Abstract
The antiproliferative activity of a set of seven natural Amaryllidaceae alkaloids and 32 derivatives against four cancer cell lines (A2780, SW1573, T47-D and WiDr) was determined. The best antiproliferative activities were achieved with alkaloids derived from pancracine (2), haemanthamine (6) and haemantidine (7). For each skeleton, some structure-activity relationships were outlined.
1. Introduction
Cancer is a major health problem all over the world. It is responsible of the death of over 8 million people every year, and almost 600,000 deaths in the United States, during 2014 [1]. Alkaloids, such as paclitaxel, vincristine or vinblastine, are known by possessing important antitumor properties and have been used in the last years for the treatment of cancer [2].
The Amaryllidaceae alkaloids have gained much interest because of their wide range of biological activities. For example, acetylcholinesterase [3], analgesic [4], antifungic [5] and antimalarial [6,7,8] activities have been reported for these alkaloids. Since the isolation of pancratistatin [9], a narciclasine-type alkaloid, and the discovery of its important antitumor properties [10], representative alkaloids of this family have been evaluated as potential cytotoxic agents [11]. More recently, some studies focus on the potential as anticancer agents of semisynthetic derivatives of lycorine [12], narciclasine [13] and crinine [14] have also been performed.
As a part of our ongoing research on Pancratium alkaloids, this work reports the antiproliferative activity of some Amaryllidaceae alkaloids, and semisynthetic derivatives with pancracine, homolycorine and haemanthamine skeletons, against four human tumor cell lines (A2780 ovary, SW1573 lung, T-47D breast and WiDr colon). Some structure-activity relationships are also presented.
2. Results and Discussion
Fresh bulbs from Pancratium canariense were chopped and macerated with MeOH for two weeks at room temperature. The bulbs were filtered, dried, and powdered for a second extraction, using a Soxhlet apparatus with MeOH. Both extracts were collected, concentrated and treated as described [15]. Tazettine (1) (3 mg), pancracine (2) (17 mg), hippeastrine (3) (1.35 g), vittatine (4) (8 mg), 11-hydroxyvittatine 5 (123 mg), haemanthamine 6 (2.01 g) and haemanthidine 7 (360 mg) were isolated (Figure 1).
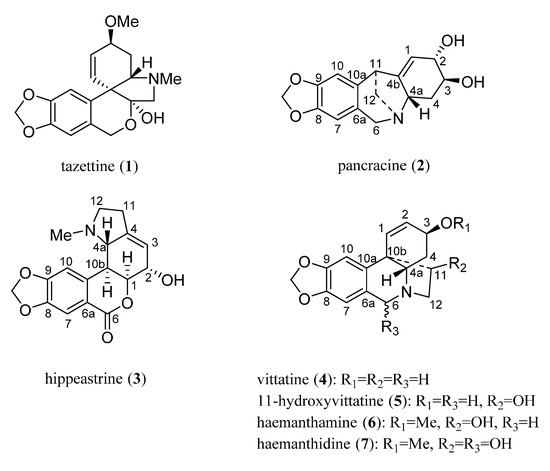
Figure 1.
Structure of Amaryllidaceae alkaloids from Pancratium canariense.
Several derivatives from the major alkaloids hippeastrine (3), 11-hydroxyvittatine (5), haemanthamine (6) and haemanthidine (7) were prepared with the aim to generate structurally diverse compounds [6,7,8].
Scheme 1 shows the preparation of montanine-type derivatives 2a and 2b by thionyl chloride mediated rearrangement of 6 and 7, respectively [16].
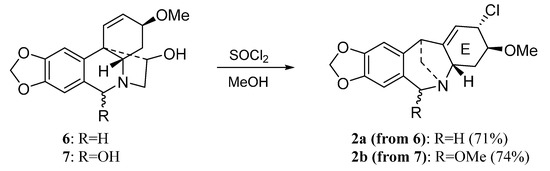
Scheme 1.
Preparation of montanine-type derivatives 2a and 2b.
Scheme 2 summarizes the modifications achieved on the structure of the homolycorine-type alkaloid hippeastrine 3 [7]. Derivatives were obtained by modifying the methylendioxy group (3a), the lactone moiety (3b), the aminomethyl group (3c), the hydroxyl group at C-2 (3d–3g), and the double bond at C3–C4 (3h,3i). Compounds 3j and 3k were also obtained under treatment with SOCl2.
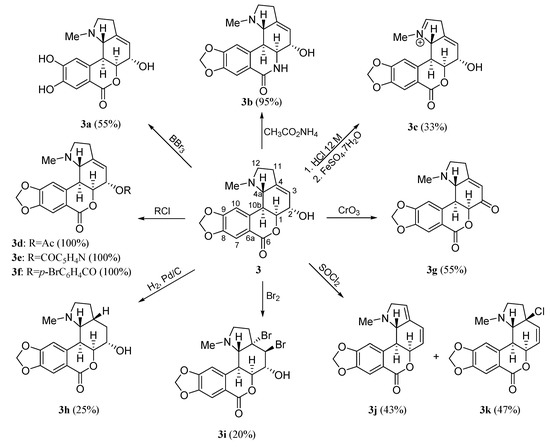
Scheme 2.
Preparation of homolycorine-type derivatives 3a–3k.
The haemanthamine-type derivatives are shown in Scheme 3, Scheme 4 and Scheme 5 [6]. These compounds have been prepared from the alkaloids 11-hydroxyvittatine 5 (compounds 5a–5c, Scheme 3), haemanthamine 6 (derivatives 6a–6k, Scheme 4) and haemanthidine 7 (compounds 7a–7e, Scheme 5). Similarly, the transformations carried out with compound 3 were performed with these alkaloids.
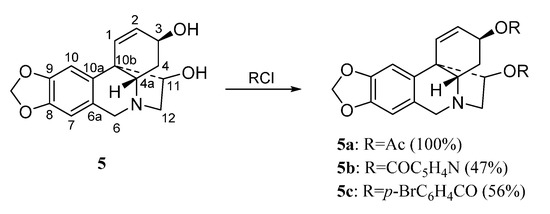
Scheme 3.
Preparation of derivatives 5a–5c from 11-hydroxyvittatine (5).
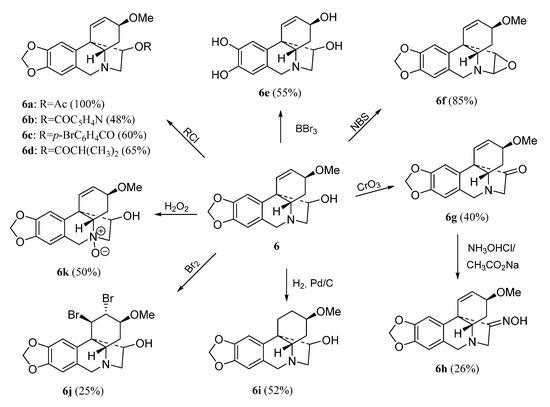
Scheme 4.
Preparation of derivatives 6a–6k from haemanthamine (6).

Scheme 5.
Preparation of derivatives 7a–7e from haemanthidine (7).
All compounds were tested for their antiproliferative activity against the human solid tumor cell lines A2780 (ovary), SW1573 (lung), T-47D (breast) and WiDr (colon) [17]. The data on antiproliferative activity shown in Table 1 allows a classification of the compounds in three groups.

Table 1.
In vitro antiproliferative activity against human solid tumor cells a.
| Compound | A2780 | SW1573 | T47-D | WiDr |
|---|---|---|---|---|
| 1 | ≥100 | ≥100 | ≥100 | ≥100 |
| 2 | 8.3 ± 0.5 | 4.3 ± 0.7 | 6.5 ± 2.0 | 9.1 ± 1.0 |
| 2a | 3.4 ± 1.0 | 3.9 ± 0.7 | 8.8 ± 1.0 | 7.5 ± 2.0 |
| 2b | 75.2 ± 25.0 | ≥100 | ≥100 | ≥100 |
| 3 | 16.5 ± 10.0 | 12.5 ± 7.0 | 52.9 ± 14.0 | 39.1 ± 20.0 |
| 3a | 41.1 ± 3.0 | 90.3 ± 11.0 | ≥ 100 | ≥100 |
| 3b | 16.8 ± 7.0 | 17.3 ± 9.0 | 26.7 ± 10.0 | 29.1 ± 11.0 |
| 3c | ≥100 | ≥100 | ≥100 | ≥100 |
| 3d | 58.7 ± 7.0 | 91.5 ± 10.0 | ≥100 | ≥100 |
| 3e | ≥100 | ≥100 | ≥100 | ≥100 |
| 3f | ≥100 | ≥100 | ≥100 | ≥100 |
| 3g | 14.6 ± 8.0 | 25.0 ± 6.0 | ≥100 | ≥100 |
| 3h | ≥100 | ≥100 | ≥100 | ≥100 |
| 3i | 67.2 ± 13.0 | ≥100 | ≥100 | ≥100 |
| 3j | ≥100 | ≥100 | ≥100 | ≥100 |
| 3k | 54.9 ± 24.0 | ≥100 | ≥100 | ≥100 |
| 4 | ≥100 | ≥100 | ≥100 | ≥100 |
| 5 | 21.0 ± 2.0 | 16.9 ± 4.0 | 12.5 ± 9.0 | 21.1 ± 6.0 |
| 5a | ≥100 | ≥100 | ≥100 | ≥100 |
| 5b | 35.9 ± 5.0 | 34.8 ± 4.0 | 51.1 ± 3.0 | 53.8 ± 3.0 |
| 5c | 100 | 100 | 100 | 100 |
| 6 | 0.68 ± 0.2 | 2.1 ± 2.0 | 0.87 ± 0.4 | 1.2 ± 0.5 |
| 6a | ≥100 | ≥100 | ≥100 | ≥100 |
| 6b | 27.2 ± 10.0 | 29.5 ± 12.0 | 72.3 ± 24.0 | 63.3 ± 35.0 |
| 6c | 19.1 ± 1.0 | 21.9 ± 4.0 | 46.1 ± 30.0 | 32.8 ± 22.0 |
| 6d | ≥100 | ≥100 | ≥100 | ≥100 |
| 6e | ≥100 | ≥100 | ≥100 | ≥100 |
| 6f | ≥100 | ≥100 | ≥100 | ≥100 |
| 6g | 1.5 ± 0.1 | 2.7 ± 0.1 | 4.4 ± 1.5 | 3.5 ± 2.0 |
| 6h | 33.2 ± 2.0 | 39.1 ± 20.0 | 78.6 ± 24.0 | 67.4 ± 34.0 |
| 6i | 31.4 ± 7.0 | 29.8 ± 3.0 | ≥100 | 58.9 ± 12.0 |
| 6j | ≥100 | ≥100 | ≥100 | ≥100 |
| 6k | 27.2 ± 5.0 | 22.0 ± 20.0 | ≥100 | ≥100 |
| 7 | 1.5 ± 0.1 | 2.0 ± 1.0 | 1.8 ± 1.0 | 2.7 ± 2.0 |
| 7a | ≥100 | ≥100 | ≥100 | ≥100 |
| 7b | 6.9 ± 1.0 | 4.5 ± 0.7 | 8.2 ± 1.2 | 10.1 ± 0.5 |
| 7c | ≥100 | ≥100 | ≥100 | ≥100 |
| 7d | ≥100 | ≥100 | ≥100 | ≥100 |
| 7e | ≥100 | ≥100 | ≥100 | ≥100 |
a Values representing GI50 are given in µM and are means of two to three experiments.
A first group is formed with the inactive compounds (GI50 values ≥ 100 µM). Tazettine (1) and vittatine (4) belong to this group. The second group includes compounds with GI50 values in the range of 10–100 µM, indicating a moderate activity. Alkaloids hippeastrine (3) and 11-hydroxyvittatine (5) are found in this group. The last and smallest group is composed of those products with GI50 ≤ 10 µM, being the most active alkaloids, haemanthamine (6) and haemanthidine (7), together with the montanine-type alkaloid pancracine (2).
Some physicochemical descriptors (MW, LogP, H-bond donors, H-bond acceptors, Rotable bonds and TPSA) of all tested compounds were calculated using Molinspiration Cheminformatics software (2014) and the corresponding values are included in Table 2.

Table 2.
Physicochemical descriptors a,b.
| Compound | MW | LogP | H-Bond Donors | H-Bond Acceptors | Rotable Bonds | TPSA |
|---|---|---|---|---|---|---|
| 1 | 331 | 1.53 | 1 | 6 | 1 | 60.40 |
| 2 | 287 | 0.54 | 2 | 5 | 1 | 62.16 |
| 2a | 319 | 2.39 | 0 | 4 | 1 | 30.94 |
| 2b | 349 | 2.55 | 0 | 5 | 2 | 40.17 |
| 3 | 315 | 1.23 | 1 | 6 | 0 | 68.24 |
| 3a | 303 | 0.37 | 3 | 6 | 0 | 90.23 |
| 3b | 314 | 0.59 | 2 | 6 | 0 | 71.03 |
| 3c | 314 | −2.91 | 1 | 6 | 0 | 68.01 |
| 3d | 357 | 1.93 | 0 | 7 | 2 | 74.32 |
| 3e | 420 | 2.37 | 0 | 8 | 3 | 87.21 |
| 3f | 498 | 4.47 | 0 | 7 | 3 | 74.32 |
| 3g | 313 | 1.04 | 0 | 6 | 0 | 65.08 |
| 3h | 317 | 1.41 | 1 | 6 | 0 | 68.24 |
| 3i | 475 | 2.29 | 1 | 6 | 0 | 68.24 |
| 3j | 297 | 2.12 | 0 | 5 | 0 | 48.01 |
| 3k | 333 | 2.60 | 0 | 5 | 0 | 48.01 |
| 4 | 271 | 1.59 | 1 | 4 | 0 | 41.93 |
| 5 | 287 | 0.67 | 2 | 5 | 0 | 62.16 |
| 5a | 371 | 2.08 | 0 | 7 | 4 | 74.32 |
| 5b | 497 | 2.38 | 0 | 9 | 6 | 100.10 |
| 5c | 653 | 7.15 | 0 | 7 | 6 | 74.32 |
| 6 | 301 | 1.29 | 1 | 5 | 1 | 51.17 |
| 6a | 343 | 1.99 | 0 | 6 | 3 | 57.25 |
| 6b | 406 | 2.14 | 0 | 7 | 4 | 70.14 |
| 6c | 484 | 4.52 | 0 | 6 | 4 | 57.25 |
| 6d | 383 | 3.46 | 0 | 6 | 4 | 57.25 |
| 6e | 275 | −0.19 | 4 | 5 | 0 | 84.15 |
| 6f | 299 | 1.74 | 0 | 5 | 1 | 43.47 |
| 6g | 299 | 1.10 | 0 | 5 | 1 | 48.01 |
| 6h | 314 | 1.55 | 1 | 6 | 1 | 63.53 |
| 6i | 301 | 1.29 | 1 | 5 | 1 | 51.17 |
| 6j | 461 | 2.22 | 1 | 5 | 1 | 51.17 |
| 6k | 317 | 1.25 | 1 | 6 | 1 | 65.00 |
| 7 | 317 | 0.83 | 2 | 6 | 1 | 71.40 |
| 7a | 401 | 2.23 | 0 | 8 | 5 | 83.55 |
| 7b | 422 | 1.68 | 1 | 8 | 4 | 90.37 |
| 7c | 683 | 7.30 | 0 | 8 | 7 | 83.55 |
| 7d | 481 | 5.17 | 0 | 8 | 7 | 83.55 |
| 7e | 291 | −0.65 | 5 | 6 | 0 | 104.38 |
a Values were calculated using Molinspiration Cheminformatics software (Molinspiration, Slovensky Grob, Slovak Republic, 2015, http://www.molinspiration.com); b Optimal range MW < 500, LogP < 5, H-bond donors < 5, H-bond acceptors < 10, Rotable bonds < 5, TPSA < 140.
With respect to the antiproliferative activity of tazzetine (1), haemanthamine (6), and haemanthidine (7), our results are consistent with those obtained by Evidente et al. [16] for their antiproliferative activity against six different cancer cell lines (A549, OE21, Hs683, U373, SKMEL and B16F10). Thus tazzetine also turned out to be inactive, and haemanthamine (6) and haemanthidine (7) had IC50 values ranging from 3.1 to 8.5 µM.
From the results of antiproliferative activity some structure-activity relationships can be outlined. The replacement of the hydroxyl groups in the E ring of pancracine (2) by a chlorine and a methoxy group (2a), respectively, produced a similar result but the introduction of a methoxy group at C-6 (2b) reduced the antiproliferative activity. Since 2a and 2b have similar Log P values, the steric hindrance at C-6 seems an important factor for the activity of this series of compounds.
Regarding to the alkaloids of the hippeastrine series, all modifications made at the hydroxyl group of hippeastrine (3) (derivatives 3d–3g) produced a significant loss of the activity, indicating the importance of a hydrogen-bond-donor (HBD) at C-2. The role of the C3-C4 double bond was evident since inactive derivatives 3h and 3i were obtained under hydrogenation or bromination. Another important group is the methylendioxy because when this group was converted into the corresponding aromatic diol 3a, the activity decreased drastically. On the other hand, the presence of the lactone ring is not essential for the activity, thus when the lactone moiety was transformed into the lactam (derivative 3b), similar activities were obtained.
Comparison of the antiproliferative activities of the natural alkaloids vittatine (4), 11-hydroxyvittatine (5), haemanthamine (6) and haemanthidine (7) indicate how important are for the activity the presence of a methoxy group at C-3 together an hydroxyl group at C-11.
These facts were confirmed with the preparation of derivatives 6a–f. Furthermore compounds 5, 6 and 7 have lower LogP than the inactive compound 4. The obtention of the inactive derivatives 6i and 6j shows the importance of the double bond at C1-C2 for the activity. Removal of the methylendioxy group also led to a less active compound (6k), indicating that this group is also important for the haemanthamine series. Haemanthidine 7, which possesses a hydroxyl group at C-6, can be considered as active as haemanthamine 6. The acylation of the hydroxyl groups at C-6 and C-11 produces a loss of antiproliferative activity (7a, 7c, 7d) but compound 7b having a free hydroxyl at C-11 and a nicotinoyl group at C-6.
Since most of the alkaloids evaluated for antiproliferative activity were also previously evaluated for antimalarial activity [6,7], a comparative antiproliferative vs. antimalarial SAR study is included. For antimalarial activity the natural alkaloids tazzettine (1) and vittatine (4) were active against Plasmodium falciparum, while they were inactive for antiproliferative activity. With respect to the compounds related to pancracine (2), we obtained identical SAR for both activities. Regarding the derivatives 3a–3k, similar SAR were determined for the modifications on the hydroxyl group at C-2, and on the double bond C-3–C-4. The aromatic diol 3a obtained from the transformation of the methylendioxy group resulted inactive for antiproliferative activity, but it showed good antimalarial activity. The same behavior was detected for compound 6e. In the derivatives obtained from the diol 5 all diesterified compounds resulted inactive for antiproliferative activity but compound 5b, with two nicotinoyl groups, which showed high antimalarial activity. For the derivatives obtained from 6, all modifications carried out on the hydroxyl group at C-11 led to a loss of antimalarial and antiproliferative activity, while the hydrogenation of the double bond produced opposite results; compound 6i resulted inactive for antiproliferative activity and had antiplasmodial activity. Finally, similar SAR for antiproliferative and antimalarial activities were obtained for the derivatives 7a–7d.
3. Experimental Section
3.1. Natural and Semisynthetic Amaryllidaceae Alkaloids
The natural alkaloids Tazettine (1) (3 mg), pancracine (2) (17 mg), hippeastrine 3 (1.35 g), vittatine 4 (8 mg), 11-hydroxyvittatine 5 (123 mg), haemanthamine 6 (2.01 g) and haemanthidine 7 (360 mg) were isolated from Pancratium canariense as describe in reference [15]. Derivatives 2a and 2b were prepared according to the procedure described in reference [17]. Homolycorine-type derivatives 3a–3k were synthesized following the reference [7] while the haemathamine-type derivatives were obtained as describe in reference [6].
3.2. Antiproliferative Assay
Growth inhibition and cytotoxicity against the human solid tumor lines A2780 (ovary), SW1573 (lung), T-47D (breast) and WiDr (colon) was screened using the sulforhodamine B (SRB) assay described in reference [18]. Cells were inoculated at densities of 7000 (A2780), 6000 (SW1573), 15,000 (T-47D) and 10,000 (WiDr) cells per well, based on their doubling times. Pure compounds were initially dissolved in DMSO at 400 times the desired final maximum test concentration (100 µM). Control cells were exposed to an equivalent concentration of DMSO. Each agent was tested in duplicate at five different tenfold dilutions. Drug incubation times were 48 h, after which cells were precipitated with 25 µL ice-cold 50% (w/v) trichloroacetic acid and fixed for 60 min at 4 °C. Then the SRB assay was performed. The optical density (OD) of each cell was measured at 490 nm using a Bio-Tek’s Elx800 NB 96-well plate reader. The percentage growth was calculated at each of the drug concentration levels based on the difference in OD at the start and end of drug exposure. Values were corrected for background OD from wells only containing medium. The resulting biological activities are expressed as GI50, the concentration of compound responsible of a 50% growth inhibition, and are shown in Table 1.
4. Conclusions
In conclusion, a set of diverse Amaryllidaceae alkaloids with different skeletons has been tested for antiproliferative activity. The compounds belonging to the pancracine and haemantine series were the most active. From the obtained result the key structural requirements for each series were outlined. The best antiproliferative activities were achieved with the natural alkaloids 6 and 7 and also with the derivatives 6g and 7b. The physicochemical descriptors (Table 2) of these compounds do not violate the optimal requirements for druggability, which suggests that these alkaloids are promising lead compounds for further research.
Acknowledgments
We gratefully acknowledge the financial support from Spanish MINECO (SAF2012-37344-C03-C01) and Instituto de Salud Carlos III (PI11/00840). These projects are also co-founded by the European Regional Development Fund (ERDF). We also thank EU Research Potential (FP7-REGPOT-2012-61367-IMBRAIN).
Author Contributions
Isolation, preparation of the derivatives and structural determination (JCC, AGR, AEB). Antiproliferative activity (JMP, LGL).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer Statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Plants as source of anticancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- McNulty, J.; Nair, J.; Little, J.; Brennan, D.; Bastida, J. Structure-activity studies on acetylcholinesterase inhibition in the lycorine series of Amaryllidaceae alkaloids. Bioorg. Med. Chem. Lett. 2010, 20, 5290–5294. [Google Scholar] [CrossRef] [PubMed]
- Citoglu, G.; Acikara, O.; Yilmaz, B.; Özbek, H. Evaluation of analgesic, anti-inflammatory and hepatoprotective effects of lycorine from Sternbergia fisheriana (Herbert) Rupr. Fitoterapia 2012, 83, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Andolfi, A.; Abou-Donia, A.H.; Touema, S.M.; Hammoda, H.M.; Shawky, E.; Motta, A. (−)-Amarbellisine, a lycorine-type alkaloid from Amaryllis belladonna L. growing in Egypt. Phytochemistry 2004, 65, 2113–2118. [Google Scholar] [CrossRef] [PubMed]
- Cedrón, J.; Gutiérrez, L.; Flores, N.; Ravelo, A.; Estévez-Braun, A. Synthesis and antimalarial activity of new haemanthamine-type derivatives. Bioorg. Med. Chem. 2012, 20, 5464–5472. [Google Scholar] [CrossRef] [PubMed]
- Cedrón, J.; Gutiérrez, L.; Flores, N.; Ravelo, A.; Estévez-Braun, A. Preparation and antimalarial activity of semisynthetic lycorenine derivative. Eur. J. Med. Chem. 2013, 63, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Cedrón, J.; Gutiérrez, L.; Flores, N.; Ravelo, A.; Estévez-Braun, A. Synthesis and antiplasmodial activity of lycorine derivatives. Bioorg. Med. Chem. 2010, 18, 4694–4701. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Gaddamidi, V.; Herald, D.L.; Singh, S.B.; Cragg, G.M.; Schmidt, J.M.; Boettner, F.E.; Williams, M.; Sagawa, Y. Antineoplastic Agents, 120. Pancratium littorale. J. Nat. Prod. 1986, 49, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, A.; Evidente, A. Chemistry, Biology, and Medicinal Potential of Narciclasine and its Congeners. Chem. Rev. 2008, 108, 1982–2014. [Google Scholar] [CrossRef] [PubMed]
- Cedrón, J.; Del Arco-Aguilar, M.; Estévez-Braun, A.; Ravelo, A. Chemistry and biology of Pancratium alkaloids. Alkaloids Chem. Biol. 2010, 68, 1–37. [Google Scholar] [PubMed]
- Wang, P.; Yuan, H.; Zhang, X.; Li, Y.; Shang, L.; Yin, Z. Novel Lycorine Derivatives as Anticancer Agents: Synthesis and in Vitro Biological Evaluation. Molecules 2014, 19, 2469–2480. [Google Scholar] [CrossRef] [PubMed]
- Ingrassia, L.; Lefranc, F.; Dewelle, J.; Pottier, L.; Mathieu, V.; Spiegl-Kreinecker, S.; Sauvage, S.; El Yazidi, M.; Dehoux, M.; Berger, W.; et al. Structure-Activity Relationship Analysis of Novel Derivatives of Narciclasine (an Amaryllidaceae Isocarbostyril Derivative) as Potential Anticancer Agent. J. Med. Chem. 2009, 52, 1100–1114. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Kireev, A.; Jenkins, A.; Romero, A.; Steelant, W.; Van Slambrouck, S.; Kornienko, A. Biological Evaluation of Structurally Diverse Amaryllidaceae Alkaloids and their Synthetic Derivatives: Discovery of Novel Leads for Anticancer Drug Design. Planta Med. 2009, 75, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Cedrón, J.; Oberti, J.; Estévez-Braun, A.; Ravelo, A.; del Arco-Aguilar, M.; López, M. Pancratium canariense as an important source of Amaryllidaceae alkaloids. J. Nat. Prod. 2009, 72, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Van Goietsenoven, G.; Andolfi, A.; Lallemand, B.; Cimmino, A.; Lamoral-Theys, D.; Gras, T.; Abou-Donia, A.; Dubois, J.; Lefranc, F.; Mathieu, V.; et al. Amaryllidaceae alkaloids belonging to different structural subgroups display activity against apoptosis-resistant cancer cells. J. Nat. Prod. 2010, 73, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Cedrón, J.; Estévez-Braun, A.; Ravelo, A.; Gutiérrez, D.; Flores, N.; Bucio, M.; Pérez-Hernández, N.; Joseph-Nathan, P. Bioactive montanine derivatives from halide-induced rearrangements of haemanthamine-type alkaloids. Absolute configuration by VCD. Org. Lett. 2009, 11, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Miranda, P.; Padrón, J.; Padrón, J.; Villar, J.; Martín, V. Synthesis and SAR Study of Cytotoxic Alkyl Chloro Dihydropyrans. Chem. Med. Chem. 2006, 1, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).