3-H-[1,2]Dithiole as a New Anti-Trypanosoma cruzi Chemotype: Biological and Mechanism of Action Studies
Abstract
:1. Introduction
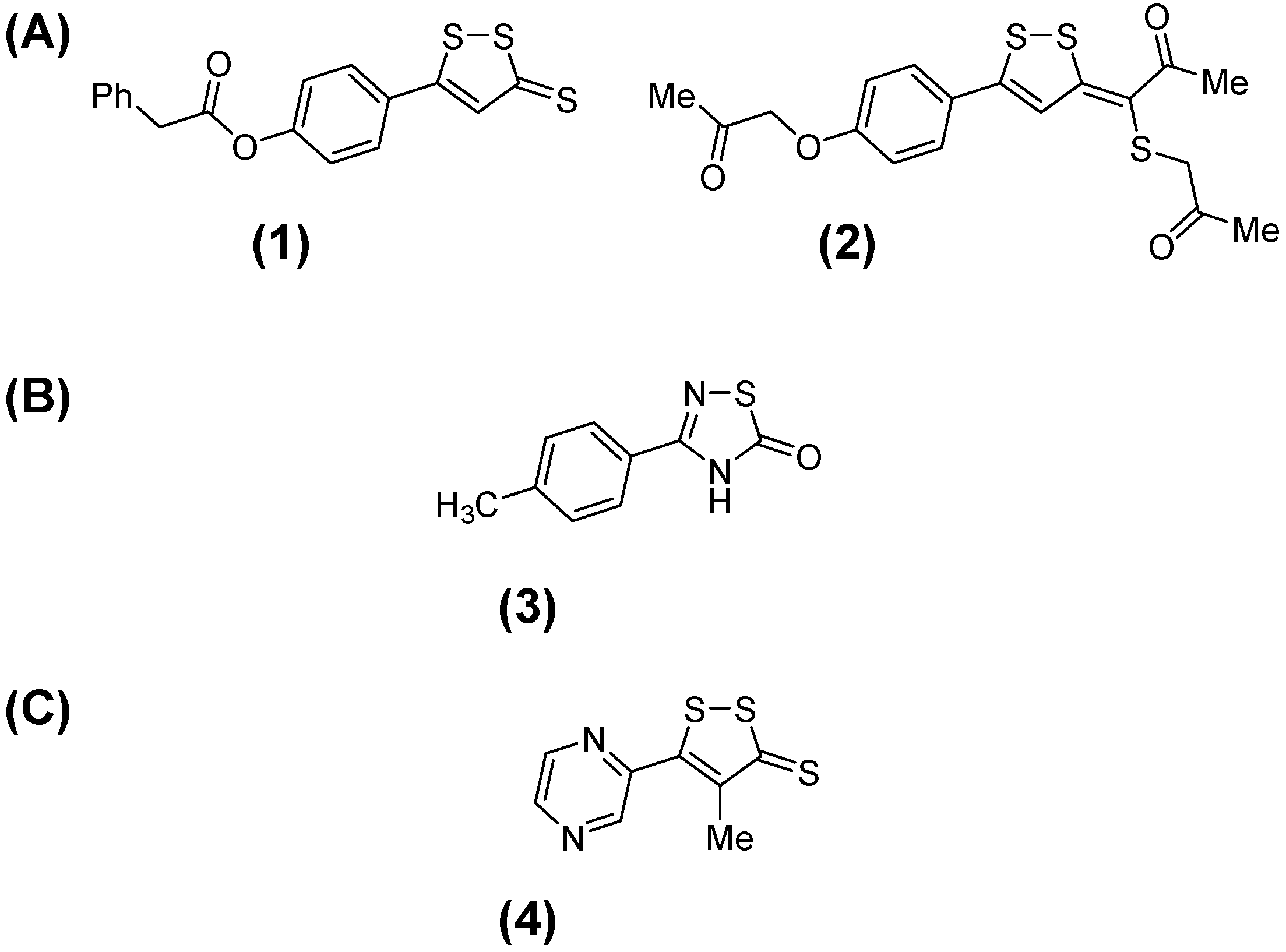
2. Results and Discussion
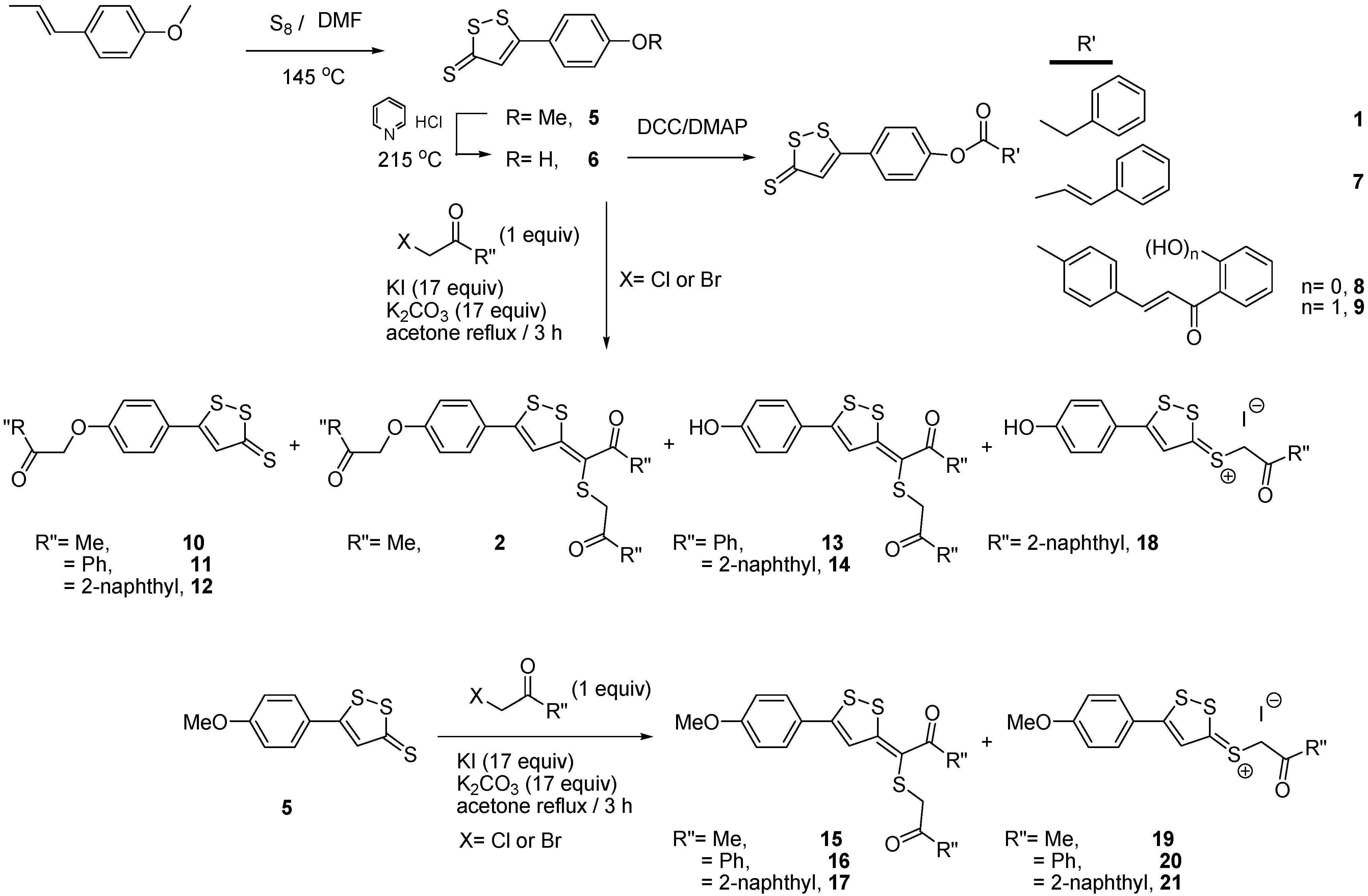
2.1. Synthesis of 3-H-[1,2]Dithiole Derivatives
2.2. In Vitro Biological Studies
| Family | Derivative | IC50 against T. cruzi (μM) | IC50 against Macrophages (μM) | SI | miLogP a |
|---|---|---|---|---|---|
| 3H-[1,2]dithiole-3-thiones | 1 | >25 | - b | - | 4.66 |
| 5 | >25 | - | - | 3.57 | |
| 6 | 25.0 ± 1.0 | 51 ± 2 | 2.0 | 3.04 | |
| 7 | >25 | - | - | 5.21 | |
| 8 | >25 | - | - | 7.26 | |
| 9 | 25.0 ± 0.5 | 63 ± 2 | 2.5 | 7.20 | |
| 10 | >25 | - | - | 3.12 | |
| 11 | >25 | - | - | 4.72 | |
| 12 | >25 | - | - | 5.91 | |
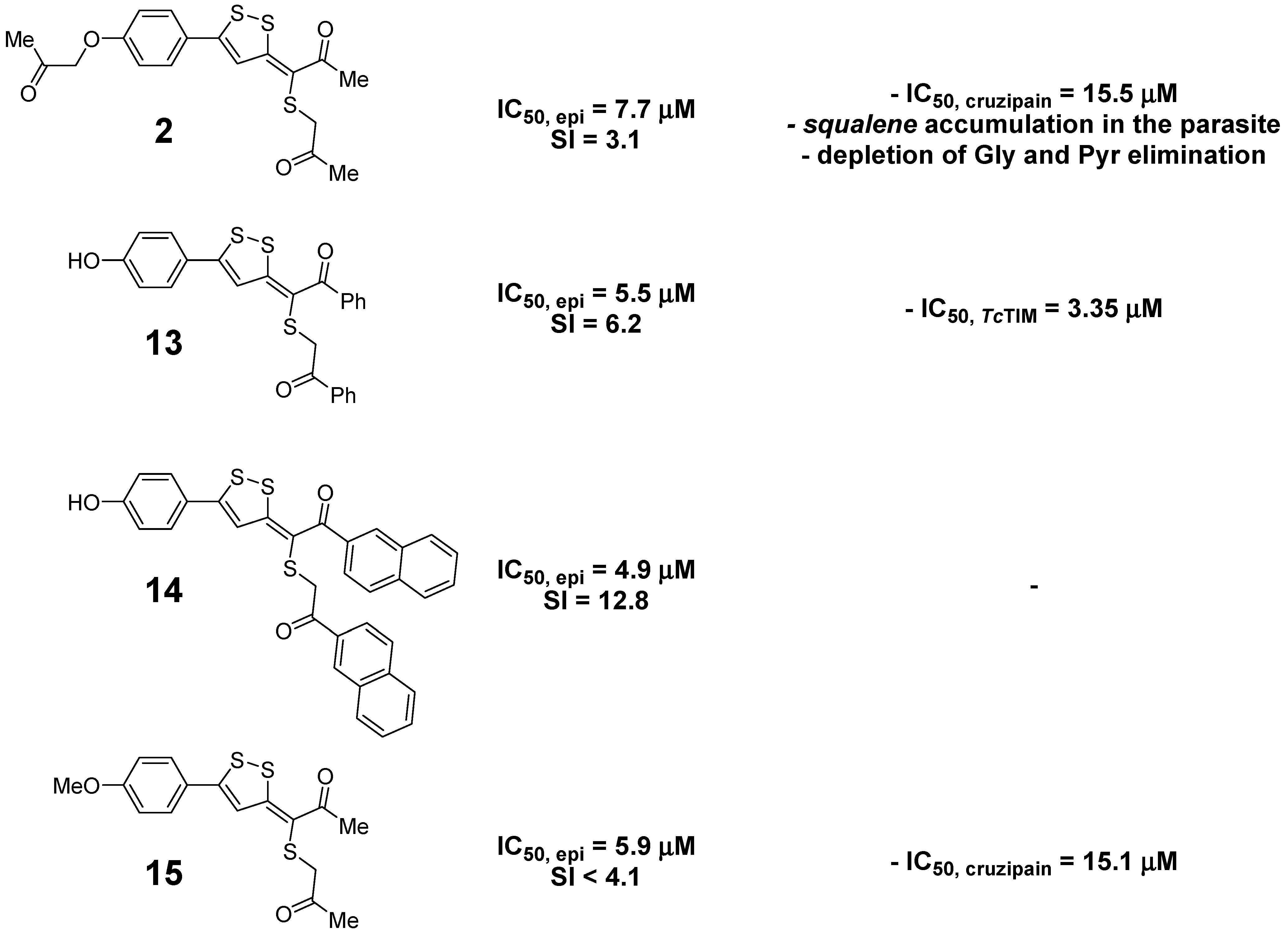
| 3-(alkylthio)propylidene-3H-[1,2]dithioles | 2 | 7.7 ± 1.4 | 24 ± 1 | 3.1 | 3.16 |
| 13 | 5.5 ± 0.9 | 34.0 ± 0.5 | 6.2 | 6.28 | |
| 14 | 4.9 ± 1.1 | 63 ± 2 | 12.8 | 8.50 | |
| 15 | 5.9 ± 1.2 | <24.0 | <4.1 | 3.61 | |
| 16 | >25 | - | - | 6.82 | |
| 17 | >25 | - | - | 8.77 | |
| [1,2]dithiolium iodide | 18 | >25 | - | 3.25 | |
| 19 | >25 | - | - | 4.07 | |
| 20 | >25 | - | - | 2.60 | |
| 21 | >25 | - | - | 3.78 |
2.3. Accessing the Mechanism of Action of the New Active Anti-T. cruzi Agents
2.3.1. Inhibition of TcTIM
| Family | Derivative | TcTIM | Cruzipain | |||
|---|---|---|---|---|---|---|
| % Inhib200 a | % Inhib25 a | IC50 (μM) | % Inhib100 a | IC50 (μM) | ||
| 3H-[1,2]dithiole-3-thiones | 1 | 77 | 33 ± 1.9 | - b | 21.3 ± 0.3 | - |
| 5 | 86 | 80 ± 2.3 | 1.2 ± 0.05 | 89.0 ± 0.5 | 4.0 ± 2.0 | |
| 6 | 71 | 35 ± 3.0 | - | 59.3 ± 0.3 | - | |
| 7 | 14 | - | - | 78.4 ± 0.6 | - | |
| 8 | ns c | - | - | 58.0 ± 0.1 | - | |
| 9 | 50 | - | - | 77.9 ± 0.6 | - | |
| 10 | 70 | 61 ± 1.0 | - | 73.9 ± 0.1 | - | |
| 11 | ns | - | - | 96.3 ± 0.7 | 17.1 ± 3.6 | |
| 12 | 70 | ni d | - | 91.5 ± 0.6 | 21.6 ± 6.1 | |
| 3-(alkylthio)propylidene-3H-[1,2]dithioles | 72 | 48 ± 4.0 | - | 91.3 ± 2.8 | 15.5 ± 2.7 | |
| 13 | 86 | 75 ± 6.0 | 3.35 ± 0.14 | 61.0 ± 0.3 | - | |
| 14 | 70 | 38 ± 3.5 | - | 66.5 ± 0.3 | - | |
| 15 | 86 | 62 ± 3.0 | - | 85.4 ± 0.2 | 15.1 ± 2.5 | |
| 16 | 77 | 63 ± 0.1 | 7.53 ± 0.10 | 62.3 ± 0.3 | - | |
| 81 | 52 ± 2.5 | - | 82.6 ± 0.6 | - | ||
| [1,2]dithiolium iodide | 70 | 75 ± 0.3 | 11.03 ±0.03 | 77.1 ± 0.1 | - | |
| 19 | 95 | 73 ± 3.2 | 3.26 ± 0.05 | 90.5 ± 0.7 | 11.3 ± 0.6 | |
| 20 | ns | - | - | 72.0 ± 0.1 | - | |
| 21 | 65 | - | - | 90.2 ± 0.6 | 17.7 ± 3.9 | |
2.3.2. Inhibition of Cruzipain
2.3.3. Inhibition of Membrane Sterol Biosynthesis
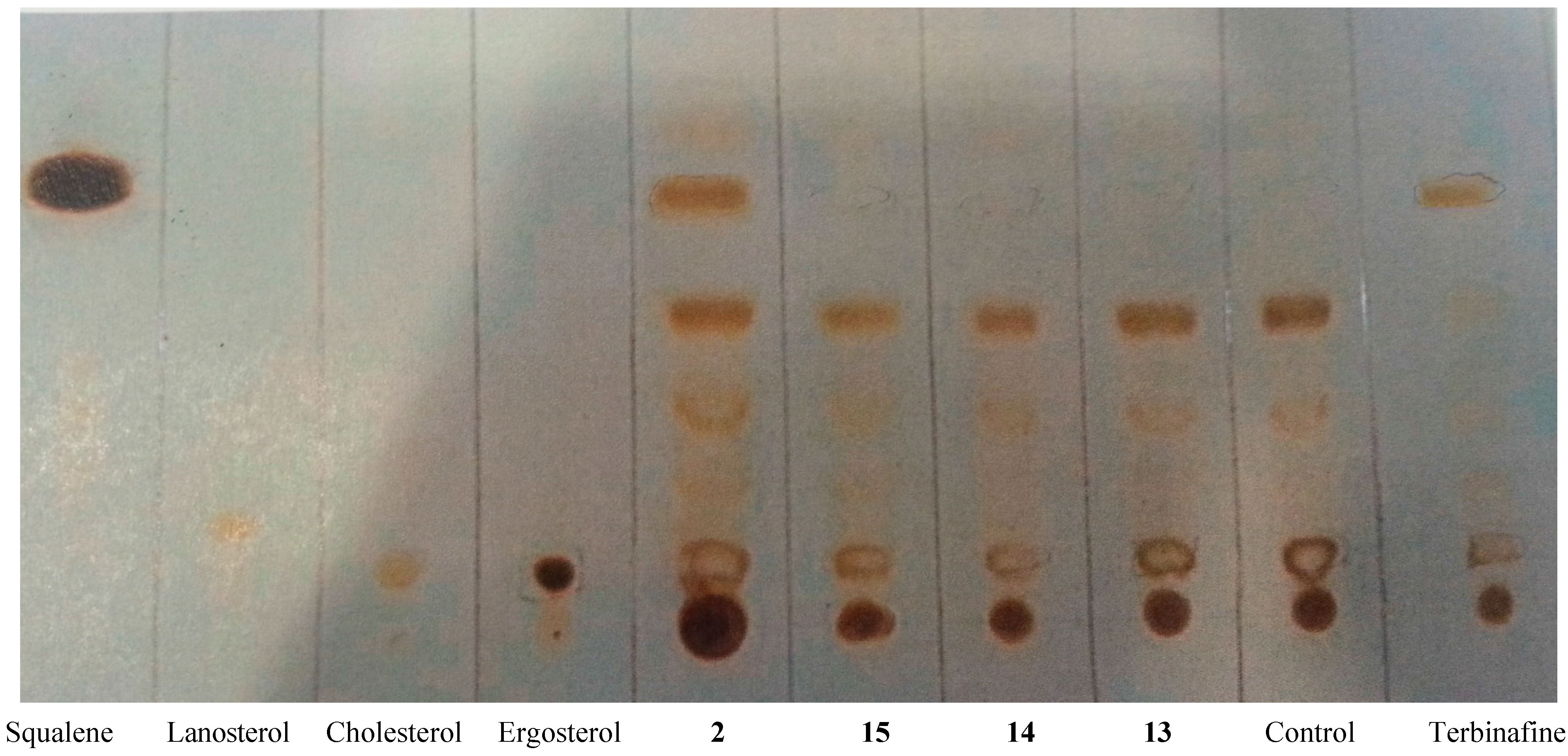
2.3.4. 1H-NMR Metabolomic Studies
| Compound a/ | Gly | Succ | Pyr | Ace | Ala | Lac |
|---|---|---|---|---|---|---|
| Metabolite b | ||||||
| 2 | 2.11 ± 0.11 c | 5.83 ± 0.24 | 8.00 ± 0.35 | 23.28 ± 0.86 | 17.76 ± 0.61 | 8.73 ± 0.37 |
| 14 | 2.48 ± 0.14 | 7.34 ± 0.65 | 9.47 ± 0.53 | 26.41 ± 1.44 | 20.65 ± 1.27 | 10.41 ± 0.58 |
| Control d | 2.48 ± 0.11 | 6.64 ± 0.18 | 8.89 ± 0.27 | 26.06 ± 2.07 | 21.38 ± 1.15 | 9.69 ± 0.68 |
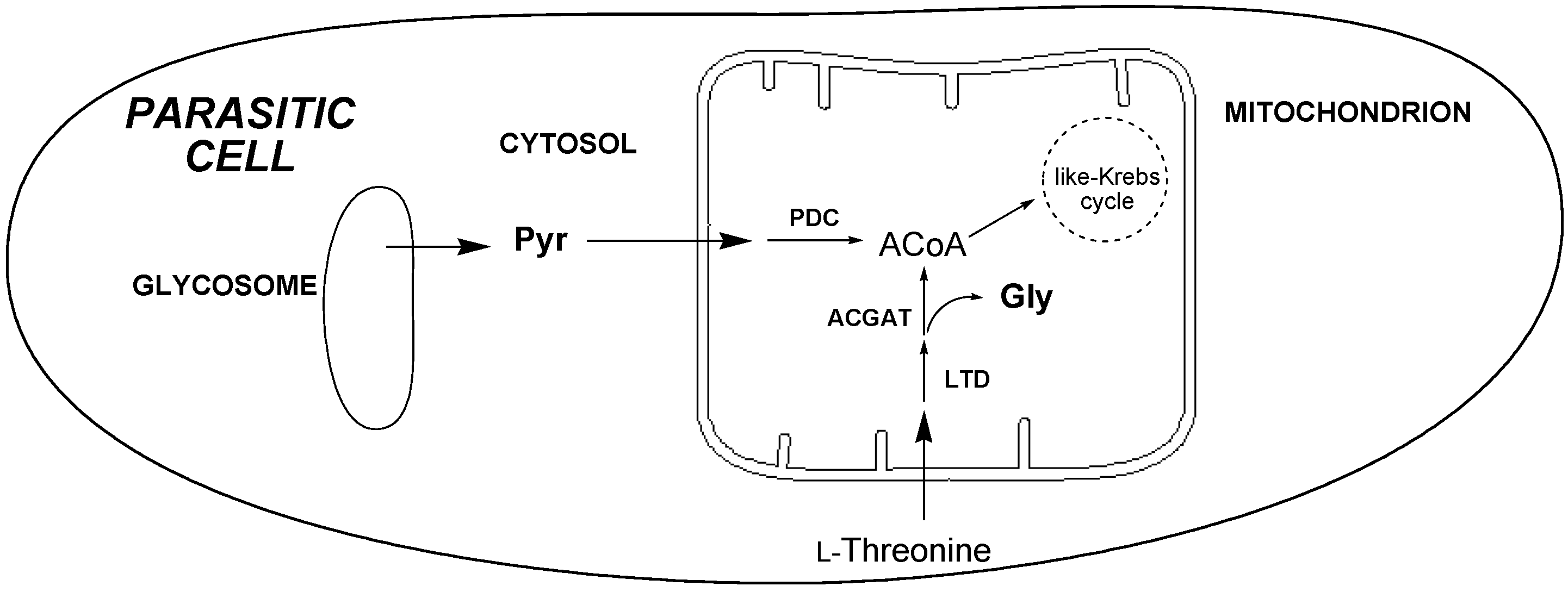
3. Experimental Section
3.1. General
3.2. General Synthetic Procedure for Compounds 12, 14, 17, 18, and 21
3.3. Anti-T. cruzi Test in Vitro
3.4. Unspecific in Vitro Cytotoxicity of Mammalian Cells
3.5. Inhibition of TcTIM
3.6. Inhibition of T. cruzi Cruzipain
3.7. Inhibition of Membrane Sterol Biosynthesis
3.8. 1H-NMR Study of the Excreted Metabolites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO). World Health Organization: Global Health Observatory Data Repository; WHO: Geneva, Switzerland, 2011; Volume 2011. [Google Scholar]
- Barrett, M.P.; Croft, S.L. Management of trypanosomiasis and leishmaniasis. Br. Med. Bull. 2012, 104, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Tekiel, V.; Alba-Soto, C.D.; González Cappa, S.M.; Postan, M.; Sánchez, D.O. Identification of vaccines candidates against Trypanosoma cruzi by immunization with sequential fractions of an epimastigote-subtracted trypomastigote cDNA expression library. Vaccine 2009, 27, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.R.; Kelly, J.M. Trypanocidal drugs: Mechanisms, resistance and new targets. Expert Rev. Mol. Med. 2009, 11, e31. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.A.; de Mecca, M.M.; Bartel, L.C. Toxic side effects of drugs used to treat Chagas’ disease (American trypanosomiasis). Hum. Exp. Toxicol. 2006, 25, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Cerecetto, H.; González, M. Anti-T. cruzi agents: Our experience in the evaluation of more than five hundred compounds. Mini Rev. Med. Chem. 2008, 8, 1355–1383. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Puyou, A.; Saavedra-Lira, E.; Becker, I.; Zubillaga, R.A.; Rojo-Dominguez, A.; Perez-Montfort, R. Using evolutionary changes to achieve species-specific inhibition of enzyme action—studies with triosephosphate isomerase. Chem. Biol. 1995, 2, 847–855. [Google Scholar] [CrossRef]
- Velanker, S.S.; Ray, S.S.; Gokhale, R.S.; Suma, S.; Balaram, H.; Balaram, P.; Murthy, M.R.N. Triosephosphate isomerase from Plasmodium falciparum: The crystal structure provides insights into antimalarial drug design. Structure 1997, 5, 751–761. [Google Scholar] [CrossRef]
- Cortés-Figueroa, A.A.; Pérez-Torres, A.; Salaiza, N.; Cabrera, N.; Escalona-Montaño, A.; Rondán, A.; Aguirre-García, M.; Gómez-Puyou, A.; Pérez-Montfort, R.; Becker, I. A monoclonal antibody that inhibits Trypanosoma cruzi growth in vitro and its reaction with intracellular triosephosphate isomerase. Parasitol. Res. 2008, 102, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, G.; Aguirre-López, B.; Varela, J.; Cabrera, M.; Merlino, A.; López, G.V.; Lavaggi, M.L.; Porcal, W.; di Maio, R.; González, M.; et al. Massive screening yields novel and selective Trypanosoma cruzi triosephosphate isomerase dimer-interface-irreversible inhibitors with anti-trypanosomal activity. Eur. J. Med. Chem. 2010, 45, 5767–5772. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, G.; Aguirre-López, B.; Cabrera, N.; Marins, E.B.; Tinoco, L.; Batthyány, C.I.; Tuena de Gómez-Puyou, M.; Puyou, A.G.; Pérez-Montfort, R.; et al. 1,2,4-Thiadiazol-5(4H)-ones: A new class of selective inhibitors of Trypanosoma cruzi triosephosphate isomerase. Study of the mechanism of inhibition. J. Enzyme Inhib. Med. Chem. 2013, 28, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, G.; Martínez, J.; Aguirre-López, B.; Cabrera, N.; Pérez-Díaz, L.; Tuena de Gómez-Puyou, M.; Gómez-Puyou, A.; Pérez-Montfort, R.; Garat, B.; et al. New chemotypes as Trypanosoma cruzi triosephosphate isomerase inhibitors: A deeper insight into the mechanism of inhibition. J. Enzyme Inhib. Med. Chem. 2014, 29, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Minini, L.; Álvarez, G.; González, M.; Cerecetto, H.; Merlino, A. Molecular docking and molecular dynamics simulation studies of Trypanosoma cruzi triosephosphate isomerase inhibitors. Insights into the inhibition mechanism and selectivity. J. Mol. Graph. Model. 2015, 58, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Couto, M.; de Ovalle, S.; Cabrera, M.; Cerecetto, H.; González, M. Searching phase II enzymes inducers, from Michael acceptor-[1,2]dithiolethione hybrids, as cancer chemopreventive agents. Future Med. Chem. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- El-Bassiouni, E.A.; Helmy, M.H.; Abdel-Hamid, M.A.; Shohayeb, M.A.; Ismail, S.S. In vitro effect of low concentrations of oltipraz on the antioxidant defence of mouse hepatocytes and Schistosoma mansoni worms. Br. J. Biomed. Sci. 2004, 61, 15–21. [Google Scholar] [PubMed]
- Bottcher, B.; Bauer, F.; Trithiones, V. Some new trithiones. Chem. Ber. 1951, 84, 458–463. [Google Scholar]
- Couto, M.; Cabrera, M.; Echeverría, G.A.; Piro, O.E.; González, M.; Cerecetto, H. A serendipitous one-step conversion of 3H-1,2-dithiole-3-thione to (E)-3-[1-(alkylthio)alkylidene]-3H-1,2-dithiole: An experimental and theoretical study. Mol. Divers. 2014, 18, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, R.O.; Agüero, F. A simple strain typing assay for Trypanosoma cruzi: Discrimination of major evolutionary lineages from a single amplification product. PLoS Negl. Trop. Dis. 2012, 6, e1777. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, G.; Varela, J.; Cruces, E.; Fernández, M.; Gabay, M.; Leal, S.M.; Escobar, P.; Sanabria, L.; Serna, E.; Torres, S.; et al. Identification of a new amide-containing thiazole as a drug candidate for treatment of Chagas’ disease. Antimicrob. Agents Chemother. 2015, 59, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Molinspiration Online Property Calculation Toolkit. Available online: http://www.molinspiration.com/cgi-bin/properties (accessed on 13 May 2015).
- Ríos, N.; Varela, J.; Birriel, E.; González, M.; Cerecetto, H.; Merlino, A.; Porcal, W. Identification of novel benzimidazole derivatives as anti-Trypanosoma cruzi agents: Solid-phase synthesis, structure-activity relationships and molecular docking studies. Future Med. Chem. 2013, 5, 1719–1732. [Google Scholar] [CrossRef] [PubMed]
- Varela, J.; Serna, E.; Torres, S.; Yaluff, G.; Vera de Bilbao, N.I.; Miño, P.; Chiriboga, X.; Cerecetto, H.; González, M. In vivo anti-Trypanosoma cruzi activity of hydro-ethanolic extract and isolated active principles from Aristeguietia glutinosa and mechanism of action studies. Molecules 2014, 19, 8488–8502. [Google Scholar] [CrossRef] [PubMed]
- Urbina, J.A. Ergosterol biosynthesis and drug development for Chagas disease. Mem. Inst. Oswaldo Cruz 2009, 104, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Mendes, T.A.; Reis Cunha, J.L.; de Almeida, L.R.; Rodrigues, L.G.F.; Lemos, L.D.; dos Santos, A.R.; da Câmara, A.C.; Galvão, L.M.; Bern, C.; Gilman, R.H.; et al. Identification of strain-specific B-cell epitopes in Trypanosoma cruzi using genome-scale epitope prediction and high-throughput immunoscreening with peptide arrays. PLoS Negl. Trop. Dis. 2013, 7, e2524. [Google Scholar] [CrossRef] [PubMed]
- Benitez, D.; Cabrera, M.; Hernández, P.; Boiani, L.; Lavaggi, M.L.; di Maio, R.; Yaluff, G.; Serna, E.; Torres, S.; Ferreira, M.E.; et al. 3-Trifluoromethylquinoxaline N,N′-dioxides as anti-trypanosomatid agents. Identification of optimal anti-T. cruzi agents and mechanism of action studies. J. Med. Chem. 2011, 54, 3624–3636. [Google Scholar] [CrossRef] [PubMed]
- Boiani, L.; Aguirre, G.; González, M.; Cerecetto, H.; Chidichimo, A.; Cazzulo, J.J.; Bertinaria, M.; Guglielmo, S. Furoxan-, alkylnitrate-derivatives and related compounds as anti-trypanosomatid agents: Mechanism of action studies. Bioorg. Med. Chem. 2008, 16, 7900–7907. [Google Scholar] [CrossRef] [PubMed]
- Bringaud, F.; Riviere, L.; Coustou, V. Energy metabolism of trypanosomatids: Adaptation to available carbon sources. Mol. Biochem. Parasitol. 2006, 149, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ostoa-Saloma, P.; Garza-Ramos, G.; Ramírez, J.; Becker, I.; Berzunza, M.; Gómez-Puyou, A.; Landa, A.; Tuena de Gómez-Puyou, M.; Pérez-Montfort, R. Cloning, expression, purification and characterization of triosephosphate isomerase from Trypanosoma cruzi. Eur. J. Biochem. 1997, 244, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Labriola, C.; Cazzulo, J.J. Purification and partial characterization of a cysteine proteinase from Trypanosoma rangeli. FEMS Microbiol. Lett. 1995, 129, 143–148. [Google Scholar] [PubMed]
- Gerpe, A.; Alvarez, G.; Benítez, D.; Boiani, L.; Quiroga, M.; Hernández, P.; Sortino, M.; Zacchino, S.; González, M.; Cerecetto, H. 5-Nitrofuranes and 5-nitrothiophenes with anti-Trypanosoma cruzi activity and ability to accumulate squalene. Bioorg. Med. Chem. 2009, 17, 7500–7509. [Google Scholar] [CrossRef] [PubMed]
- Benítez, D.; Casanova, G.; Cabrera, G.; Galanti, N.; Cerecetto, H.; González, M. Initial studies on mechanism of action and cell death of active N-oxide-containing heterocycles in Trypanosoma cruzi epimastigotes in vitro. Parasitology 2014, 141, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.J.; Manssour Fraga, C.A. New insights for multifactorial disease therapy: The challenge of the symbiotic drugs. Curr. Drug Ther. 2008, 3, 1–13. [Google Scholar]
- Sample Availability: Samples of the studied compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couto, M.; Sánchez, C.; Dávila, B.; Machín, V.; Varela, J.; Álvarez, G.; Cabrera, M.; Celano, L.; Aguirre-López, B.; Cabrera, N.; et al. 3-H-[1,2]Dithiole as a New Anti-Trypanosoma cruzi Chemotype: Biological and Mechanism of Action Studies. Molecules 2015, 20, 14595-14610. https://doi.org/10.3390/molecules200814595
Couto M, Sánchez C, Dávila B, Machín V, Varela J, Álvarez G, Cabrera M, Celano L, Aguirre-López B, Cabrera N, et al. 3-H-[1,2]Dithiole as a New Anti-Trypanosoma cruzi Chemotype: Biological and Mechanism of Action Studies. Molecules. 2015; 20(8):14595-14610. https://doi.org/10.3390/molecules200814595
Chicago/Turabian StyleCouto, Marcos, Carina Sánchez, Belén Dávila, Valentina Machín, Javier Varela, Guzmán Álvarez, Mauricio Cabrera, Laura Celano, Beatriz Aguirre-López, Nallely Cabrera, and et al. 2015. "3-H-[1,2]Dithiole as a New Anti-Trypanosoma cruzi Chemotype: Biological and Mechanism of Action Studies" Molecules 20, no. 8: 14595-14610. https://doi.org/10.3390/molecules200814595
APA StyleCouto, M., Sánchez, C., Dávila, B., Machín, V., Varela, J., Álvarez, G., Cabrera, M., Celano, L., Aguirre-López, B., Cabrera, N., De Gómez-Puyou, M. T., Gómez-Puyou, A., Pérez-Montfort, R., Cerecetto, H., & González, M. (2015). 3-H-[1,2]Dithiole as a New Anti-Trypanosoma cruzi Chemotype: Biological and Mechanism of Action Studies. Molecules, 20(8), 14595-14610. https://doi.org/10.3390/molecules200814595