Photochemical and Photophysical Properties of Phthalocyanines Modified with Optically Active Alcohols
Abstract
:1. Introduction
2. Results and Discussion
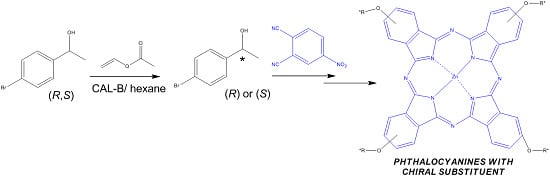
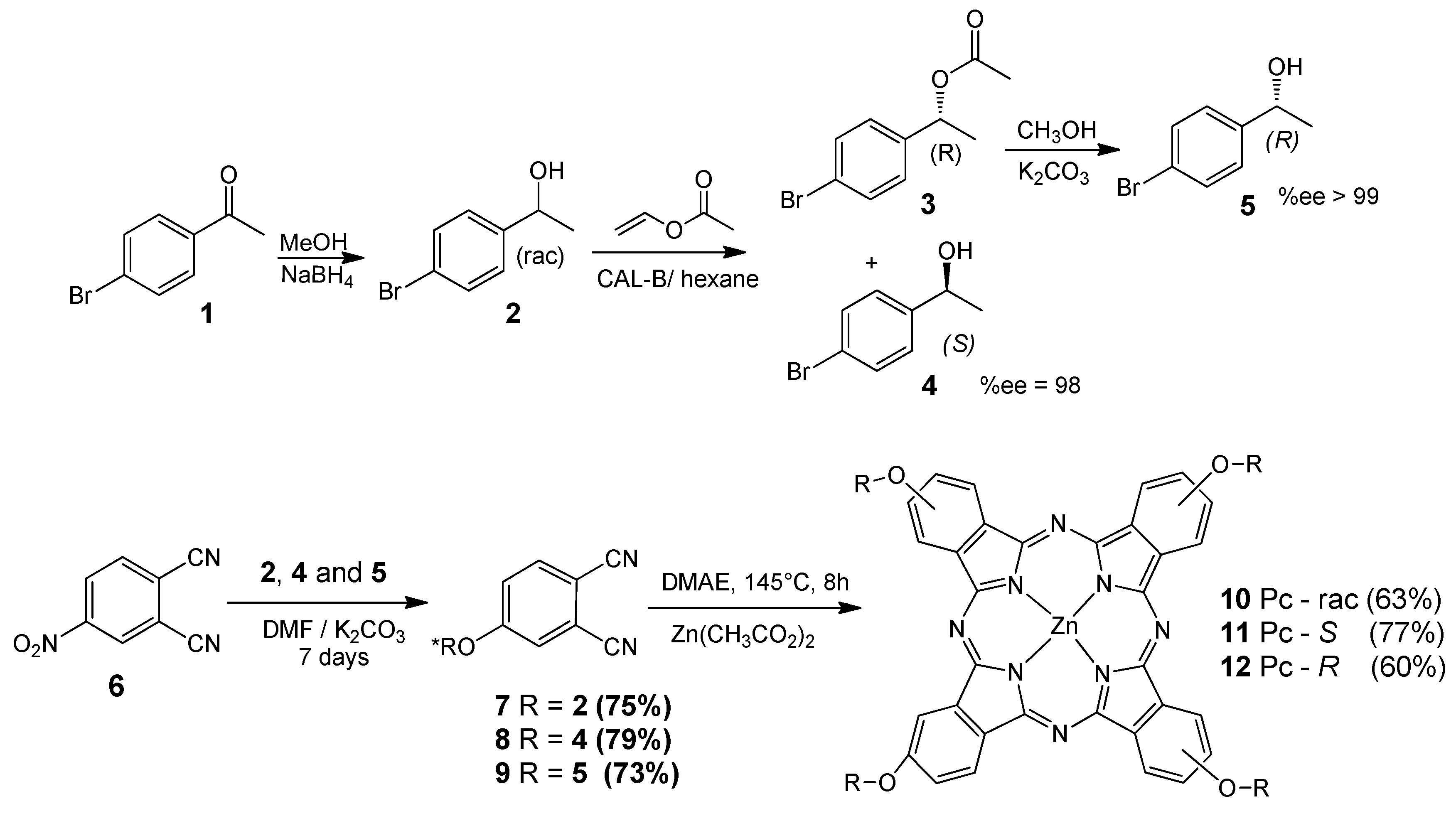
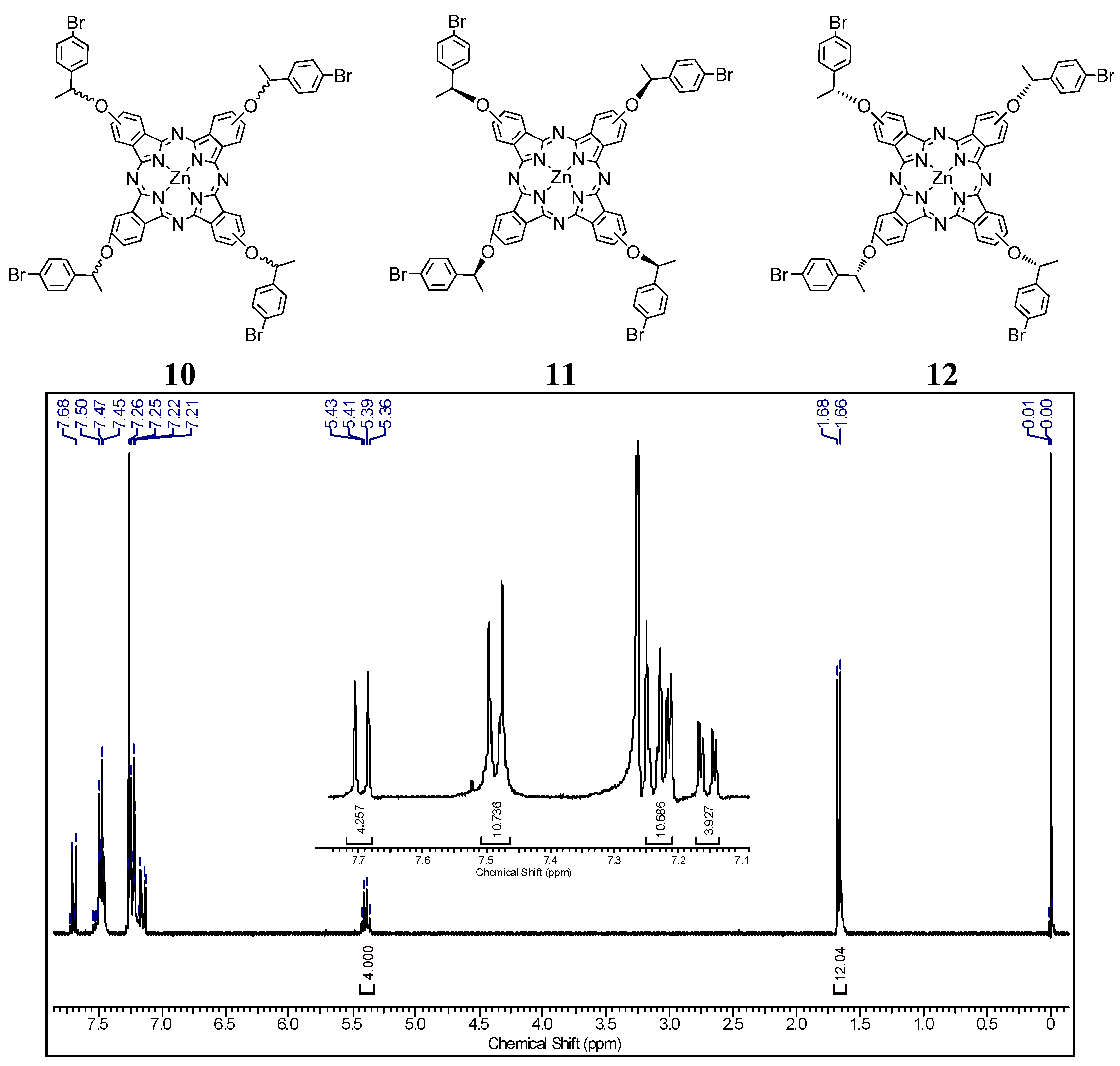
2.1. Preparation and Structural Characterization
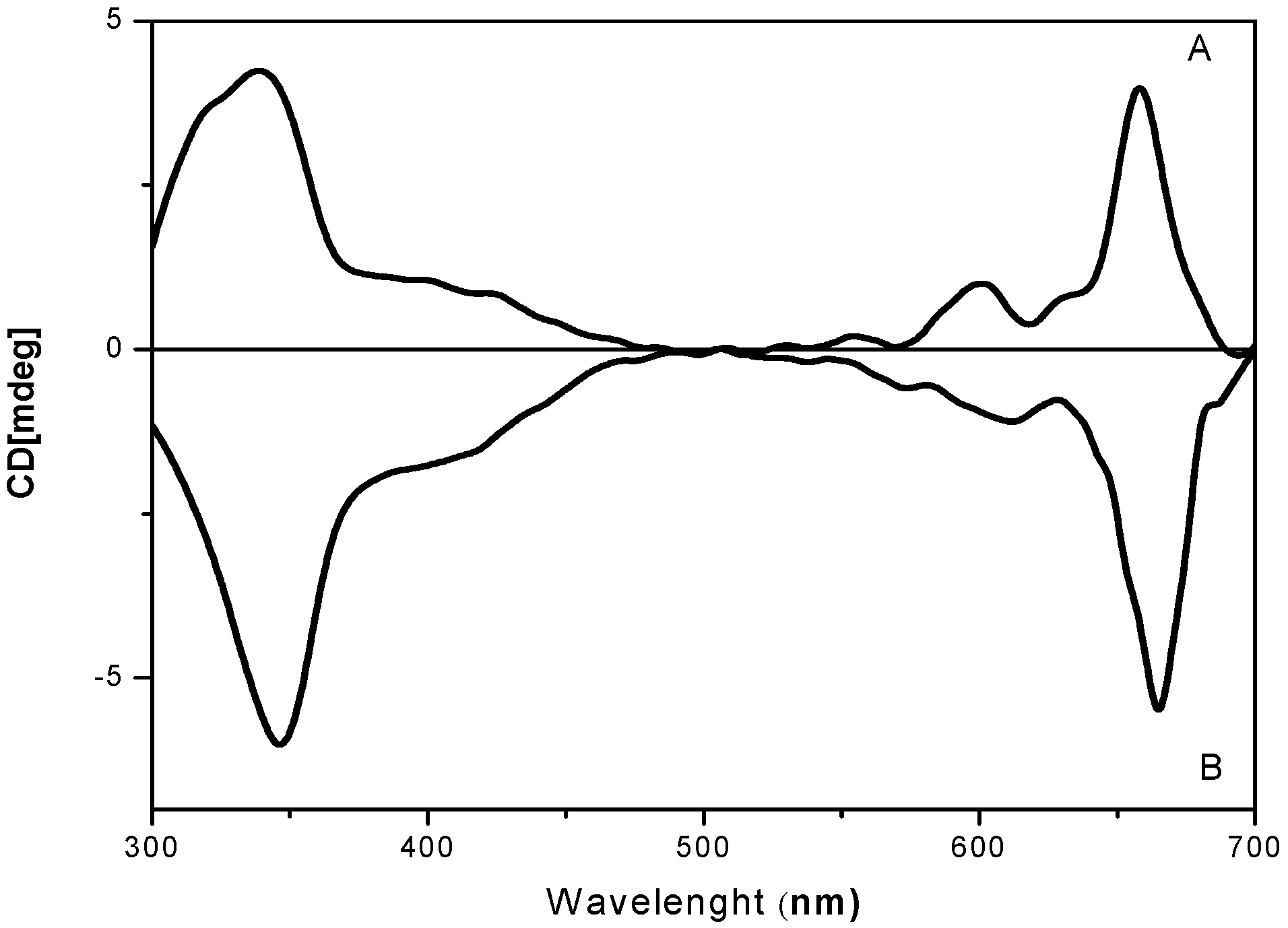
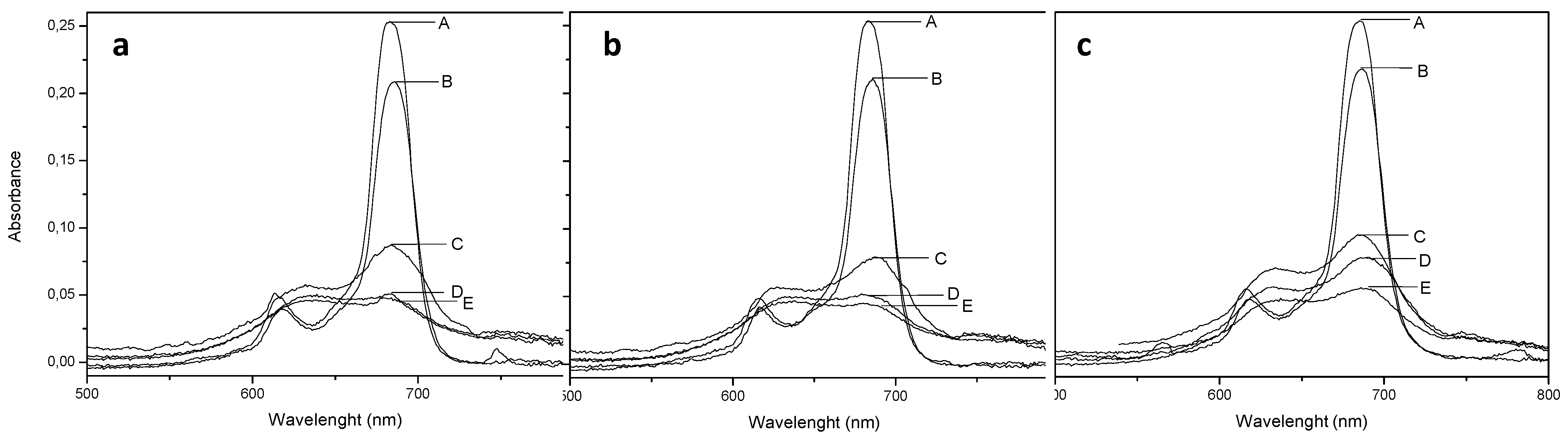
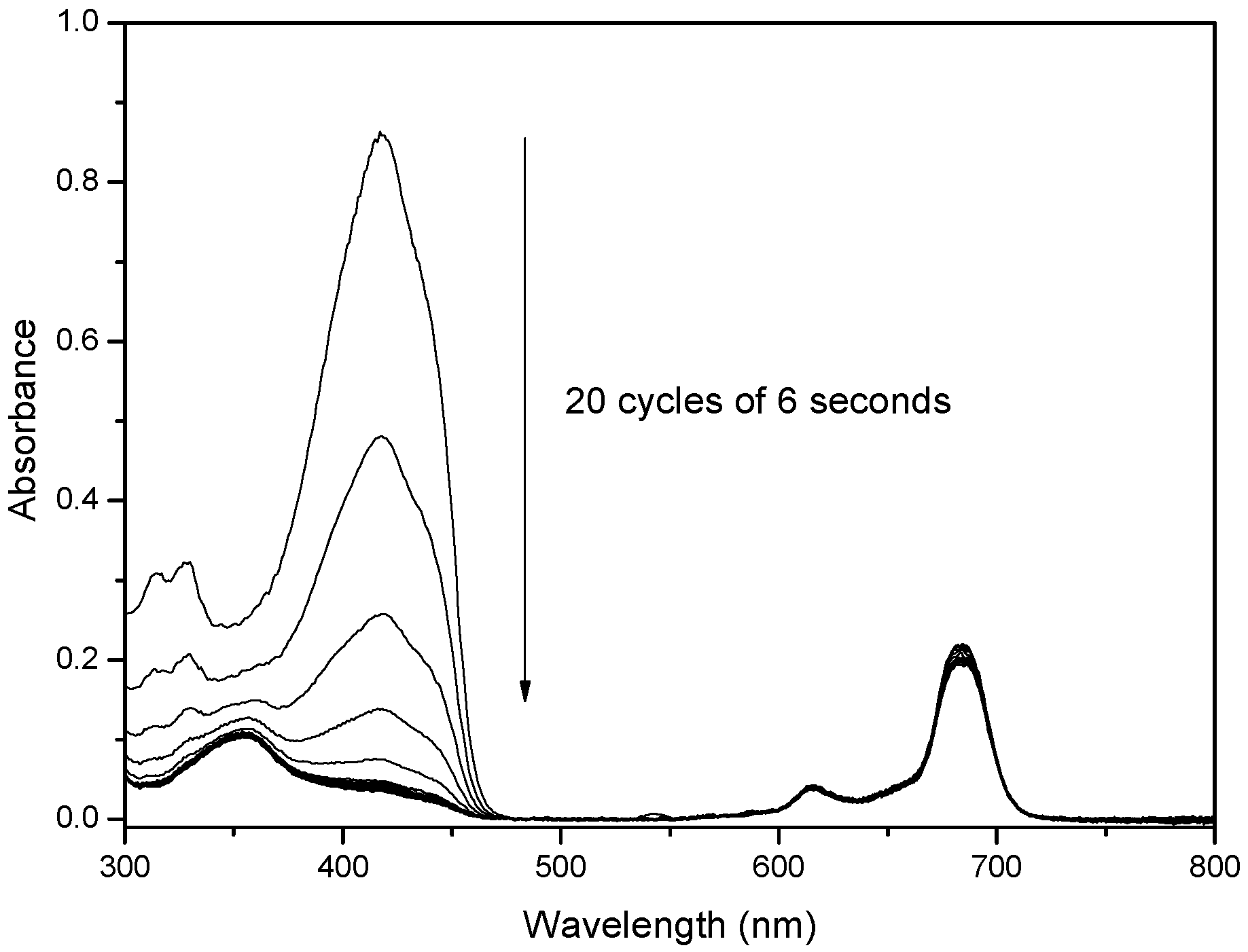
2.2. UV-Vis Behavior and Aggregation Processes
| Pc | λmax (nm) | log Ɛ (cm−1·M−1, 684 nm) | Log P | ΦΔ | ΦF |
|---|---|---|---|---|---|
| 10 | 356, 615, 684 | 5.02 | 1.97 ± 0.01 | 0.67 | 0.10 |
| 11 | 356, 615, 684 | 5.01 | 2.37 ± 0.09 | 0.65 | 0.10 |
| 12 | 356, 615, 684 | 4.91 | 2.78 ± 0.02 | 0.76 | 0.12 |
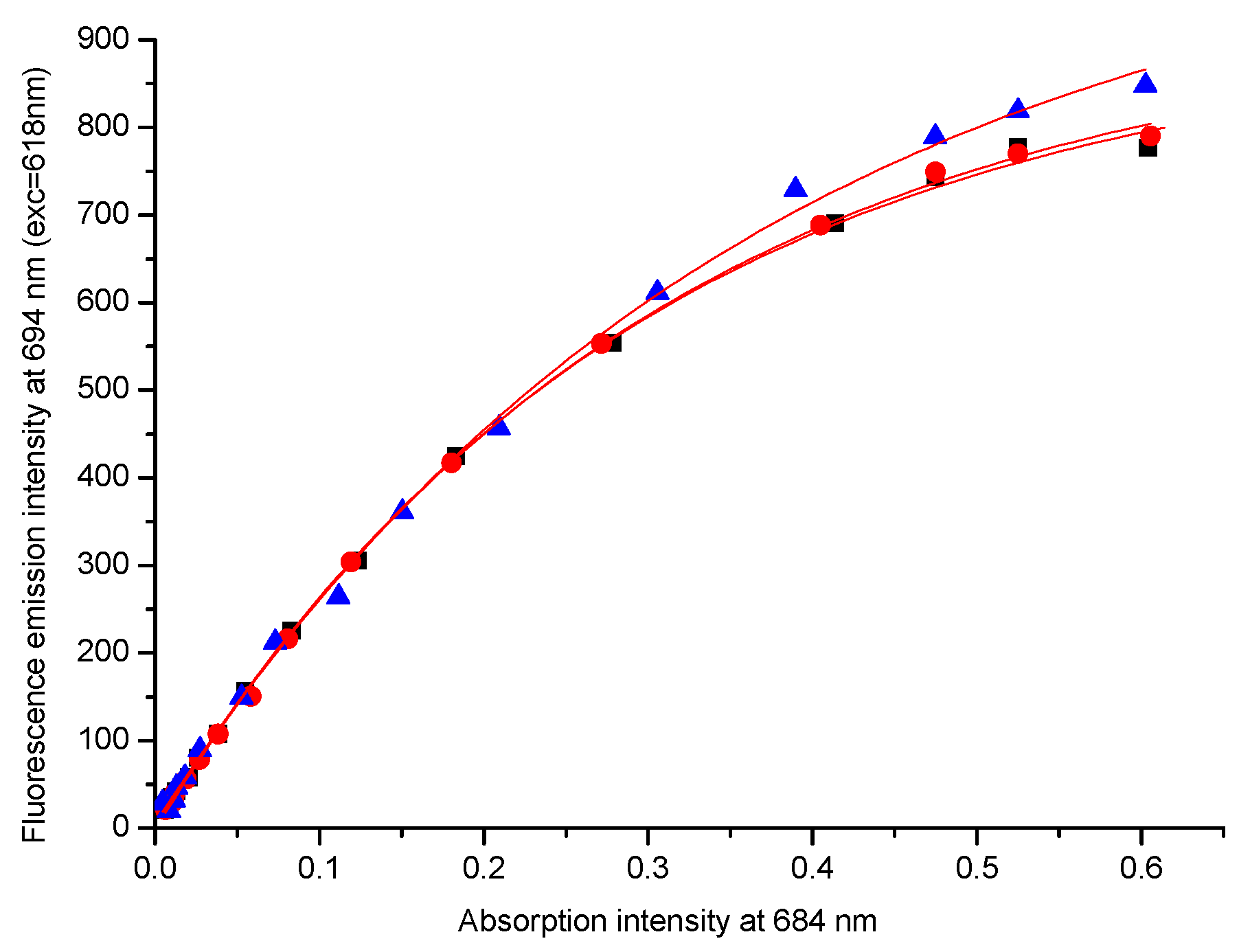
2.3. Generation of Oxygen Reactive Species and Fluorescence Quantum Yield
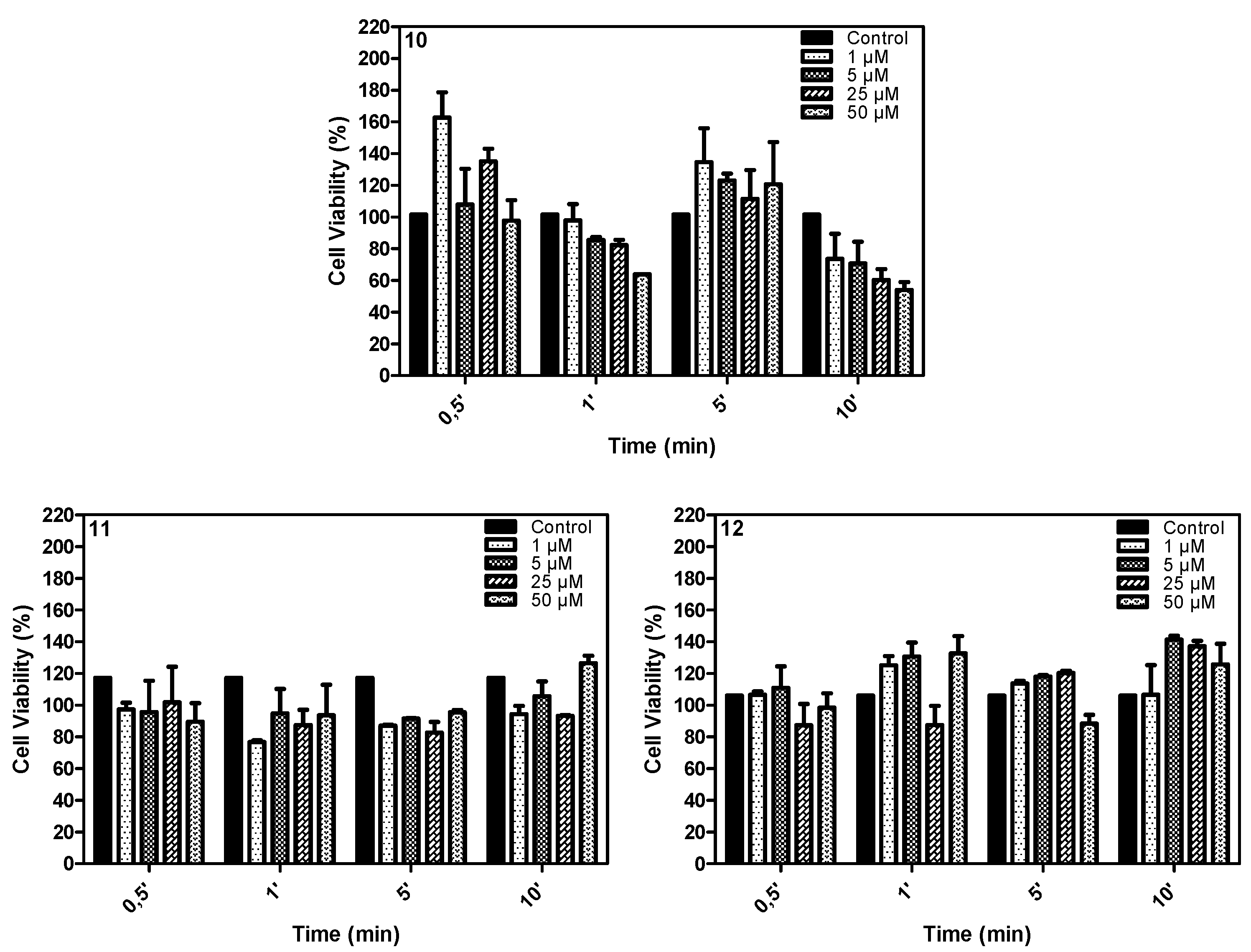
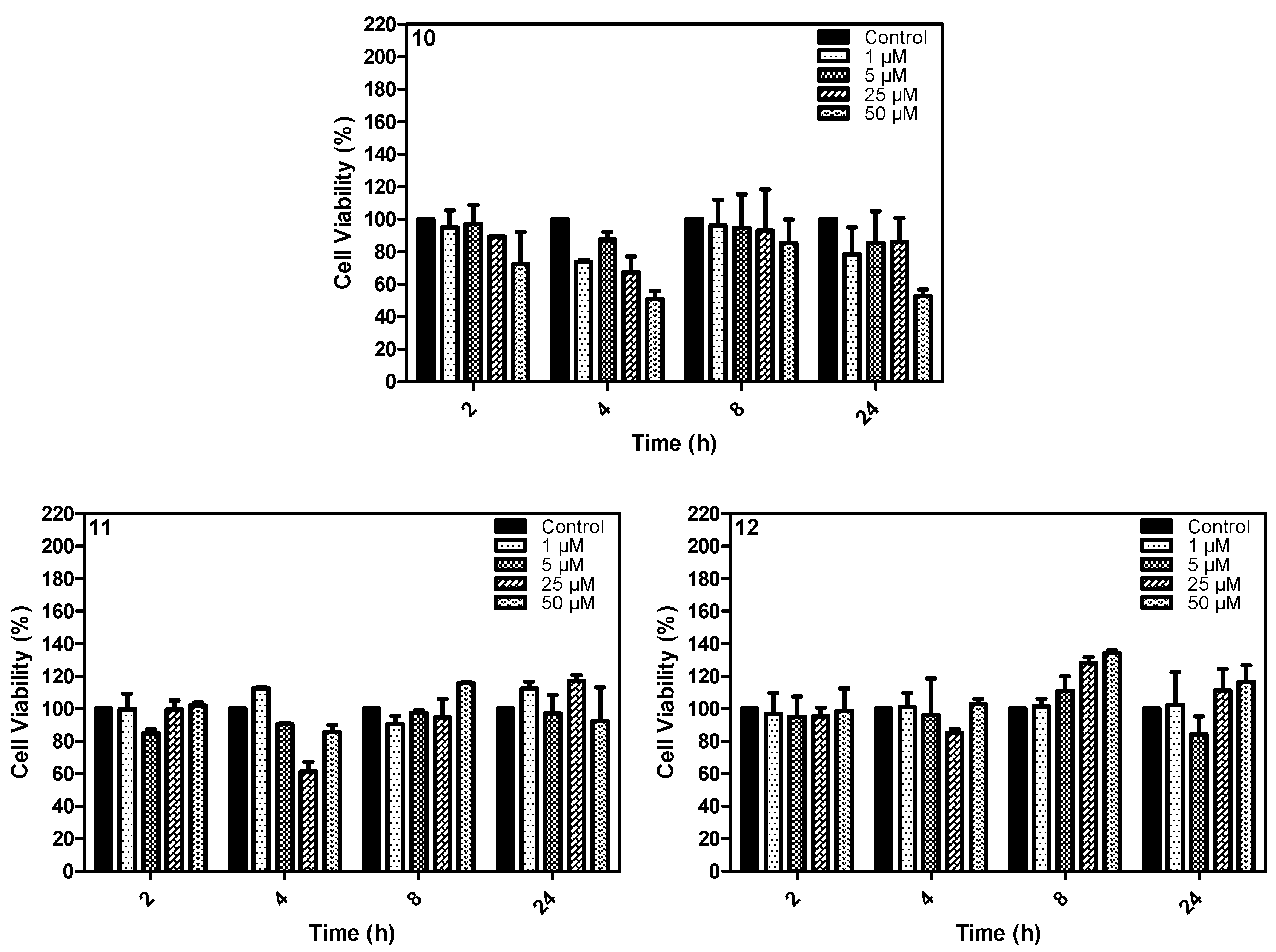
2.4. In Vitro Study
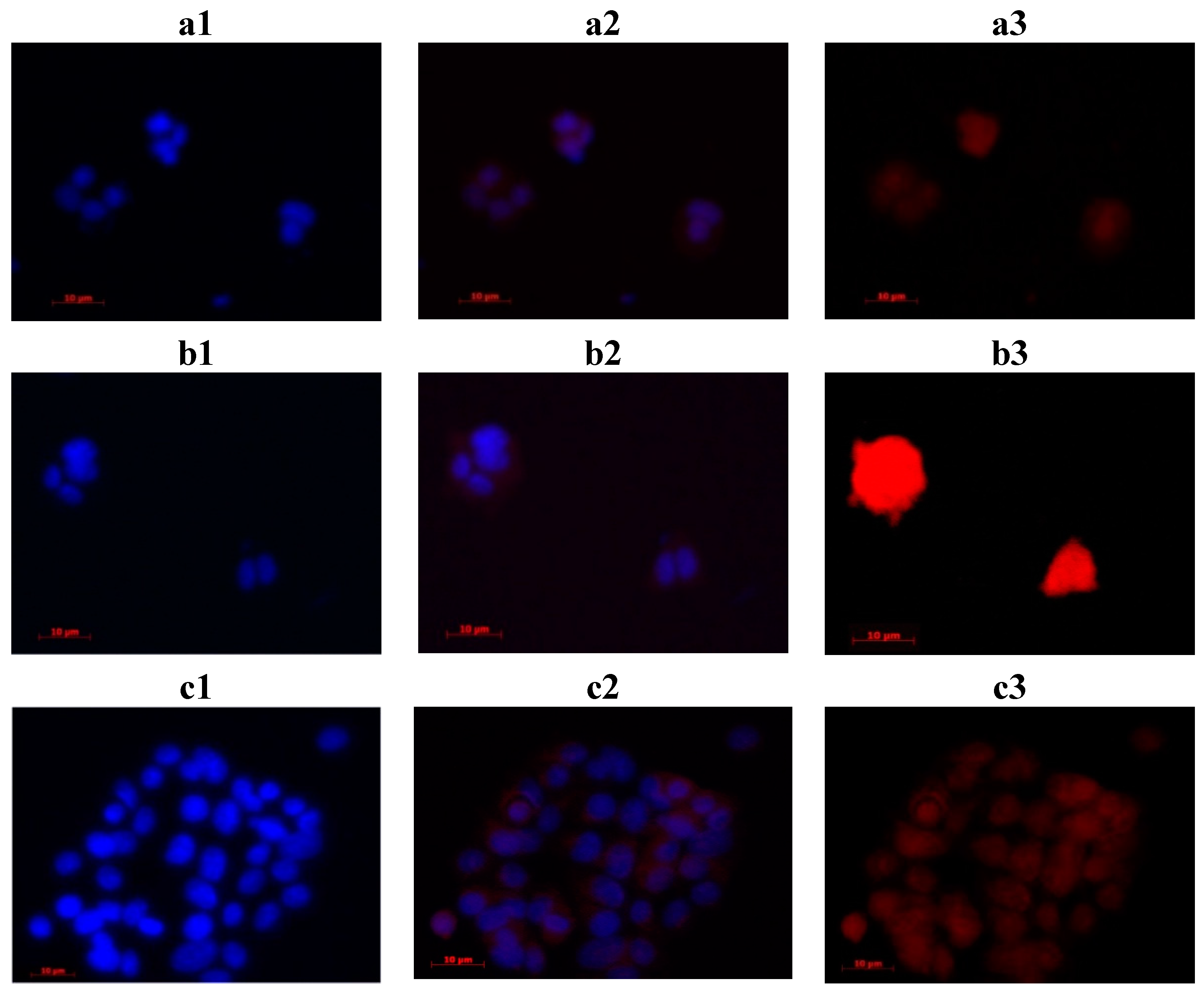
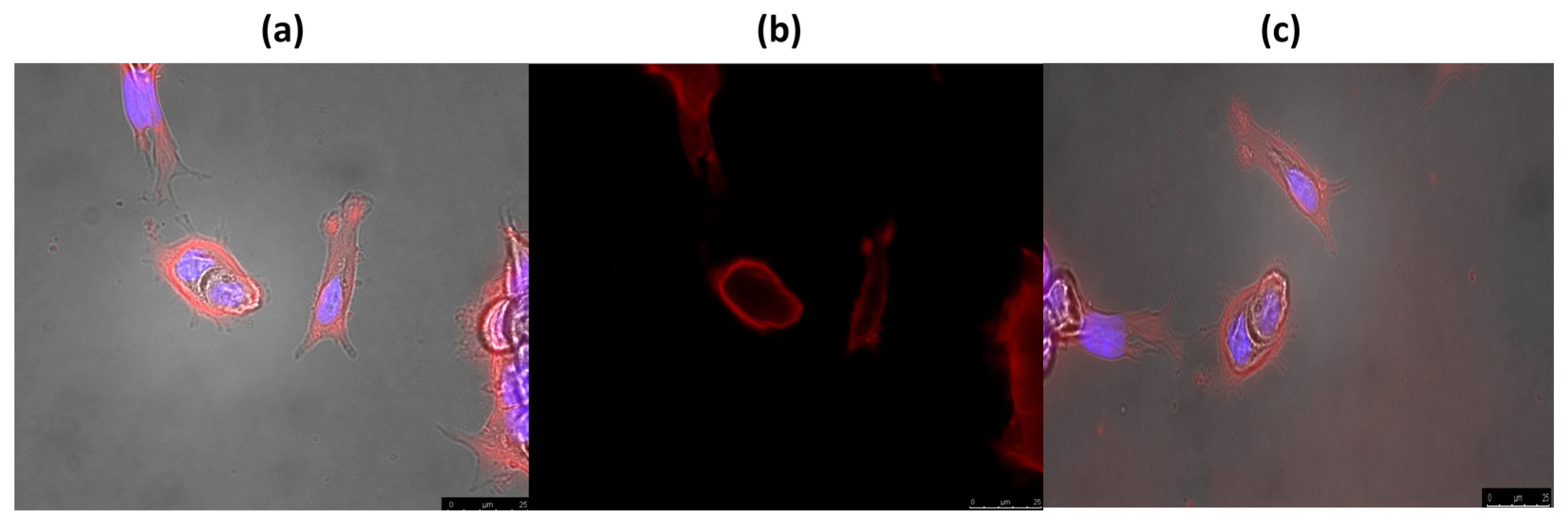
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.2.1. Kinetic Resolution of 1-(4-Bromophenyl)ethanol
3.2.2. Reaction of 4-Nitrophthalonitrile with 1-(4-Bromophenyl)ethanol (R, S and Racemic Forms)
2.2.3. Syntheses of Optically Active Phthalocyanine 10, 11 and 12
3.3. Photophysical and Photochemical Studies
3.3.1. Singlet Oxygen Quantum Yields
3.3.2. Fluorescence Quantum Yields
3.3.3. Determination of Partition Coefficients (PO/W)
3.4. Cell Interaction Study
3.4.1. Cell Culture
3.4.2. Fluorescence Microscopy
3.4.3. Cell Viability—MTT
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mack, J.; Kobayashi, N. Low Symmetry Phthalocyanines and Their Analogues. Chem. Rev. 2011, 111, 281–321. [Google Scholar] [CrossRef] [PubMed]
- Mckeown, N.B. The Porphyrin Handbook: Phthalocyanines: Synthesis of Symmetrical Phthalocyanines; Kadish, K., Cuilard, R., Smith, K., Eds.; Academic Press: Burlington, MA, USA, 2003; Volume 15, cap 98; pp. 61–124. [Google Scholar]
- Sesalan, B.S.; Koca, A.; Gul, A. Water soluble novel phthalocyanines containing dodeca-amino groups. Dyes Pigments 2008, 79, 259–264. [Google Scholar] [CrossRef]
- Zhang, X.F. Tetrabenzotriazacorrole: Its synthesis, reactivity, physical properties, and applications. Coord. Chem. Rev. 2015, 285, 52–64. [Google Scholar] [CrossRef]
- De Oliveira, K.T.; de Assis, F.F.; Ribeiro, A.O.; Neri, C.R.; Fernandes, A.U.; Baptista, M.S.; Lopes, N.P.; Serra, O.A.; Iamamoto, Y. Synthesis of Phthalocyanines−ALA Conjugates: Water-Soluble Compounds with Low Aggregation. J. Org. Chem. 2009, 74, 7962–7965. [Google Scholar] [CrossRef] [PubMed]
- Pietrangeli, D.; Rosa, A.; Pepe, A.; Ricciardi, G. Symmetrically Substituted nido-Carboranylphthalocyanines: Facile Synthesis, Characterization, and Solution Properties. Evidence for Intra- and Intermolecular H+/K+ Exchange. Inorg. Chem. 2011, 50, 4680–4682. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Kuroda, T.; Ohta, K.; Hanabusa, K.; Shirai, H.; Kobayashi, N. Self-Organization of Hydrogen-Bonded Optically Active Phthalocyanine Dimers. Langmuir 2003, 19, 4825–4830. [Google Scholar] [CrossRef]
- Raminelli, C.; Comasseto, J.V.; Andrade, L.H.; Porto, A.L.M. Kinetic resolution of propargylic and allylic alcohols by Candida antarctica lipase (Novozyme 435). Tetrahedron Asymmetry 2004, 15, 3117–3122. [Google Scholar] [CrossRef]
- Souza, R.O.M.A.; Antunes, O.A.C.; Kroutil, W.; Kappe, C.O. Kinetic Resolution of rac-1-Phenylethanol with Immobilized Lipases: A Critical Comparison of Microwave and Conventional Heating Protocols. J. Org. Chem. 2009, 74, 6157–6162. [Google Scholar] [CrossRef] [PubMed]
- Pietrangeli, D.; Rosa, A.; Pepe, A.; Altieri, S.; Bortolussi, S.; Postuma, I.; Protti, N.; Ferrari, C.; Cansolino, L.; Clerici, A.M.; et al. Water-soluble carboranyl-phthalocyanines for BNCT. Synthesis, characterization, and in vitro tests of the Zn(II)-nido-carboranyl-hexylthiophthalocyanine. Dalton Trans. 2015, 44, 11021–11028. [Google Scholar] [CrossRef] [PubMed]
- Masilela, M.; Kleyi, P.; Tshentu, Z.; Priniotakis, G.; Westbroek, P.; Nyokong, T. Photodynamic inactivation of Staphylococcus aureus using low symmetrically substituted phthalocyanines supported on a polystyrene polymer. Dyes Pigments 2013, 96, 500–508. [Google Scholar] [CrossRef]
- Özçeşmeci, M.; Ecevit, O.B.; Sürgün, S.; Hamuryudan, E. Tetracationic fluorinated zinc(ii)phthalocyanine: Synthesis, characterization and DNA-binding properties. Dyes Pigments 2013, 96, 52–58. [Google Scholar] [CrossRef]
- Zhang, X.F.; Guo, W. Imidazole functionalized magnesium phthalocyanine photosensitizer: Modified photophysics, singlet oxygen generation and photooxidation mechanism. J. Phys. Chem. A 2012, 116, 7651–7657. [Google Scholar] [CrossRef] [PubMed]
- Karaca, H.; Sezer, S.; Tanyeli, C. Synthesis of l-prolinol substituted novel optically active phthalocyanines. Dyes Pigments 2011, 90, 100–105. [Google Scholar] [CrossRef]
- Ogunsipe, A.; Chen, J.Y.; Nyokong, T. Photophysical and photochemical studies of zinc(II) phthalocyanine derivatives-effects of substituents and solvents. New J. Chem. 2004, 28, 822–827. [Google Scholar] [CrossRef]
- Ogunsipe, A.; Nyokong, T. Photophysical and photochemical studies of sulphonated non-transition metal phthalocyanines in aqueous and non-aqueous media. J. Photochem. Photobiol. A Chem. 2005, 173, 211–220. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.F.; Xulin, L. Metal-free phthalocyanine aggregation and binding with amines: Specific and general solvent effects on absorption and fluorescence properties. J. Mol. Struct. 2015, 1084, 319–325. [Google Scholar] [CrossRef]
- Kuznetsova, N.A.; Shevchenko, E.N.; Makarov, D.A.; Slivka, L.K.; Solovyova, L.I.; Kaliya, O.L.; Lukyanets, E.A. Synthesis and photophysicochemical studies of non-metal 2,3,9,10,16,17,23,24-octacarboxyphthalocyanine. J. Porphyr. Phthalocyanines 2012, 16, 1244–1251. [Google Scholar] [CrossRef]
- Lei, S.B.; Wang, C.; Yin, S.X.; Wang, H.N.; Xi, F.; Liu, H.W.; Xu, B.; Wan, L.J.; Bai, C.J. Surface Stabilized Porphyrin and Phthalocyanine Two-Dimensional Network Connected by Hydrogen Bonds. J. Phys. Chem. B 2001, 105, 10838–10841. [Google Scholar] [CrossRef]
- Pietrangeli, D.; Rosa, A.; Ristori, S.; Salvati, A.; Altieri, S.; Ricciardi, G. Carboranyl-porphyrazines and derivatives for boron neutron capture therapy: From synthesis to in vitro tests. Coord. Chem. Rev. 2013, 257, 2213–2231. [Google Scholar] [CrossRef]
- Zhang, X.F.; Lin, Y.; Guo, W.; Zhu, J. Spectroscopic insights on imidazole substituted phthalocyanine photosensitizers: Fluorescence properties, triplet state and singlet oxygen generation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Tebello, N. Effects of substituents on the photochemical and photophysical properties of main group metal phthalocyanines. Coord. Chem. Rev. 2007, 251, 1707–1722. [Google Scholar]
- Zhang, X.F.; Shao, X.; Tian, H.; Sun, X.; Han, K. Synthesis, fluorescence, excited triplet state properties and singlet oxygen generation of para-(tert-butylphenoxy) substituted phthalocyanines containing group IVA central elements. Dyes Pigments 2013, 99, 480–488. [Google Scholar] [CrossRef]
- Zhang, X.F.; Guo, W. Indole substituted zinc phthalocyanine: Improved photosensitizing ability and modified photooxidation mechanism. J. Photochem. Photobiol. A 2011, 225, 117–124. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, A.A.; Nascimento, F.B.; De Souza, T.F.M.; Omori, A.T.; Manieri, T.M.; Cerchiaro, G.; Ribeiro, A.O. Photochemical and Photophysical Properties of Phthalocyanines Modified with Optically Active Alcohols. Molecules 2015, 20, 13575-13590. https://doi.org/10.3390/molecules200813575
Ramos AA, Nascimento FB, De Souza TFM, Omori AT, Manieri TM, Cerchiaro G, Ribeiro AO. Photochemical and Photophysical Properties of Phthalocyanines Modified with Optically Active Alcohols. Molecules. 2015; 20(8):13575-13590. https://doi.org/10.3390/molecules200813575
Chicago/Turabian StyleRamos, Aline A., Francisco B. Nascimento, Thaiza F. M. De Souza, Alvaro T. Omori, Tânia M. Manieri, Giselle Cerchiaro, and Anderson O. Ribeiro. 2015. "Photochemical and Photophysical Properties of Phthalocyanines Modified with Optically Active Alcohols" Molecules 20, no. 8: 13575-13590. https://doi.org/10.3390/molecules200813575
APA StyleRamos, A. A., Nascimento, F. B., De Souza, T. F. M., Omori, A. T., Manieri, T. M., Cerchiaro, G., & Ribeiro, A. O. (2015). Photochemical and Photophysical Properties of Phthalocyanines Modified with Optically Active Alcohols. Molecules, 20(8), 13575-13590. https://doi.org/10.3390/molecules200813575