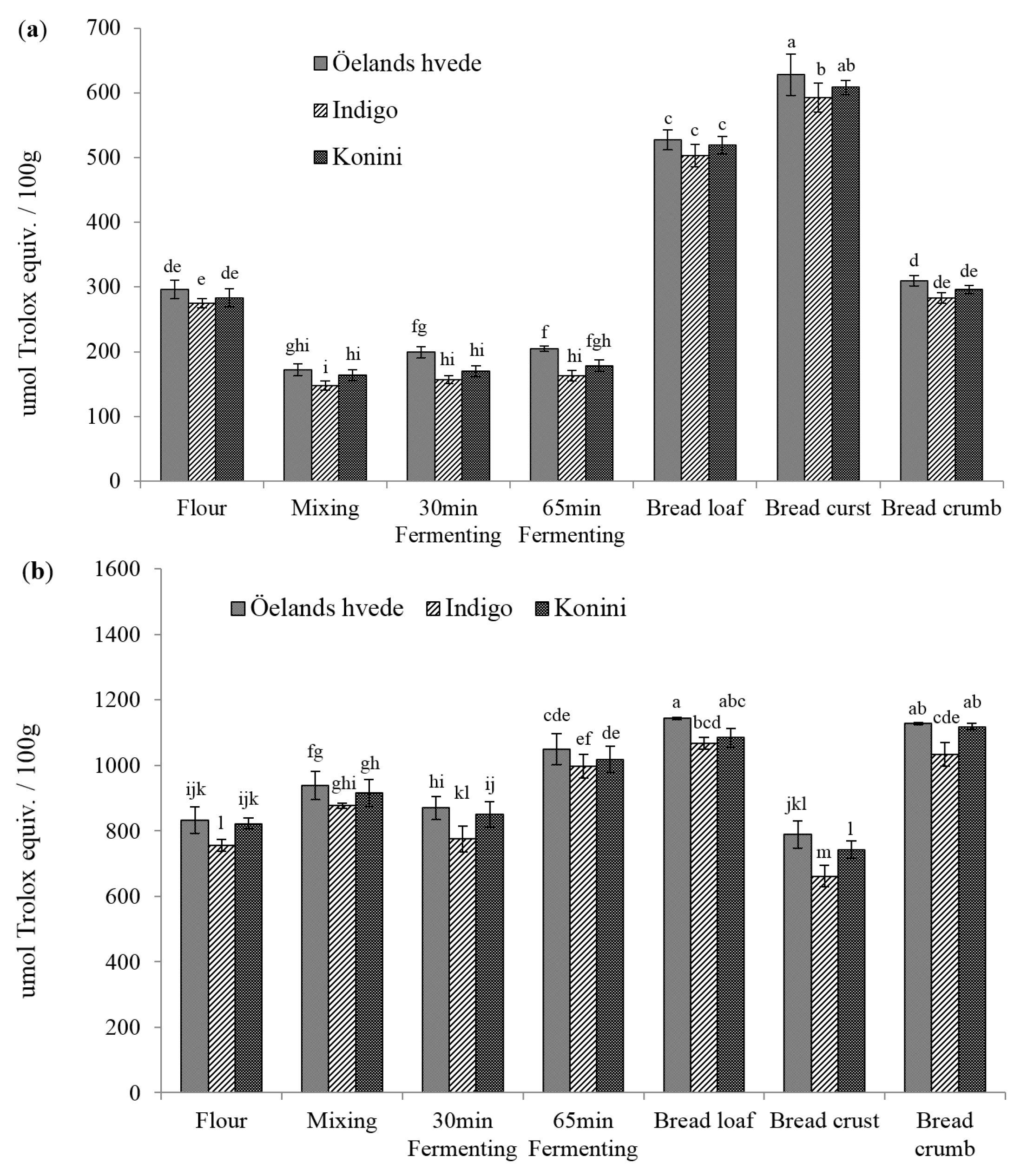
2.3. Identification and Quantification of Phenolic Acids in Soluble and Insoluble Phenolic Extracts
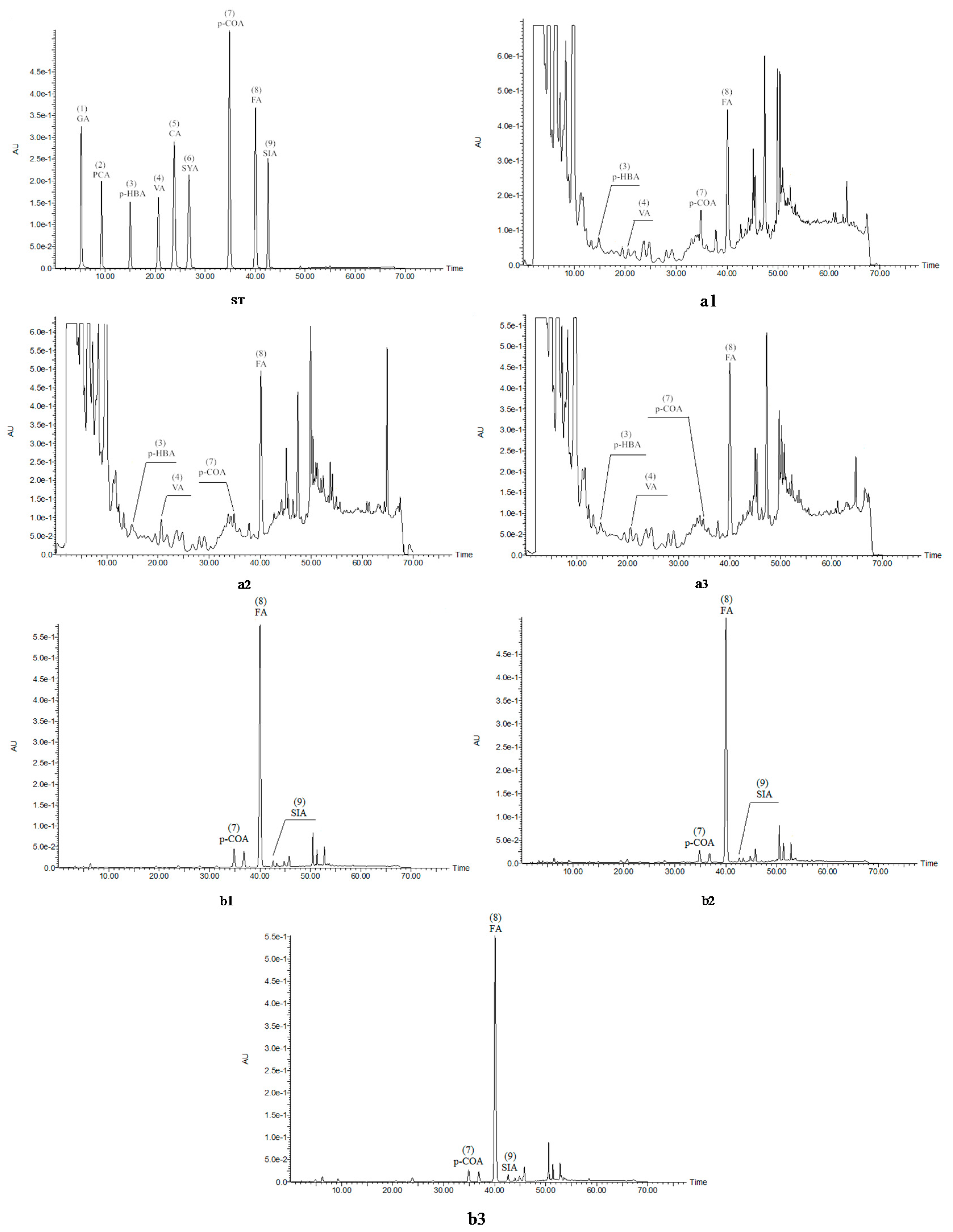
| Steps | Sample Name | p-Hydroxybenzoic Acid | Vanillic Acid | p-Coumaric Acid | Ferulic Acid | Total Phenolic Acid |
|---|---|---|---|---|---|---|
| Flour | Öelands hvede | nd | nd | nd | 2.50 ± 0.20 j | 2.50 ± 0.20 |
| Indigo | nd | nd | nd | 2.02 ± 0.07 j | 2.02 ± 0.07 | |
| Konini | nd | nd | nd | 2.25 ± 0.01 j | 2.25 ± 0.01 | |
| Mixing | Öelands hvede | nd | nd | nd | 10.01 ± 1.23 hi | 10.01 ± 1.23 |
| Indigo | nd | nd | nd | 9.04 ± 0.24 i | 9.04 ± 0.24 | |
| Konini | nd | nd | nd | 10.58 ± 0.69 ghi | 10.58 ± 0.69 | |
| 30 min Fermenting | Öelands hvede | nd | 2.44 ± 0.53 d | 1.31 ± 0.17 ab | 11.25 ± 0.67 egh | 15.01 ± 1.37 |
| Indigo | nd | nd | nd | 10.57 ± 1.55 ghi | 10.57 ± 1.55 | |
| Konini | nd | nd | nd | 12.55 ± 1.13 ef | 12.55 ± 1.13 | |
| 65 min Fermenting | Öelands hvede | 4.19 ± 0.71 ef | 3.49 ± 0.09 c | nd | 13.34 ± 1.68 cde | 21.03 ± 2.49 |
| Indigo | nd | nd | nd | 13.25 ± 2.06 de | 13.25 ± 2.06 | |
| Konini | 2.66 ± 0.37 f | nd | nd | 15.01 ± 0.69 bcd | 17.67 ± 0.44 | |
| Bread Loaf | Öelands hvede | 11.07 ± 2.42 cd | 4.37 ± 0.29 dc | 1.51 ± 0.07 a | 13.53 ± 1.49 cde | 30.47 ± 4.27 |
| Indigo | 7.39 ± 2.41 de | 5.20 ± 0.83 ab | 1.26 ± 0.06 b | 14.40 ± 0.42 bcd | 28.25 ± 3.66 | |
| Konini | 11.87 ± 1.93 bc | 3.44 ± 0.32 c | 1.15 ± 0.01 b | 15.53 ± 0.98 ab | 32.00 ± 2.53 | |
| Bread Crust | Öelands hvede | nd | 3.73 ± 0.27 c | nd | 12.41 ± 0.90 ef | 16.15 ± 1.17 |
| Indigo | nd | 5.19 ± 0.04 ab | nd | 12.01 ± 1.01 efg | 17.21 ± 1.05 | |
| Konini | nd | 6.00 ± 0.28 a | 0.70 ± 0.02 c | 15.11 ± 0.26 abc | 21.82 ± 0.55 | |
| Bread Crumb | Öelands hvede | 16.29 ± 1.68 a | nd | nd | 14.76 ± 0.74 abcd | 31.05 ± 1.82 |
| Indigo | 8.64 ± 1.87 cd | nd | nd | 14.65 ± 0.03 abcd | 23.29 ± 2.01 | |
| Konini | 15.24 ± 1.37 ab | nd | nd | 16.44 ± 0.57 a | 31.68 ± 1.54 |
| Steps | Sample Name | Protocatechuic Acid | p-Hydroxybenzoic Acid | Vanillic Acid | Caffeic Acid | Syringic Acid | p-Coumaric Acid | Ferulic Acid | Sinapic Acid | Total Phenolic Acid |
|---|---|---|---|---|---|---|---|---|---|---|
| Flour | Öelands hvede | nd | 22.73 ± 1.29 b | 9.29 ± 0.54 f | 4.54 ± 0.18 k | 9.03 ± 0.58 b | 26.13 ± 1.19 a | 532.15 ± 30.00 defgh | 69.36 ± 2.69 cd | 673.25 ± 36.47 |
| Indigo | nd | 25.69 ± 1.17 a | 24.82 ± 0.01 a | 6.53 ± 0.15 ef | 9.95 ± 0.23 a | 19.14 ± 1.50 def | 447.02 ± 24.14 kl | 35.97 ± 2.80 k | 569.12 ± 29.99 | |
| Konini | nd | 24.60 ± 1.24 a | 19.20 ± 0.97 b | 7.10 ± 0.30 bc | 9.05 ± 0.09 b | 21.64 ± 0.68 c | 466.66 ± 0.28 jk | 52.48 ± 2.24 hij | 600.74 ± 5.81 | |
| Mixing | Öelands hvede | 20.67 ± 1.00 a | 10.63 ± 0.73 fg | 7.44 ± 0.49 h | 4.85 ± 0.02 jk | 9.46 ± 0.57 ab | 26.50 ± 0.39 a | 573.64 ± 11.84 bcd | 72.37 ± 0.88 bc | 725.56 ± 15.92 |
| Indigo | 19.37 ± 0.60 ab | 24.48 ± 1.68 a | 9.48 ± 0.32 f | 4.52 ± 0.00 k | 5.97 ± 0.17 f | 19.63 ± 1.25 de | 482.89 ± 9.02 ijk | 51.74 ± 2.88 ij | 618.07 ± 15.94 | |
| Konini | 14.37 ± 0.84 e | 20.59 ± 1.19 c | 13.58 ± 0.18 de | 5.70 ± 0.28 g | 7.24 ± 0.36 de | 22.57 ± 1.16 bc | 514.29 ± 4.29 hi | 60.38 ± 0.28 ef | 658.73 ± 8.57 | |
| 30 min | Öelands hvede | 15.42 ± 0.43 d | 7.83 ± 0.39 h | 6.17 ± 0.17 h | nd | nd | 21.02 ± 0.96 cd | 411.57 ± 13.46 lm | 64.66 ± 2.88 de | 526.66 ± 18.23 |
| Indigo | 15.46 ± 0.51 d | 11.63 ± 0.31 f | 8.68 ± 0.73 fg | nd | nd | 14.56 ± 0.54 ij | 363.99 ± 18.02 n | 40.82 ± 3.24 k | 455.14 ± 23.35 | |
| Konini | 11.13 ± 0.68 f | 13.79 ± 0.42 e | 12.76 ± 0.51 e | nd | nd | 13.57 ± 0.03 j | 386.39 ± 11.13 mn | 49.10 ± 2.84 j | 486.74 ± 15.60 | |
| 65 min | Öelands hvede | 17.56 ± 0.04 c | 11.70 ± 0.61 f | 12.86 ± 0.92 e | 5.20 ± 0.08 ij | 9.40 ± 0.46 ab | 26.57 ± 0.56 a | 526.64 ± 31.76 efghi | 71.83 ±1.05 bc | 681.75 ± 35.47 |
| Indigo | 19.97 ± 0.23 ab | 12.19 ± 0.24 f | 19.18 ± 0.69 b | 6.21 ± 0.28 f | 6.83 ± 0.32 e | 16.90 ± 0.25 gh | 488.22 ± 2.63 hijk | 54.86 ± 0.48 ghi | 624.36 ± 5.12 | |
| Konini | 14.99 ± 0.22 de | 15.77 ± 0.91 d | 18.17 ± 0.98 b | 6.86 ± 0.05 cde | 7.82 ± 0.27 cd | 18.61 ± 0.19 efg | 523.25 ± 7.22 fghi | 60.34 ± 2.47 ef | 665.81 ± 12.31 | |
| Load | Öelands hvede | 3.75 ± 0.12 ghi | 10.94 ± 0.27 fg | 14.68 ± 0.30 cd | 5.30 ± 0.22 hi | 9.63 ± 0.16 ab | 26.64 ± 0.30 a | 586.92 ± 30.32 bc | 75.51 ± 2.09 b | 733.38 ± 33.79 |
| Indigo | 4.12 ± 0.08 gh | 9.99 ± 0.32 g | 19.32 ± 0.59 b | 6.62 ± 0.16 de | 6.97 ± 0.27 e | 17.31 ± 0.33 fgh | 517.14 ± 1.40 hi | 56.12 ± 1.48 fghi | 637.57 ± 5.16 | |
| Konini | 3.10 ± 0.08 i | 5.29 ± 0.15 ij | 18.65 ± 0.62 b | 7.33 ± 0.26 ab | 8.12 ± 0.09 c | 19.39 ± 1.47 de | 564.64 ± 37.89 cdef | 65.31 ± 1.81 de | 691.84 ± 43.37 | |
| Crust | Öelands hvede | nd | 4.80 ± 0.21 ij | 15.45 ± 0.21 c | 5.66 ± 0.19 gh | nd | 27.87 ± 0.79 a | 631.54 ± 42.35 a | 80.92 ± 3.40 a | 766.23 ± 47.15 |
| Indigo | nd | 4.44 ± 0.10 j | 19.49 ± 0.66 b | 7.17 ± 0.24 bc | nd | 19.30 ± 1.07 de | 551.35 ± 6.76 cdefg | 57.45 ± 4.15 fgh | 659.20 ± 12.98 | |
| Konini | nd | 4.25 ± 0.27 j | 18.77 ± 0.63 b | 7.63 ± 0.04 a | nd | 20.70 ± 4.12 cd | 616.95 ± 21.59 ab | 71.57 ± 3.64 bc | 739.88 ± 26.59 | |
| Crumb | Öelands hvede | 3.95 ± 0.14 ghi | 6.17 ± 0.59 i | 14.51 ± 0.11 cd | 5.19 ± 0.05 ij | 9.80 ± 0.16 a | 23.93 ± 1.64 b | 569.32 ± 26.73 cde | 70.96 ± 4.66 bc | 703.82 ± 34.08 |
| Indigo | 4.33 ± 0.17 g | 5.62 ± 0.21 ij | 18.33 ± 1.00 b | 6.56 ± 0.15 ef | 6.97 ± 0.38 e | 17.10 ± 0.63 gh | 498.80 ± 7.71 hij | 51.14 ± 0.89 ij | 608.85 ± 11.08 | |
| Konini | 3.25 ± 0.13 hi | 4.32 ± 0.27 j | 18.65 ± 1.21 b | 6.96 ± 1.64 bcd | 8.18 ± 0.13 c | 16.05 ± 0.98 hi | 554.64 ± 24.93 cdefg | 58.22 ± 0.79 fg | 670.28 ± 28.61 |
2.4. Total Anthocyanin Content (TAC)
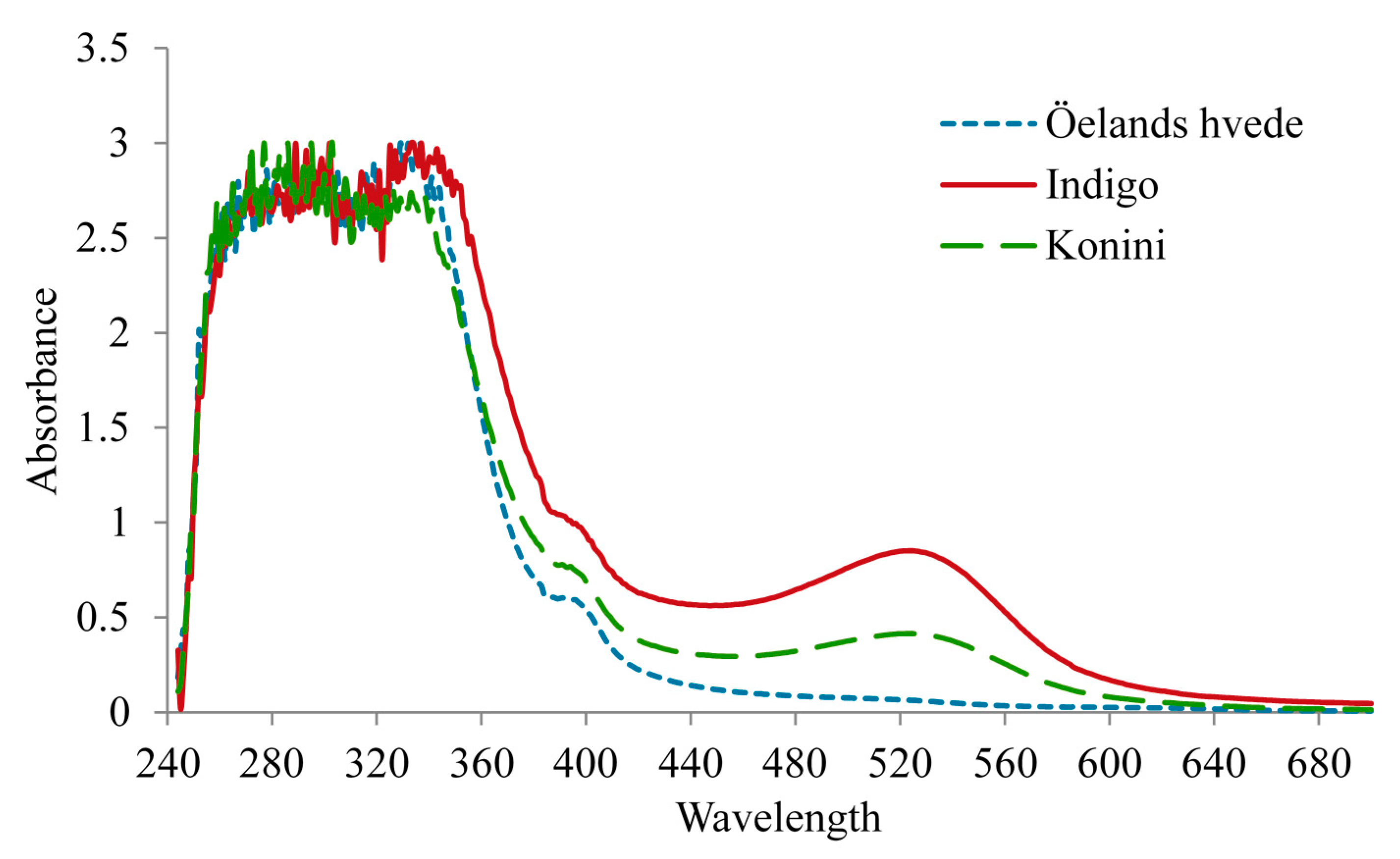
| Bread-Making Stages | Öelands hvede | Indigo | Konini |
|---|---|---|---|
| Flour * | 1.45 ± 0.06 k | 10.84 ± 0.27 a | 5.61 ± 0.12 e |
| Mixing * | 1.06 ± 0.05 l | 8.50 ± 0.14 d | 4.38 ± 0.05 h |
| 30 min Fermenting * | 1.35 ± 0.03 k | 8.95 ± 0.09 c | 5.05 ± 0.14 fg |
| 65 min Fermenting * | 1.39 ± 0.03 l | 9.47 ± 0.31 b | 5.20 ± 0.04 f |
| Bread Loaf * | 1.02 ± 0.03 l | 4.85 ± 0.03 g | 2.60 ± 0.03 i |
| Bread Crust * | 0.86 ± 0.06 l | 4.39 ± 0.14 h | 2.33 ± 0.05 j |
| Bread Crumb * | 0.55 ± 0.02 m | 5.47 ± 0.03 e | 2.77 ± 0.06 i |
2.5. Total Anthocyanin Content Using pH Differential Method
| Bread-Making Stages | Öelands hvede | Indigo | Konini |
|---|---|---|---|
| Flour * | nd | 10.41 ± 0.39 a | 5.31 ± 0.02 d |
| Mixing * | nd | 8.09 ± 0.37 c | 4.22 ± 0.07 f |
| 30 min Fermenting * | nd | 8.60 ± 0.47 bc | 4.65 ± 0.25 ef |
| 65 min Fermenting * | nd | 8.97 ± 0.26 b | 4.80 ± 0.08 de |
| Bread Loaf * | nd | 4.64 ± 0.24 ef | 2.15 ± 0.04 g |
| Bread Crust * | nd | 4.16 ± 0.21 f | 1.99 ± 0.07 g |
| Bread Crumb * | nd | 4.81 ± 0.26 de | 2.18 ± 0.08 g |
2.6. Antioxidant Activity of Anthocyanin Extracts
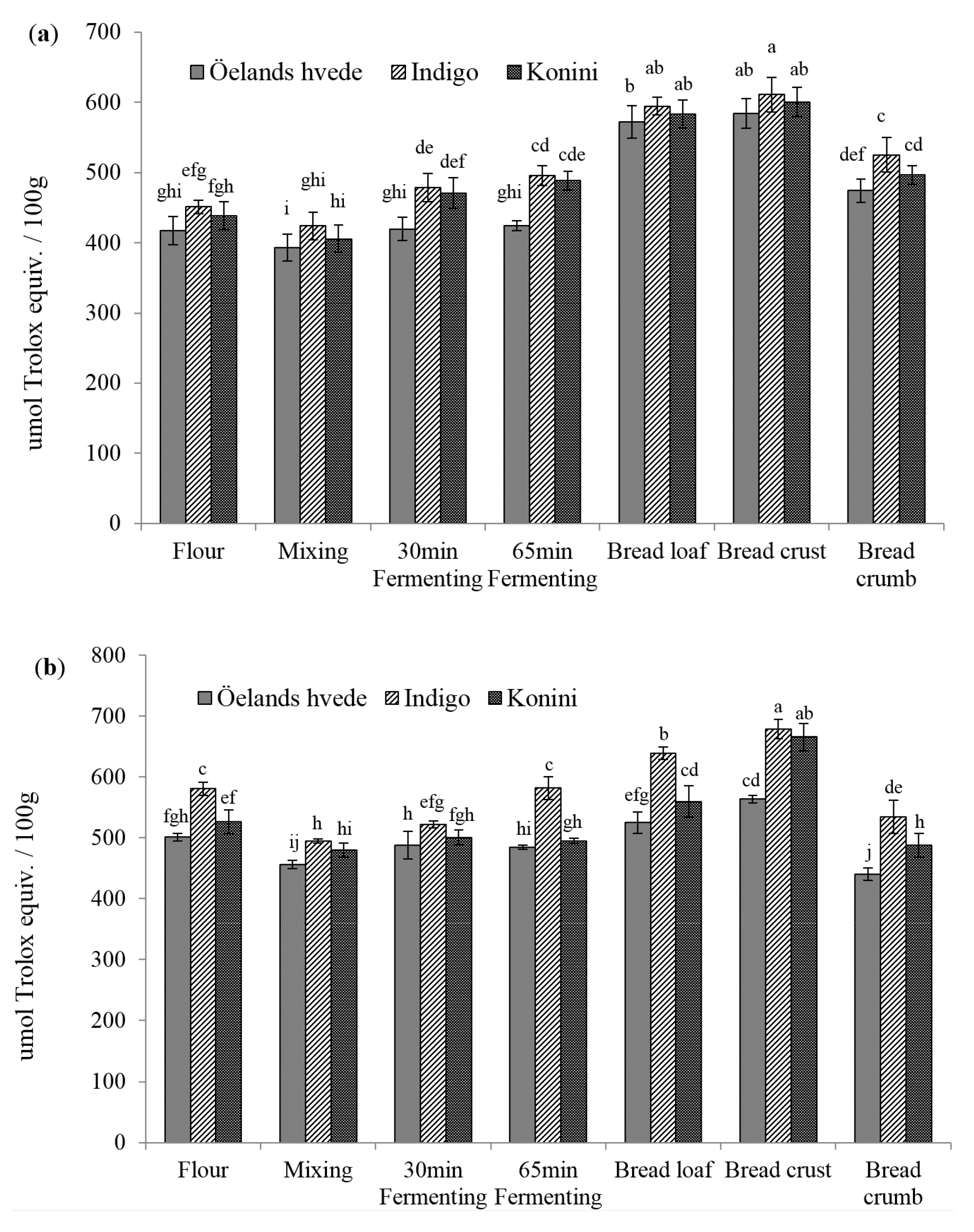
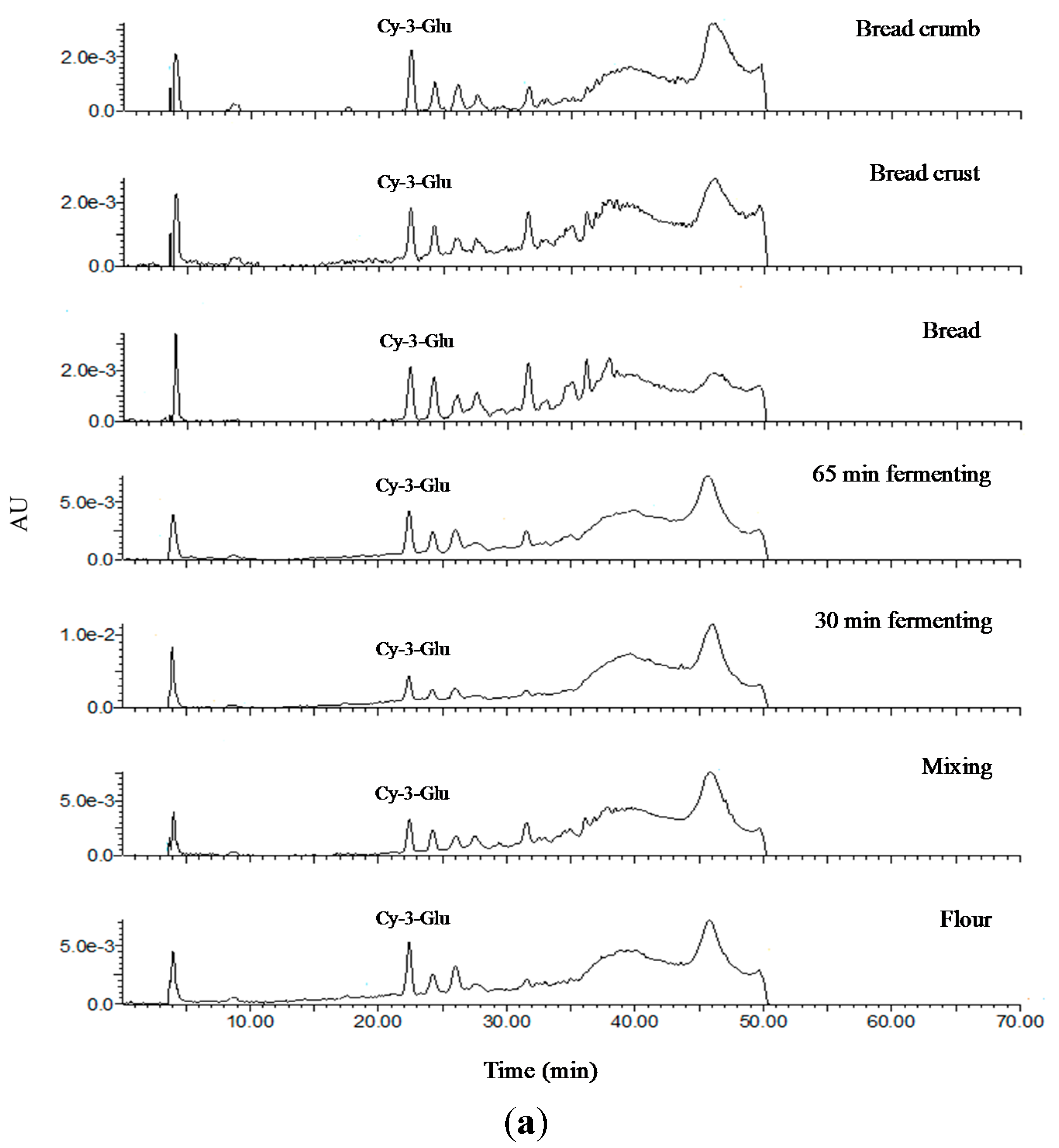
2.7. Analysis of Anthocyanin Composition
3. Experimental Section
3.1. Chemicals and Standards
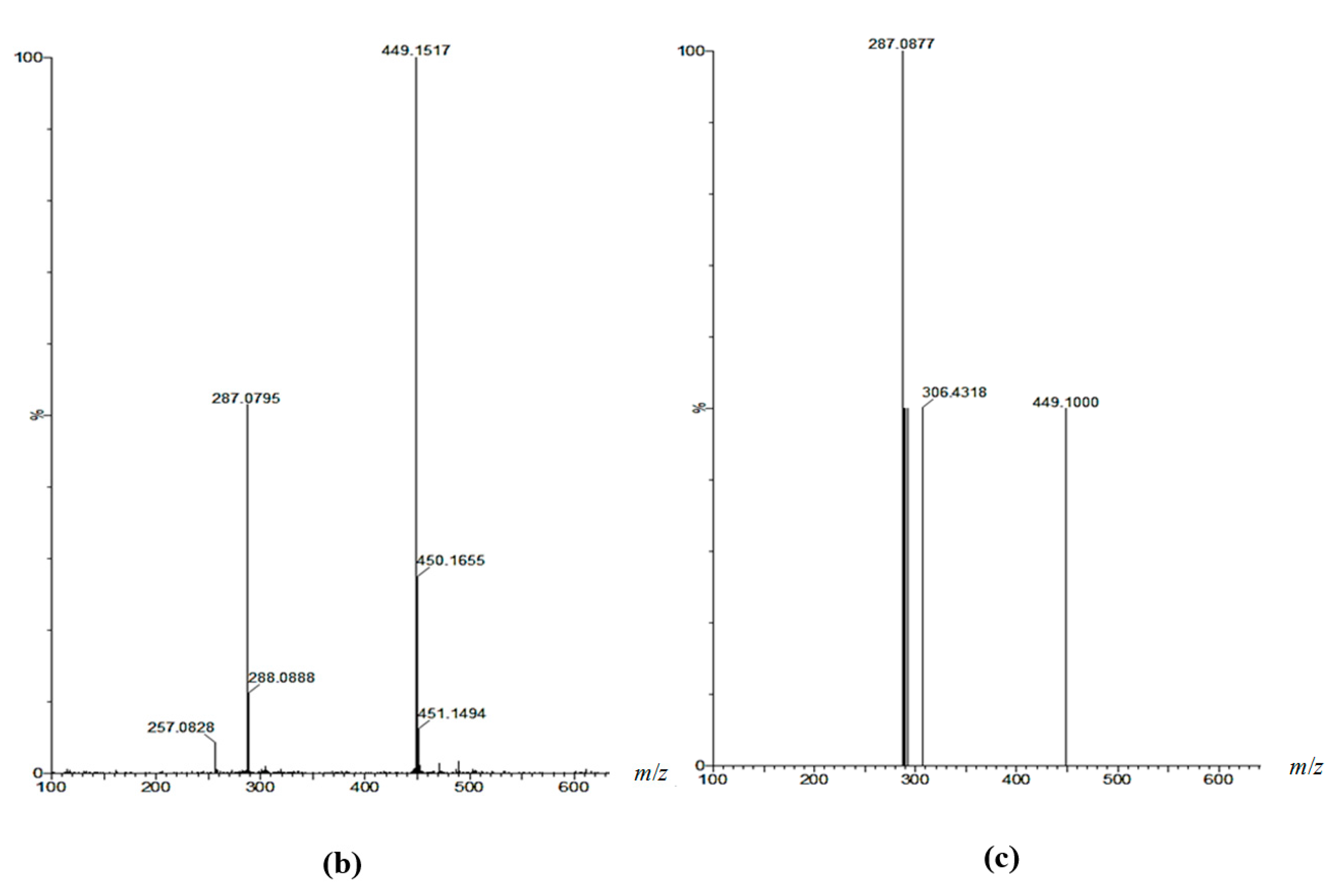
3.2. Sample Description

3.3. Bread-Making Procedure
3.4. Sample Preparation
3.5. Extraction of Soluble Phenolic Compounds
3.6. Extraction of Insoluble Phenolic Compounds
3.7. Extraction of Anthocyanins
3.8. Determination of Phenolic Content
3.9. DPPH Radical Scavenging Capacity Activity Assay
3.10. ABTS Radical Cation Decolorization Assay
3.11. Absorbance Spectra of Anthocyanin Extract at Different Wavelengths
3.12. Direct Measurement of Total Anthocyanin Content
3.13. Total Anthocyanin Content using the pH Differential Method
3.14. Identification and Quantification of Phenolic Compounds with High Performance Liquid Chromatographu
3.15. Identification of Anthocyanins in Purple Wheat with HPLC-QTOP-MS/MS
3.16. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Quideau, S.; Deffieux, D.; Couat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological anticities, and synthesis. Angew. Chem. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Okarter, N.; Liu, C.; Sorrells, M.E.; Liu, R.H. Phytochemical content and antioxidant activity of six diverse varieties of whole wheat. Food Chem. 2010, 199, 249–257. [Google Scholar] [CrossRef]
- Challacombe, C.A.; Abdel, E.M.; Seetharaman, K.; Duizer, L.M. Influence of phenolic acid content on sensory perception of bread and crackers made from red or white wheat. J. Cereal Sci. 2012, 56, 181–188. [Google Scholar] [CrossRef]
- Beta, T.; Nam, S.; Dexter, J.E.; Sapirstein, H.D. Phenolic content and antioxidant activity of pearled wheat and roller-milled fractions. Cereal Chem. 2005, 82, 390–393. [Google Scholar] [CrossRef]
- Liu, Q.; Qiu, Y.; Beta, T. Comparison of antioxidant activities of different colored wheat grains and analysis of phenolic compounds. J. Agric. Food Chem. 2010, 58, 9235–9241. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.L.; Ng, A.; Waldron, K.W. The phenolic acid and polysaccharide composition of cell walls of bran layers of mature wheat (Triticum aestivum L. cv. Avalon) grains. J. Food Sci. Agric. 2005, 85, 2539–2547. [Google Scholar] [CrossRef]
- Katina, K.; Arendt, E.; Liukkonen, K.H.; Autio, K.; Flander, L.; Poutanen, K. Potential of sourdough for healthier cereal products. Trends Food Sci. Tech. 2005, 16, 104–112. [Google Scholar] [CrossRef]
- Li, W.; Pickard, M.D.; Beta, T. Effect of thermal processing on antioxidant properties of purple wheat bran. Food Chem. 2007, 104, 1080–1086. [Google Scholar] [CrossRef]
- Adom, K.K.; Sorrells, M.E.; Liu, R.H. Phytochemical profiles and antioxidant activity of wheat varieties. J. Agric. Food Chem. 2003, 51, 7825–7834. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jimenez, J.; Diaz-Rubio, M.E.; Mesias, M.; Morales, F.J.; Saura-Calixto, F. Evidence for the formation of maillardized insoluble dietary fiber in bread: A specific kind of dietary fiber in thermally processed food. Food Res. Int. 2014, 55, 391–396. [Google Scholar] [CrossRef]
- Gelinas, P.; McKinnon, C.M. Effect of wheat variety, farming site, and bread-baking on total phenolics. Int. J. Food Sci. Tech. 2006, 41, 329–332. [Google Scholar] [CrossRef]
- Garcia-Conesa, M.T.; Wilson, P.D.; Plumb, G.W.; Ralph, J.; Williamson, G. Antioxidant properties of 4,4′-dihydroxy-3,3′-dimethoxy-β,β′-bicinnamic acid (8-8-diferulic acid, non-cyclic form). J. Sci. Food Agric. 1999, 79, 379–384. [Google Scholar] [CrossRef]
- Rondini, L.; Peyrat-Maillard, M.N.; Marsset-Baglieri, A.; Fromentin, G.; Durand, P.; Tome, D. Bound ferulic acid from bran is more bioavailable than the free compound in rat. J. Agric. Food Chem. 2004, 52, 4338–4343. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, S.; Bunzel, M. Development and application of a methodology to determine free ferulic acid and ferulic acid ester-linked to different types of carbohydrates in cereal products. Cereal Chem. 2012, 89, 247–254. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Nutritional and Pharmacological Effects of Food Phenolics. In Foodphenolics: Sources, Chemistry, Effects and Applications; Technomic Publishing Company, Inc.: Lancester, PA, USA, 1995; pp. 171–191. [Google Scholar]
- Renard, C.M.G.C.; Baron, A.; Guyot, S.; Drilleau, J.F. Interactions between apple cell walls and native apple polyphenols: Quantification and some consequences. Int. J. Biol. Macromol. 2001, 29, 115–125. [Google Scholar] [CrossRef]
- Almajano, M.P.; Delgado, M.E.; Gordon, M.H. Changes in the antioxidant properties of protein solutions in the presence of epigallocatechin gallate. Food Chem. 2007, 101, 126–130. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Quek, S.Y.; Perera, C.O. Properties of bread dough with added fibre polysaccharides and phenolic antioxidants: A review. J. Food Sci. 2010, 75, R163–R174. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.G.; Shibamoto, T. Toxicology and antioxidant activities of non-enzymatic browning reaction products: Review. Food Rev. Int. 2002, 18, 151–175. [Google Scholar] [CrossRef]
- Chandrasekara, N.; Shahidi, F. Effect of roasting on phenolic content and antioxidant activities of whole cashew nuts, kernels, and tests. J. Agric. Food Chem. 2011, 59, 5006–5014. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, R.C.; Mennella, C.; Barba, F.; Russo, M.; Russo, G.L.; Krome, K.; Erbersdobler, H.F.; Faist, V.; Fogliano, V. Characterization of coloured compounds obtained by enzymatic extraction of bakery products. Food Chem. Toxicol. 2003, 41, 1367–1374. [Google Scholar] [CrossRef]
- Yu, L.; Nanguet, A.L.; Beta, T. Comparison of antioxidant properties of refined and whole wheat flour and bread. Antioxidants 2013, 2, 370–383. [Google Scholar] [CrossRef]
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Nicoli, M.C.; Lerici, R.C. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci. Technol. 2001, 11, 340–346. [Google Scholar] [CrossRef]
- Han, H.M.; Koh, B.K. Antioxidant activity of hard wheat flour, dough and bread prepared using various processes with the addition of different phenolic acids. J. Sci. Food Agric. 2011, 91, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Yu, L.; Lu, Y.; Niu, Y.; Liu, L.; Costa, J.; Yu, L. Phytochemical compositions, and antioxidant properties, and antiproliferative activities of wheat flour. Food Chem. 2012, 135, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Beta, T. Phenolic acid composition and antioxidant potential of insoluble and soluble dietary fibre extracts derived from selected whole-grain cereals. Food Res. Int. 2013, 51, 518–525. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, Q.; Beta, T. Antioxidant properties of commercial wild rice and analysis of soluble and insoluble phenolic acids. Food Chem. 2010, 121, 140–147. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Yao, Y.; Yan, J.; He, Z. Phenolic acid profiles of Chinese wheat cultivars. J. Cereal Sci. 2012, 56, 629–635. [Google Scholar] [CrossRef]
- Whent, M.; Huang, H.; Xie, Z.; Lutterodt, H.; Yu, L.; Fuerst, E.P.; Morris, C.F.; Yu, L.; Luthria, D. Phytochemical composition, anti-inflammatory, and antiproliferative activity of whole wheat flour. J. Agric. Food Chem. 2012, 60, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Angioloni, A.; Collar, C. Polyphenol composition and “in vitro” antiradical activity of single and multigrain breads. J. Cereal Sci. 2011, 53, 90–96. [Google Scholar] [CrossRef]
- Hansen, H.B.; Andreasen, M.F.; Nielsen, M.M.; Larsen, L.M.; Knudsen, K.E.B.; Meyer, A.S.; Christensen, L.P.; Hansen, A. Changes in dietary fibre, phenolic acids and activity of endogenous enzymes during rye bread-making. Eur. Food Res. Technol. 2002, 214, 33–42. [Google Scholar] [CrossRef]
- Moore, J.; Luther, M.; Cheng, Z.; Yu, L. Effects of baking conditions, dough fermentation, and bran particle size on antioxidant properties of whole-wheat pizza crusts. J. Agric. Food Chem. 2009, 57, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Handbook of Food Analytical Chemistry Pigments, Colorant, Flavors, Textrure, and Bioactive Food Components; Wrolstad, R.E., Acree, T.E., Decker, E.A., Penner, M., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Smith, D., Sporns, P., Eds.; Wiley: Interscience, Hoboken, NJ, USA, 2005. [Google Scholar]
- Jing, P.; Noriega, V.; Schwartz, S.J.; Giusti, M. Effects of growing conditions on purple corncob (Zea mays L.) anthocyanins. J. Agric. Food Chem. 2007, 55, 8625–8629. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Hucl, P. Composition and stability of anthocyanins in blue-grained wheat. J. Agric. Food Chem. 2003, 51, 2174–2180. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, F.S.; Li, W.; Beta, T. Measurement of anthocyanins and other phytochemicals in purple wheat. Food Chem. 2008, 109, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Canadian Grain Commission. Canadian short process method. Methods and definitions. In Proceedings of the Quality of Western Canadian Wheat Exports Grain Research Laboratory, Canadian Grain Commission, Winnipeg, MB, USA, 1 February–31 July 1997; pp. 22–26.
- Young, J.C.; Abdel-Aal, E.M. Anthocyanidins. In Analysis of Bioactive Components in Small Grain Cereals; Shewry, P.R., Ward, J.L., Eds.; AACC International Press: St. Paul, MN, USA, 2010; Chapter 12; pp. 141–165. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. Assoc. Off. Anal. Chem. Int. 2005, 88, 1269–1278. [Google Scholar]
- Bicudo, M.O.P.; Ribani, R.H.; Beta, T. Anthocyanins, phenolic acids and antioxidant properties of juçara fruits (Euterpe edulis M.) along the on-tree ripening process. Plant Food Hum. Nutr. 2014, 69, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Beta, T. Identification and Antioxidant Properties of Phenolic Compounds during Production of Bread from Purple Wheat Grains. Molecules 2015, 20, 15525-15549. https://doi.org/10.3390/molecules200915525
Yu L, Beta T. Identification and Antioxidant Properties of Phenolic Compounds during Production of Bread from Purple Wheat Grains. Molecules. 2015; 20(9):15525-15549. https://doi.org/10.3390/molecules200915525
Chicago/Turabian StyleYu, Lilei, and Trust Beta. 2015. "Identification and Antioxidant Properties of Phenolic Compounds during Production of Bread from Purple Wheat Grains" Molecules 20, no. 9: 15525-15549. https://doi.org/10.3390/molecules200915525
APA StyleYu, L., & Beta, T. (2015). Identification and Antioxidant Properties of Phenolic Compounds during Production of Bread from Purple Wheat Grains. Molecules, 20(9), 15525-15549. https://doi.org/10.3390/molecules200915525





