The Enhanced Inhibitory Effect of Different Antitumor Agents in Self-Microemulsifying Drug Delivery Systems on Human Cervical Cancer HeLa Cells
Abstract
:1. Introduction
2. Results
2.1. Formulation and Evaluation of Self-Micro-Emulsifying Drug Delivery Systems
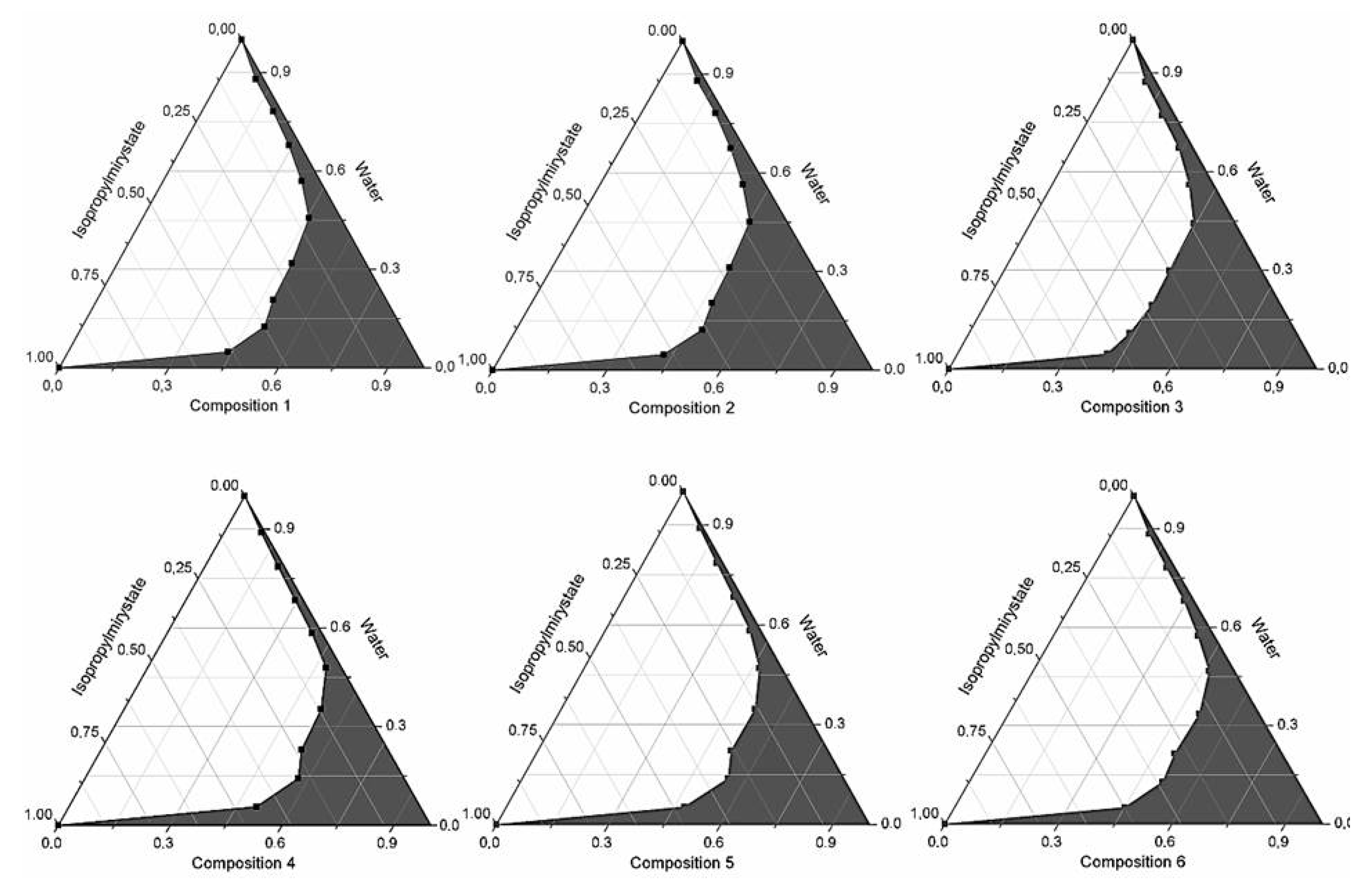
| Composition | API (mg) | Ratio of Surface Active Agents in SMEDDSs (Total Volume of Composition: 1 mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Kolliphor RH40 | Labrasol | Capryol 90 | Lauroglycol FCC | Transcutol HP | Isopropyl Myristate | |||
| 1 | 0.001–0.05 | Cisplatin | 2 | 2 | 1 | 0 | 2 | 1 |
| 0.1–5.00 | Ifosfamide | 2 | 2 | 1 | 0 | 2 | 1 | |
| 0.0005–0.05 | Bleomycin sulfate | 2 | 2 | 1 | 0 | 2 | 1 | |
| 2 | 0.001–0.05 | Cisplatin | 2 | 2 | 1 | 0 | 4 | 1 |
| 0.1–5.00 | Ifosfamide | 2 | 2 | 1 | 0 | 4 | 1 | |
| 0.0005–0.05 | Bleomycin sulfate | 2 | 2 | 1 | 0 | 4 | 1 | |
| 3 | 0.001–0.05 | Cisplatin | 2 | 2 | 1 | 0 | 6 | 1 |
| 0.1–5.00 | Ifosfamide | 2 | 2 | 1 | 0 | 6 | 1 | |
| 0.0005–0.05 | Bleomycin sulfate | 2 | 2 | 1 | 0 | 6 | 1 | |
| 4 | 0.001–0.05 | Cisplatin | 1 | 1 | 0 | 1 | 1 | 1 |
| 0.1–5.00 | Ifosfamide | 1 | 1 | 0 | 1 | 1 | 1 | |
| 0.0005–0.05 | Bleomycin sulfate | 1 | 1 | 0 | 1 | 1 | 1 | |
| 5 | 0.001–0.05 | Cisplatin | 1 | 1 | 0 | 1 | 2 | 1 |
| 0.1–5.00 | Ifosfamide | 1 | 1 | 0 | 1 | 2 | 1 | |
| 0.0005–0.05 | Bleomycin sulfate | 1 | 1 | 0 | 1 | 2 | 1 | |
| 6 | 0.001–0.05 | Cisplatin | 1 | 1 | 0 | 1 | 4 | 1 |
| 0.1–5.00 | Ifosfamide | 1 | 1 | 0 | 1 | 4 | 1 | |
| 0.0005–0.05 | Bleomycin sulfate | 1 | 1 | 0 | 1 | 4 | 1 | |
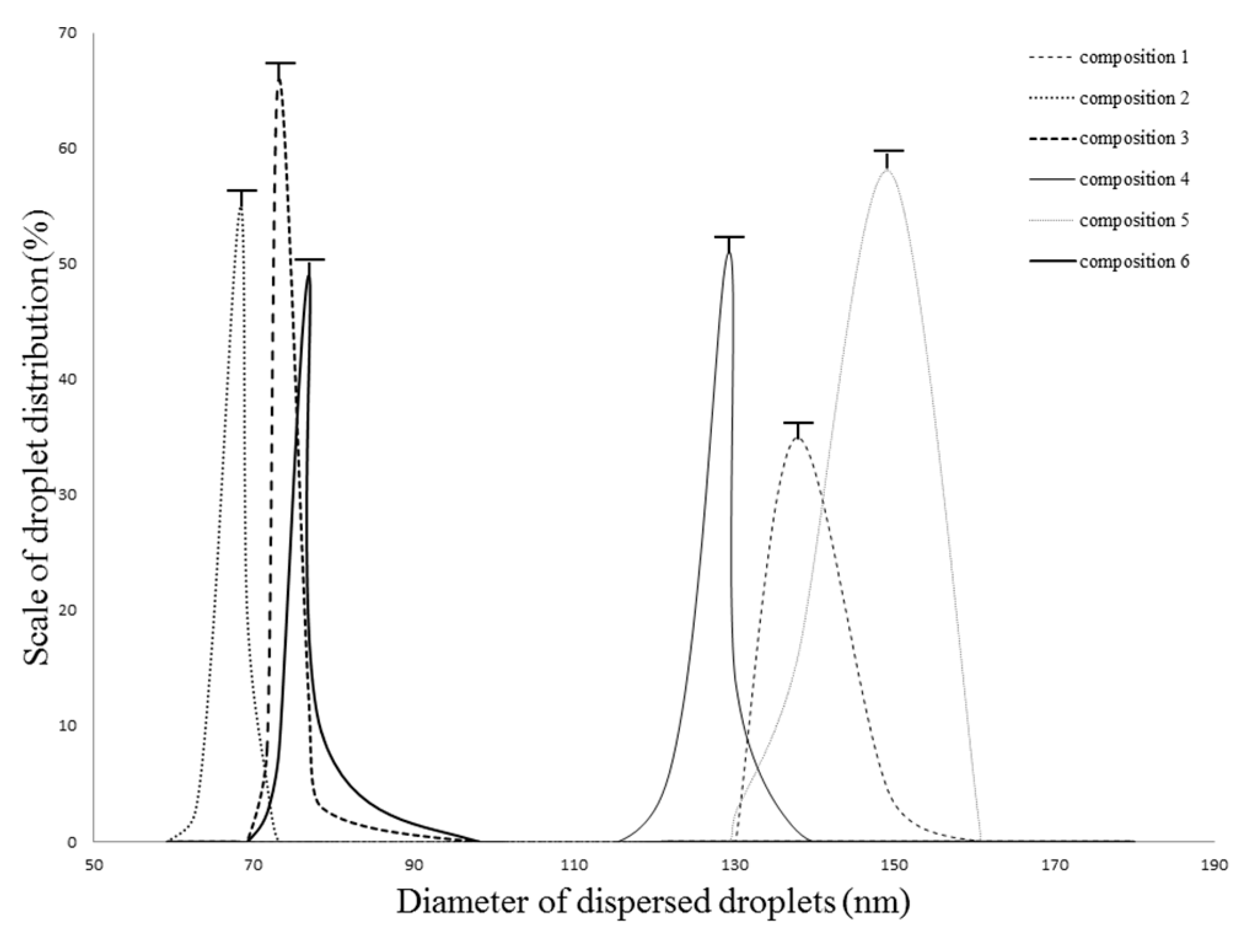
2.2. Droplet Size and Zeta Potential Determination
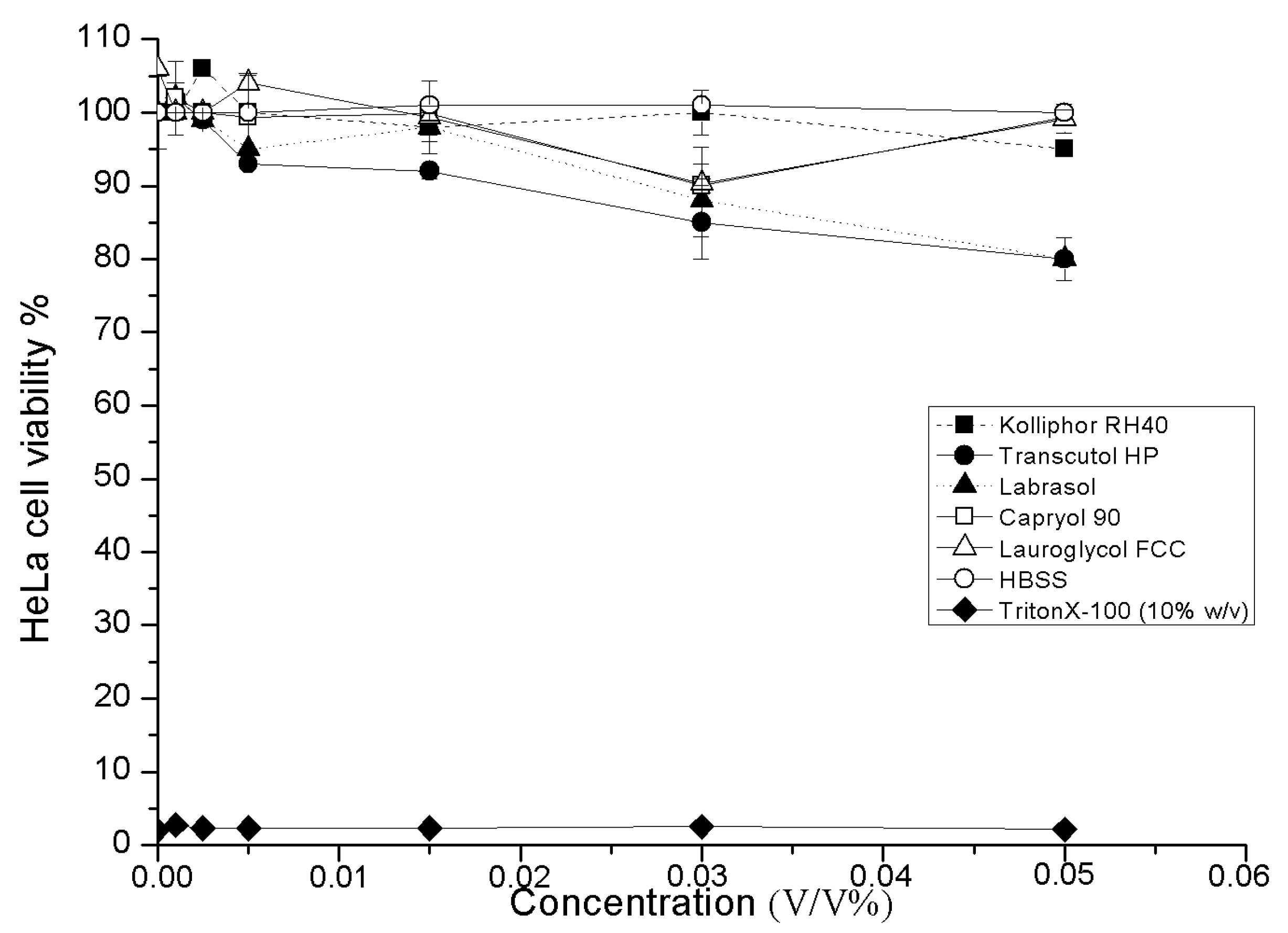
2.3. Cell Viability Test of SMEDDS Ingredients
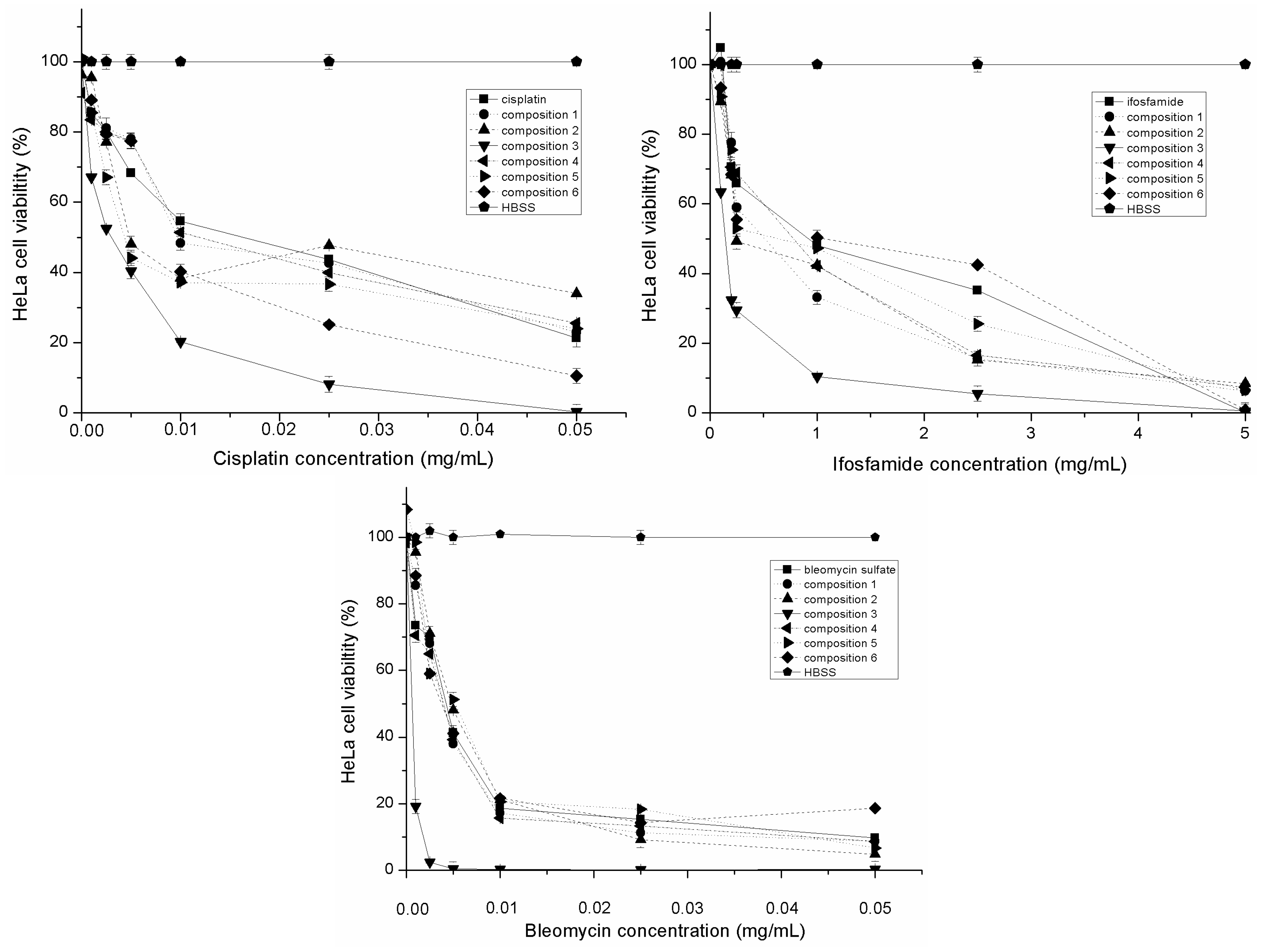
2.4. Evaluation of Inhibitory Effect of SMEDDS Containing Antitumor Agent
| API(Bold/Compositions) | IC50 (μg/mL) | ±SD | IC50 (μg/mL) in Presence of TNF-α, IL-1-β | ±SD |
|---|---|---|---|---|
| Cisplatin | 16.68 | 0.10 | 25.96 | 0.56 |
| Composition 1 | 9.63 | 0.11 | 27.88 | 0.21 |
| Composition 2 | 4.81 | 0.21 | 8.69 | 1.00 |
| Composition 3 | 2.82 | 0.15 | 5.84 | 0.45 |
| Composition 4 | 11.03 | 0.96 | 9.00 | 0.01 |
| Composition 5 | 4.26 | 0.85 | 32.70 | 0.21 |
| Composition 6 | 5.35 | 0.32 | 14.29 | 041 |
| Bleomycin sulfate | 4.21 | 0.11 | 5.69 | 0.11 |
| Composition 1 | 3.84 | 0.15 | 4.37 | 0.19 |
| Composition 2 | 3.99 | 0.50 | 6.70 | 0.32 |
| Composition 3 | 0.61 | 0.012 | 0.82 | 0.01 |
| Composition 4 | 4.71 | 0.12 | 6.81 | 0.01 |
| Composition 5 | 5.12 | 0.33 | 7.56 | 0.02 |
| Composition 6 | 4.93 | 0.22 | 6.33 | 0.01 |
| Ifosfamide | 993.50 | 25.00 | 1936.02 | 5.40 |
| Composition 1 | 521.70 | 20.40 | 641.10 | 4.21 |
| Composition 2 | 247.30 | 35.60 | 479.00 | 4.52 |
| Composition 3 | 148.50 | 1.00 | 191.10 | 3.66 |
| Composition 4 | 607.30 | 10.90 | 823.20 | 0.99 |
| Composition 5 | 802.41 | 30.55 | 873.14 | 0.99 |
| Composition 6 | 1044.70 | 1.11 | 1098.70 | 1.90 |
| BIP | 3.36 | 0.10 | 4.93 | 0.18 |
| Composition 1 | 2.76 | 0.23 | 3.56 | 0.11 |
| Composition 2 | 2.01 | 0.21 | 3.67 | 0.34 |
| Composition 3 | 0.33 | 0.05 | 0.95 | 0.09 |
| Composition 4 | 3.31 | 0.08 | 3.95 | 0.17 |
| Composition 5 | 2.99 | 0.23 | 4.51 | 0.35 |
| Composition 6 | 2.74 | 0.32 | 3.45 | 0.37 |
2.5. Evaluation of Inhibitory Effect of SMEDDS Containing Antitumor Agent in the Presence of Inflammatory Mediators
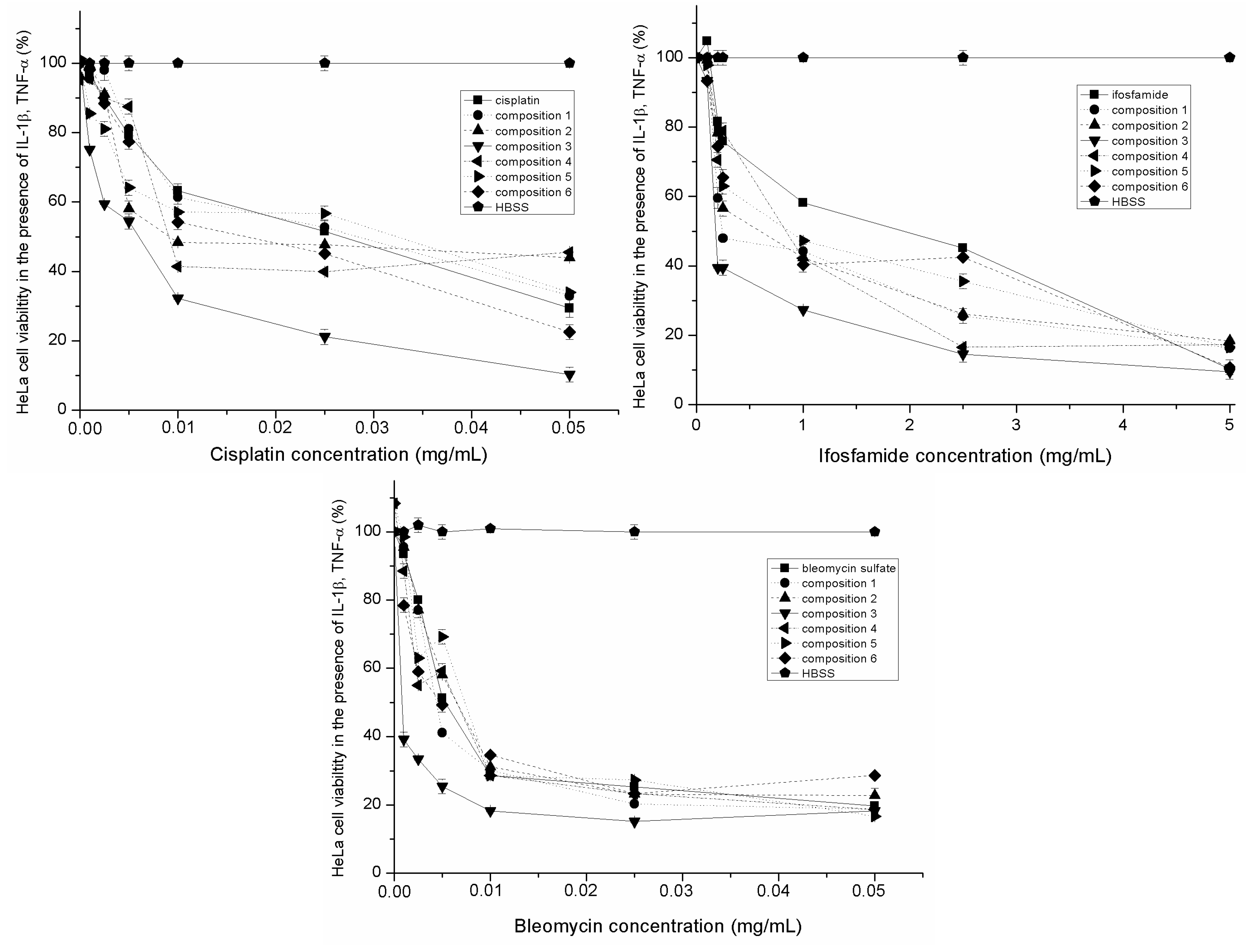
2.6. Discussion
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Formulation and Evaluation of Self-Micro-Emulsifying Drug Delivery Systems
3.2.2. Droplet Size and Zeta Potential Determination
3.2.3. Cell Culturing
3.2.4. In Vitro Cell Viability Assay
3.2.5. Inhibitory Effect of Different SMEDDS Containing Antitumor Agents
3.2.6. Inhibitory Effect of Different SMEDDS Containing Antitumor Agents in the Presence of Inflammatory Mediators
3.2.7. Statistical Analysis
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Rein, D.T.; Kurbacher, C.M.; Breidenbach, M.; Schöndorf, T.; Schmidt, T.; König, E.; Göhring, U.J.; Blohmer, J.U.; Mallman, P. Weekly carboplatin and docetaxel for locally advanced primary and recurrent cervical cancer: A phase study. Gynecol. Oncol. 2002, 87, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Adefuye, A.; Katz, A.A.; Sales, K.J. The regulation of inflammatory pathways and infectious disease of the cervix by seminar fluid. Patholog. Res. Int. 2014, 2014, 748740. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, D.; Petrelli, F.; Coinu, A.; Raspagliesi, F.; Barni, S. A systematic review comparing cisplatin and carboplatin plus paclitaxel-based chemotherapy for recurrent or metastatic cervical cancer. Gynecol. Oncol. 2014, 133, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Pectasides, D.; Kamposioras, K.; Papaxoinis, G.; Pestasides, E. Chemotherapy for recurrent cervical cancer. Cancer. Treat. Rev. 2008, 34, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Herod, J.; Burton, A.; Buxton, J.; Tobias, J.; Luesley, D.; Jordan, S.; Dunn, J.; Poole, C.J. A randomised, prospective, phase III clinical trial of primary bleomycin, ifosfamide and cisplatin (BIP) chemotherapy followed by radiotherapy versus radiotherapy alone in inoperable cancer of the cervix. Ann. Oncol. 2000, 11, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, C.; Fina, D.; Caruso, R. Cyclooxygenase-2-dependent and -independent inhibition of proliferation of colon cancer cells by 5-aminosalicylic acid. Biochem. Pharmacol. 2008, 75, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Raz, A. Is inhibition of cyclooxygenase required for the anti-tumorigenic effects of nonsteroidal, anti-inflammatory drugs (NSAIDs)? In vitro versus in vivo results and the relevance for the prevention and treatment of cancer. Biochem. Pharmacol. 2000, 63, 343–347. [Google Scholar] [CrossRef]
- Chan, A.T.; Giovannucci, E.L.; Meyerhardt, J.A.; Schernhammer, E.S.; Wu, K.; Fuchs, C.S. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology 2008, 134, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Holm, R.; Rades, T.; Müllertz, A. Characterising Lipid Lipolysis and Its Implication in Lipid-Based Formulation Development. AAPS J. 2012, 14, 860–871. [Google Scholar] [CrossRef] [PubMed]
- El Maghraby, G.M. Self-microemulsifying and microemulsion systems for transdermal delivery of indomethacin: Effect of phase transition. Colloids Surf. B 2010, 75, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Tambaro, R.; Scambia, G.; di Maio, M.; Pisano, C.; Barletta, E.; Iaffaioli, V.M.; Pignata, S. The role of chemotherapy in locally advanced, metastatic and recurrent cervical cancer. Crit. Rev. Oncol. Hematol. 2004, 52, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Adamczyk, J.; Palak, J.; Stoklosa, S.; Kubis, B.; Pastuszek, P.; Slota, E.; Wnuk, M. Curcumin-mediated decrease in the expression of nucleolar organizer regions in cervical cancer (HeLa) cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 1, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhai, W.; Jiang, Q.; Huang, R.; Liu, L.; Dai, J.; Gong, W.; Du, S.; Wu, Q. Curcumin-pipeine mixtures in self-microemulsifying drug delivery system for ulcerative colitis therapy. Int. J. Pharm. 2015, 490, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Elfström, K.M.; Arheim-Dahlström, L.; von Karsa, L.; Dillner, J. Cervical cancer screening in Europe: Quality assurance and organization of programmes. Eur. J. Cancer 2015, 58, 950–968. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.C.; Lai, C.H.; Homg, J.H.; Hsueh, S.; Huang, K.G.; Chou, H.H.; Tseng, C.J.; Tsai, C.S.; Chang, J.T.; Lin, C.T.; et al. Randomized trial of neoadjuvant cisplatin, vincristine, bleomycin, and radical hysterectomy versus radiation therapy for bulky stage IB and IIA cervical cancer. J. Clin. Oncol. 2000, 18, 1741–1747. [Google Scholar]
- Rahangdale, L.; Lippman, Q.K.; Garcia, K.; Budwit, D.; Smith, J.S.; van Le, L. Topical 5-fuouracil for treatment of cervical intraepithelial neoplasia 2: A randomized controlled trial. Am. J. Obstet. Gynecol. 2014, 210. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Lipid formulations for oral administration of drugs: Nonemulsifying, self-emulsifying and “self-microemulsifying” drug delivery systems. Eur. J. Pharm. Sci. 2000, 11, 93–98. [Google Scholar] [CrossRef]
- Ujhelyi, Z.; Fenyvesi, F.; Váradi, J.; Fehér, P.; Kiss, T.; Veszelka, S.; Deli, M.; Vecsernyés, M.; Bácskay, I. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur. J. Pharm. Sci. 2012, 47, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Sha, X.; Yan, G.; Wu, Y.; Li, J.; Fang, X. Effect of self-microemulsifying drug delivery systems containing Labrasol on tight junctions in Caco-2 cells. Eur. J. Pharm. Sci. 2005, 24, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Chiragkumar, D.; Fiona, M.W.; Stephan, A.S.; Richard, W.P.; Charles, F.; Vivian, B.S. Effectiveness of a topical local anaesthetic spray as analgesia for dressing changes: A double-blinded randomised pilot trial comparing an emulsion with an aqueous lidocaine formulation. Burns 2014, 40, 106–112. [Google Scholar]
- Tay, S.K.; Lai, F.M.; Soh, L.T.; Ho, T.H.; Ang, P.T.; Au, E. Combined chemotherapy using cisplatin, ifosfamide and bleomycin (BIP) in the treatment of advanced and recurrent cervical carcinoma. Aust. N. Z. J. Obstet. Gynaecol. 1992, 32, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.; Bansal, V.; Chandra, A.; Madan, J.; Jain, U.K.; Chandra, R.; Jain, S.M. Bleomycin sulphate loaded nanostructured lipid particles augment oral bioavailability, cytotoxicity and apoptosis in cervical cancer cells. Colloids Surf. B 2014, 118, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, X.; Song, L.; Zhu, J.; Yu, R. The Inhibitory Effect of a Novel Polypeptide Fraction from Area subcrenata on Cancer-Related Inflammation in Human Cervical Cancer HeLa Cells. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Deivendran, S.; Marzook, K.H.; Radhakrishna Pillai, M. The role of inflammation in cervical cancer. Adv. Exp. Med. Biol. 2014, 816, 377–399. [Google Scholar] [PubMed]
- Adefuye, A.O.; Sales, K.J.; Katz, A.A. Seminal plasma induces the expression of IL-1α in normal and neoplastic cervical cells via EP2/EGFR/PI3K/AKT pathway. J. Mol. Signal. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Ujhelyi, Z.; Róka, E.; Fenyvesi, F.; Fehér, P.; Váradi, J.; Réti-Nagy, K.; Vecsernyés, M.; Bácskay, I. Assessment of the hemolytic activity and cytotoxicity of different PEG-based solubilizing agents. Pharmazie 2013, 68, 383–384. [Google Scholar] [PubMed]
- Urban, C.; Schurtenberger, P. Characterization of turbid colloidal suspensions using light scattering techniques combined with cross-correlation methods. J. Colloid Interface Sci. 1998, 207, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Bigansoli, E.; Cavenaghi, L.A.; Rossi, R.; Brunati, M.C.; Nolli, M.L. Use of a Caco-2 cell culture model for the characterization of intestinal absorption of antibiotics. Farmaco 1999, 54, 594–599. [Google Scholar] [CrossRef]
- Palamakula, A.; Khan, M.A. Evaluation of cytotoxicity of oils used in coenzyme Q10 Self-emulsifying Drug Delivery Systems (SEDDS). Int. J. Pharm. 2004, 273, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, N.; Garrigue, J.S.; Razafindratsita, A.; Lambert, G.; Benita, S. Excipient effects on in vitro cytotoxicity of a novel paclitaxel self-emulsifying drug delivery system. J. Pharm. Sci. 2003, 92, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ujhelyi, Z.; Kalantari, A.; Vecsernyés, M.; Róka, E.; Fenyvesi, F.; Póka, R.; Kozma, B.; Bácskay, I. The Enhanced Inhibitory Effect of Different Antitumor Agents in Self-Microemulsifying Drug Delivery Systems on Human Cervical Cancer HeLa Cells. Molecules 2015, 20, 13226-13239. https://doi.org/10.3390/molecules200713226
Ujhelyi Z, Kalantari A, Vecsernyés M, Róka E, Fenyvesi F, Póka R, Kozma B, Bácskay I. The Enhanced Inhibitory Effect of Different Antitumor Agents in Self-Microemulsifying Drug Delivery Systems on Human Cervical Cancer HeLa Cells. Molecules. 2015; 20(7):13226-13239. https://doi.org/10.3390/molecules200713226
Chicago/Turabian StyleUjhelyi, Zoltán, Azin Kalantari, Miklós Vecsernyés, Eszter Róka, Ferenc Fenyvesi, Róbert Póka, Bence Kozma, and Ildikó Bácskay. 2015. "The Enhanced Inhibitory Effect of Different Antitumor Agents in Self-Microemulsifying Drug Delivery Systems on Human Cervical Cancer HeLa Cells" Molecules 20, no. 7: 13226-13239. https://doi.org/10.3390/molecules200713226
APA StyleUjhelyi, Z., Kalantari, A., Vecsernyés, M., Róka, E., Fenyvesi, F., Póka, R., Kozma, B., & Bácskay, I. (2015). The Enhanced Inhibitory Effect of Different Antitumor Agents in Self-Microemulsifying Drug Delivery Systems on Human Cervical Cancer HeLa Cells. Molecules, 20(7), 13226-13239. https://doi.org/10.3390/molecules200713226











