The Influence of the Combination of Carboxylate and Phosphinate Pendant Arms in 1,4,7-Triazacyclononane-Based Chelators on Their 68Ga Labelling Properties
Abstract
:1. Introduction
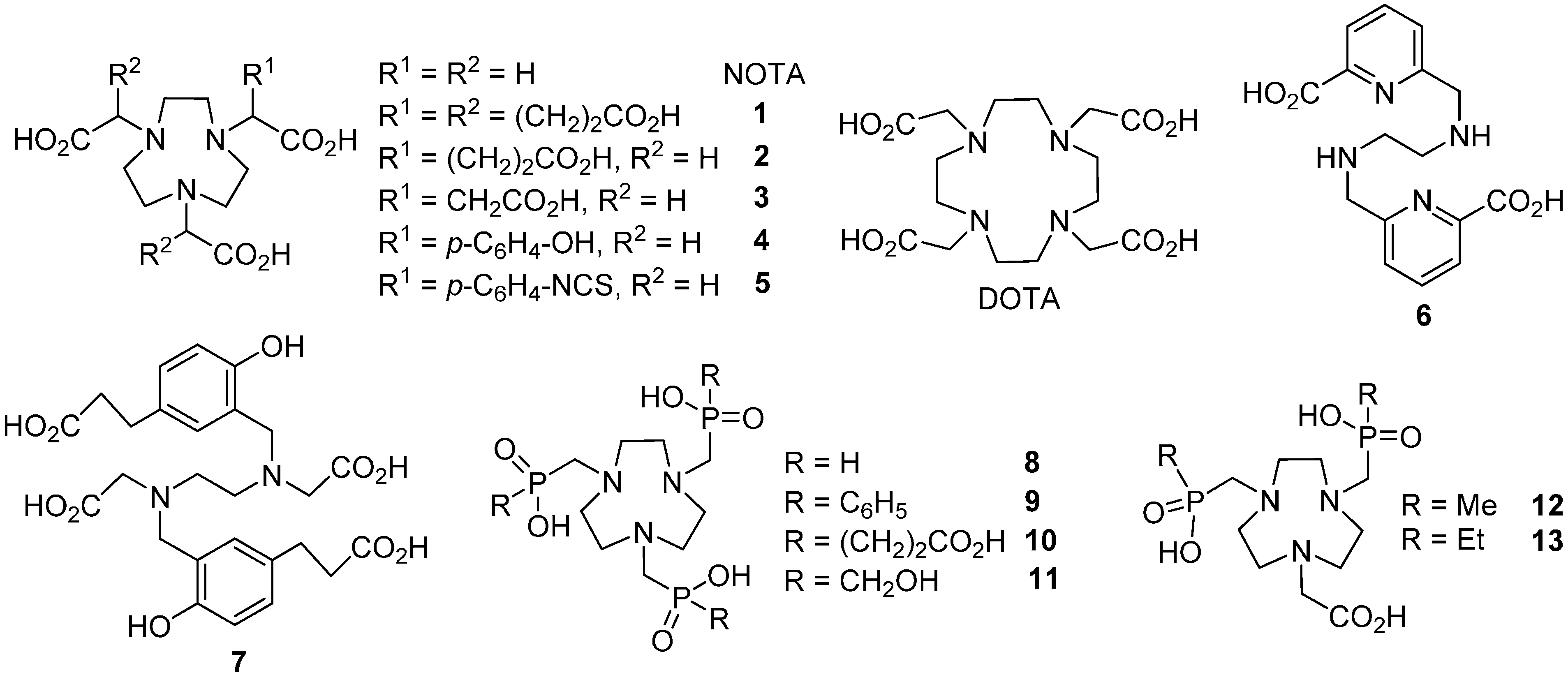
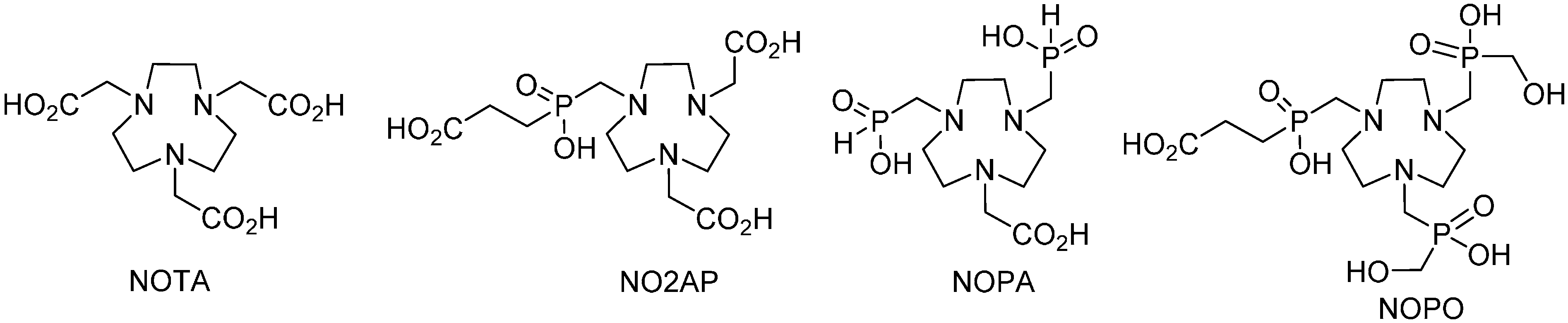
2. Results and Discussion
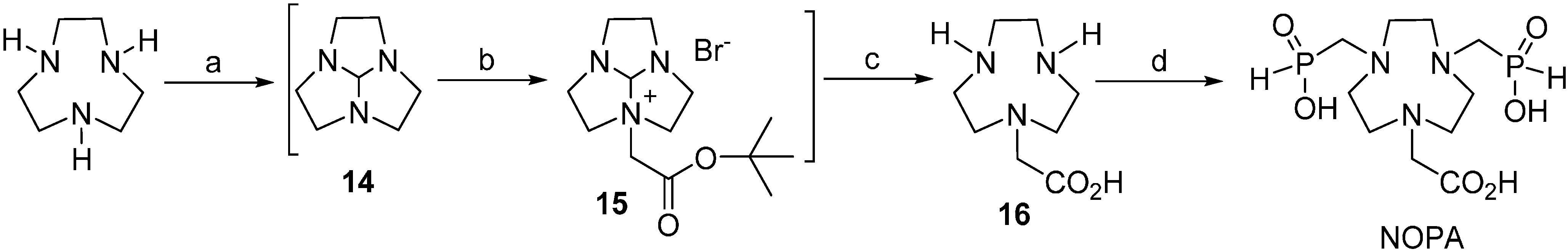
2.1. Ligand Synthesis

2.2. Equilibrium Studies
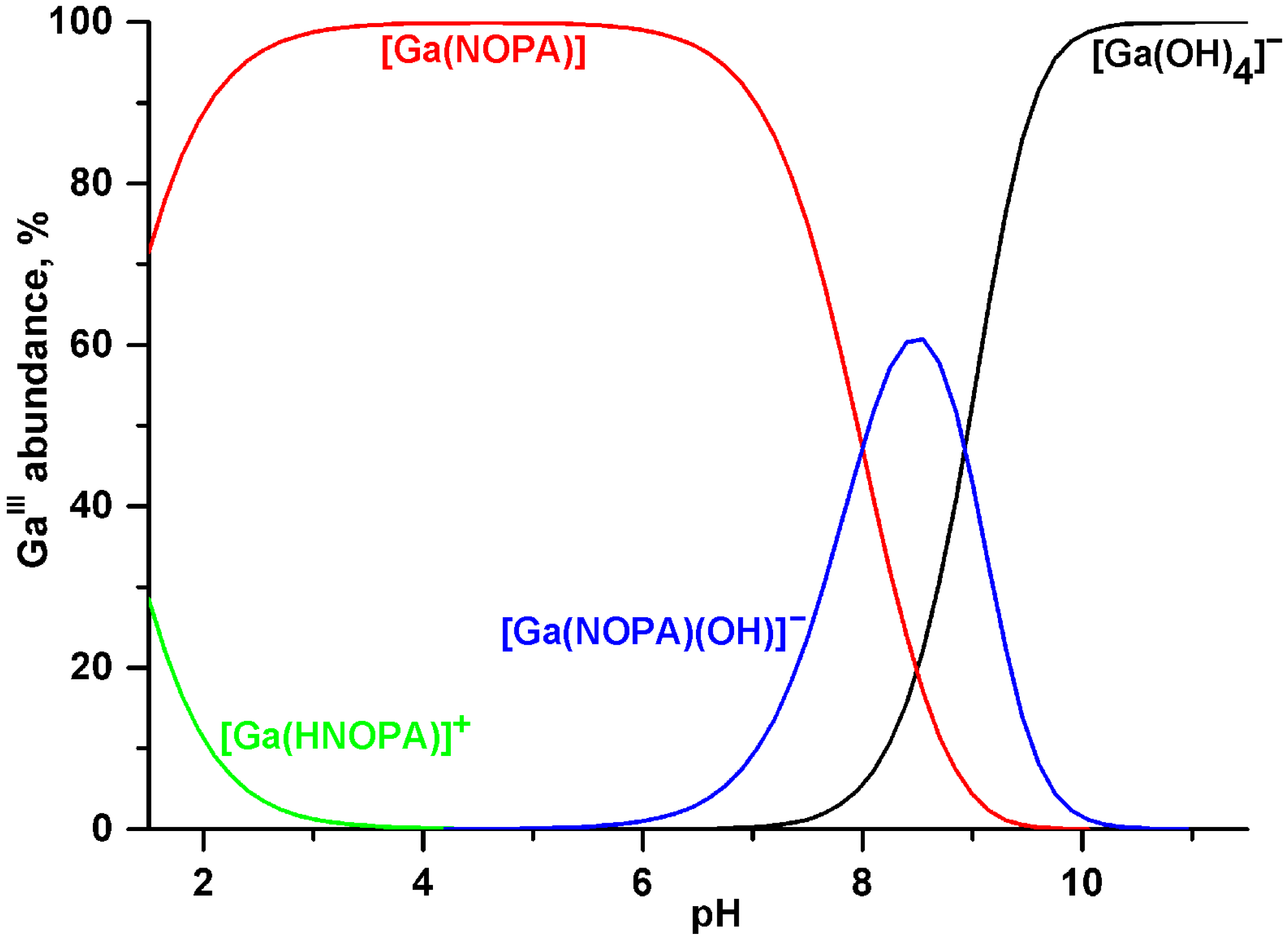
| Constant | Ligand | |||||
|---|---|---|---|---|---|---|
| NOPA a | 12 b [34] | 8 [25] | 10 [15] | NOPO [32] | NOTA [44] | |
| logK1 | 12.06 | 11.7 | 10.48 | 11.48 | 11.96 | 13.17 |
| 12.058(4) | ||||||
| logK2 | 3.90 | 4.24 | 3.28 | 5.44 | 5.22 | 5.74 |
| 15.958(6) | ||||||
| logK3 | 1.95 | 2.10 | 4.84 | 3.77 | 3.22 | |
| 17.910(6) | ||||||
| logK4 | 4.23 | 1.54 | 1.96 | |||
| logK5 | 3.45 | |||||
| logK6 | 1.66 | |||||
| logKGaLc | 24.04 | 21.91 | 26.24 | 25.0 | 29.60 [25] | |
| 24.04(6) | ||||||
2.3. 68Ga Radiolabelling
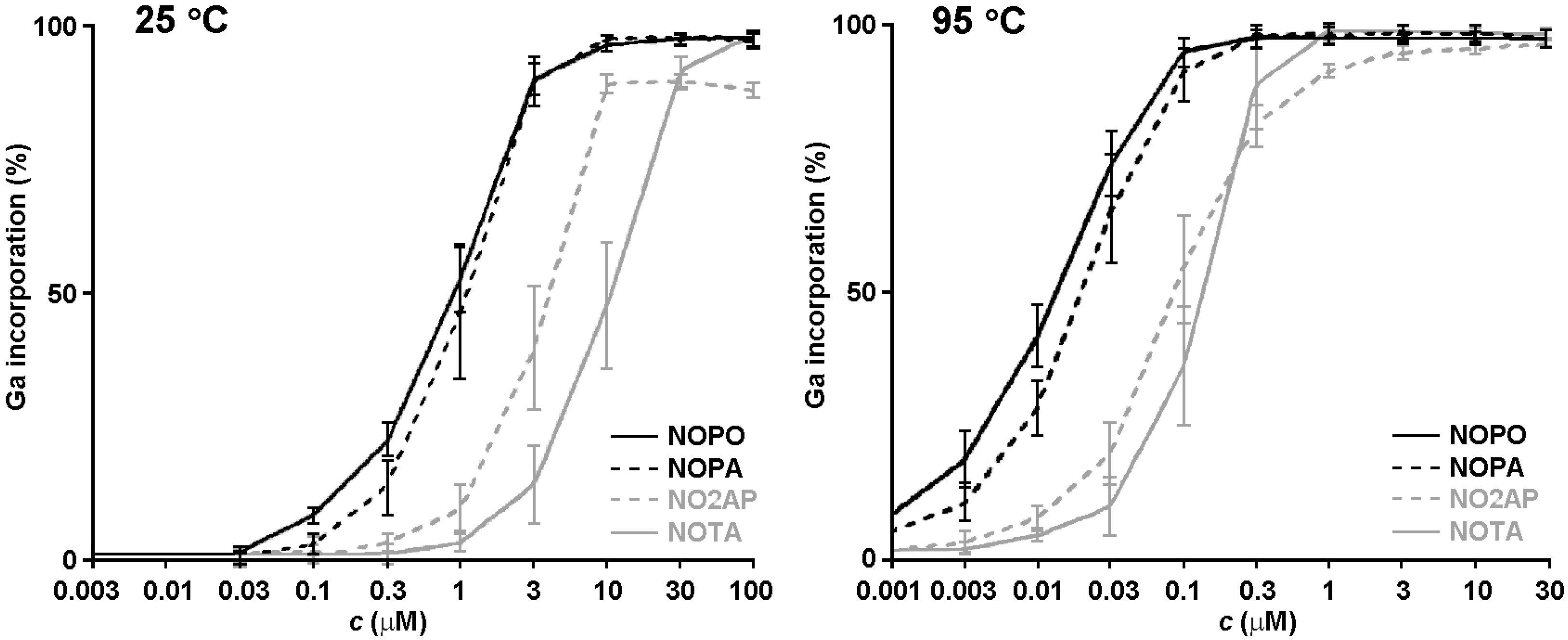
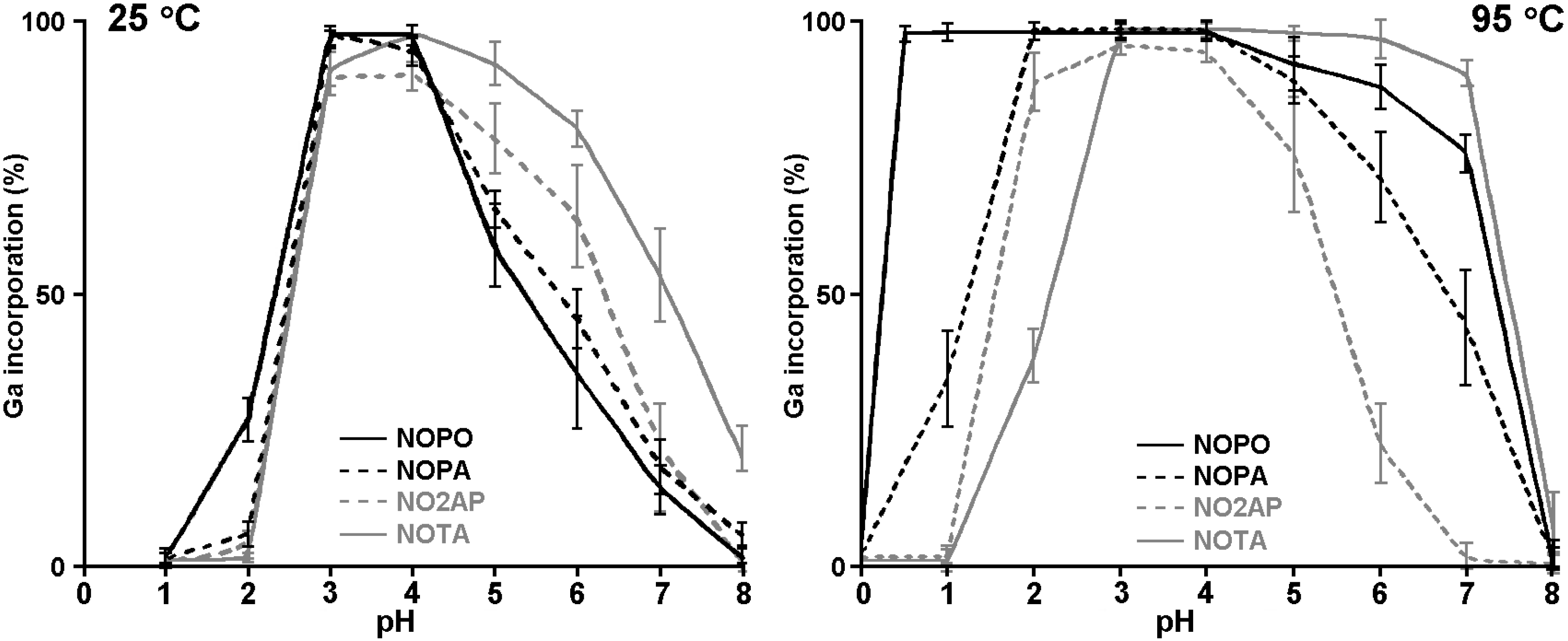
3. Experimental Section
3.1. General Information
3.2. Syntheses
3.2.1. Synthesis of (1,4,7-Triazacyclononan-1-yl)acetic Acid (16)
3.2.2. Synthesis of 1,4,7-Triazacyclononane-7-(carboxymethyl)-1,4-bis(methylenephosphinic acid) (NOPA)
3.2.3. Synthesis of 1,4,7-Triazacyclononane-4,7-bis(t-butyloxycarbonylmethyl)-1-[methylene(2-carboxyethyl)phosphinic acid] (20)
3.2.4. Synthesis of 1,4,7-Triazacyclononane-4,7-bis(carboxymethyl)-1-[methylene(2-carboxy-ethyl)phosphinic acid] (NO2AP)
3.3. Potentiometry
3.4. 68Ga Labelling
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Charkraborty, S.; Liu, S. 99mTc and 111In-labeling of small biomolecules: Bifunctional chelators and related coordination chemistry. Curr. Top. Med. Chem. 2010, 10, 1113–1134. [Google Scholar] [CrossRef]
- Rösch, F. Past, present and future of 68Ge/68Ga generators. Appl. Radiat. Isot. 2013, 76, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.R.; Pomper, M.G. Clinical applications of gallium-68. Appl. Radiat. Isot. 2013, 76, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. Continued rapid growth in 68Ga applications: Update 2013 to June 2014. J. Label. Compd. Radiopharm. 2015, 58, 99–121. [Google Scholar] [CrossRef] [PubMed]
- Geijer, H.; Breimer, L.H. Somatostatin receptor PET/CT in neuroendocrine tumours: Update on systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Van Essen, M.; Sundin, A.; Krenning, E.P.; Kwekkeboom, D.J. Neuroendocrine tumours: The role of imaging for diagnosis and therapy. Nat. Rev. Endocrinol. 2014, 10, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Ramogida, C.F.; Orvig, C. Tumour targeting with radiometals for diagnosis and therapy. Chem. Commun. 2013, 49, 4720–4739. [Google Scholar] [CrossRef] [PubMed]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Hermann, P.; Havlíčková, J.; Kotek, J.; Kubíček, V.; Plutnar, J.; Loktionova, N.; Riss, P.J.; Rösch, F.; Lukeš, I. A triazacyclononane based bifunctional phosphinate ligand for preparation of multimeric 68Ga PET tracers. Chem. Eur. J. 2010, 16, 7174–7185. [Google Scholar] [CrossRef] [PubMed]
- Šimeček, J.; Zemek, O.; Hermann, P.; Wester, H.J.; Notni, J. A monoreactive bifunctional triazacyclononane-phosphinate chelator with high selectivity for gallium-68. ChemMedChem 2012, 7, 1375–1378. [Google Scholar] [CrossRef] [PubMed]
- Andre, J.P.; Mäcke, H.R.; Zehnder, M.; Macko, L.; Akyel, K.G. 1,4,7-triazacyclononane-1-succinic acid-4,7-diacetic acid (NODASA): A new bifunctional chelator for radio gallium-labelling of biomolecules. Chem. Commun. 1998, 1301–1302. [Google Scholar] [CrossRef]
- Eisenwiener, K.P.; Prata, M.I.M.; Buschmann, I.; Zhang, H.W.; Santos, A.C.; Wenger, S.; Reubi, J.C.; Mäcke, H.R. NODAGATOC, a new chelator-coupled somatostatin analogue labeled with [Ga-67/68] and [In-111] for SPECT, PET, and targeted therapeutic applications of somatostatin receptor (hsst2) expressing tumors. Bioconjugate Chem. 2002, 13, 530–541. [Google Scholar] [CrossRef]
- Riss, P.J.; Kroll, C.; Nagel, V.; Rösch, F. NODAPA-OH and NODAPA-(NCS)n: Synthesis, 68Ga-radiolabelling and in vitro characterisation of novel versatile bifunctional chelators for molecular imaging. Bioorg. Med. Chem. Lett. 2008, 18, 5364–5367. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.N.; Liu, W.; Hao, G.; Kumar, A.; Gupta, A.; Oz, O.K.; Hsieh, J.T.; Sun, X. Multivalent bifunctional chelator scaffolds for gallium-68 based positron emission tomography imaging probe design: Signal amplification via multivalency. Bioconjugate Chem. 2011, 22, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Guerra Gomez, F.L.; Uehara, T.; Rokugawa, T.; Higaki, Y.; Suzuki, H.; Hanaoka, H.; Akizawa, H.; Arano, Y. Synthesis and evaluation of diastereoisomers of 1,4,7-triazacyclononane-1,4,7-tris-(glutaric acid) (NOTGA) for multimeric radiopharmaceuticals of galium. Bioconjugate Chem. 2012, 23, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Waldron, B.P.; Parker, D.; Burchardt, C.; Yufit, D.S.; Zimny, M.; Rösch, F. Structure and stability of hexadentate complexes of ligands based on AAZTA for efficient PET labelling with gallium-68. Chem. Commun. 2013, 49, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Ferreira, C.L.; Cawthray, J.F.; Price, E.W.; Patrick, B.O.; Wester, D.W.; Adam, M.J.; Orvig, C. Acyclic chelate with ideal properties for Ga-68 PET imaging agent elaboration. J. Am. Chem. Soc. 2010, 132, 15726–15733. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J.; Ma, Y.; Ballinger, J.R.; Tavaré, R.; Koers, A.; Sunassee, K.; Zhou, T.; Nawaz, S.; Mullen, G.E.D.; Hider, R.C.; et al. Efficient bifunctional gallium-68 chelators for positron emission tomography: Tris(hydroxypyridinone) ligands. Chem. Commun. 2011, 47, 7068–7070. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Šimeček, J.; Hermann, P.; Wester, H.J. TRAP, a powerful and versatile framework for gallium-68 radiopharmaceuticals. Chem. Eur. J. 2011, 17, 14718–14722. [Google Scholar] [CrossRef] [PubMed]
- Šimeček, J.; Hermann, P.; Wester, H.J.; Notni, J. How is 68Ga labeling of macrocyclic chelators influenced by metal ion contaminants in 68Ge/68Ga generator eluates? ChemMedChem 2013, 8, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Hull, W.E.; Wängler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68Ga-Complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjugate Chem. 2012, 23, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. PET imaging with a [68Ga] gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Ferreira, C.L.; Yapp, D.T.T.; Gill, R.K.; Price, E.W.; Adam, M.J.; Orvig, C. RGD conjugates of the H2dedpa scaffold: Synthesis, labeling and imaging with 68Ga. Nucl. Med. Biol. 2012, 29, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Pohle, K.; Wester, H.J. Be spoilt for choice with radiolabelled RGD peptides: Preclinical evaluation of 68Ga-TRAP(RGD)3. Nucl. Med. Biol. 2013, 40, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Šimeček, J.; Schulz, M.; Notni, J.; Plutnar, J.; Kubíček, V.; Havlíčková, J.; Hermann, P. Complexation of metal ions with TRAP (1,4,7-triazacyclononane phosphinic acid) ligands and NOTA: Phosphinate-containing ligands as unique chelators for trivalent galium. Inorg. Chem. 2012, 51, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Šimeček, J.; Wester, H.J. Phosphinic acid functionalized polyazacycloalkane chelators for radiodiagnostics and radiotherapeutics: Unique characteristics and applications. ChemMedChem 2014, 9, 1107–1115, (corrigendum: ibid. 2014, 9, 2614). [Google Scholar] [CrossRef] [PubMed]
- Bazakas, K.; Lukeš, I. Synthesis and complexing properties of polyazamacrocycles with pendant N-methylenephosphinic acid. J. Chem. Soc. Dalton Trans. 1995. [Google Scholar] [CrossRef]
- Cole, E.; Parker, D.; Ferguson, G.; Gallagher, J.F.; Kaitner, B. Synthesis and structure of chiral metal complexes of polyazacycloalkane ligands incorporating phosphinic acid donors. J. Chem. Soc. Chem. Commun. 1991, 1473–1475. [Google Scholar] [CrossRef]
- Hacht, B. Gallium(III) ion hydrolysis under physiological conditions. Bull. Korean Chem. Soc. 2008, 29, 372–376. [Google Scholar]
- Notni, J.; Hermann, P.; Dregely, I.; Wester, H.J. Convenient synthesis of 68Ga-labeled gadolinium(III) complexes: Towards bimodal responsive probes for functional imaging with PET/MRI. Chem. Eur. J. 2013, 19, 12602–12606. [Google Scholar] [CrossRef] [PubMed]
- Šimeček, J.; Notni, J.; Kapp, T.G.; Kessler, H.; Wester, H.J. Benefits of NOPO as chelator in gallium-68 peptides, exemplified by preclinical characterization of 68Ga-NOPO−c(RGDfK). Mol. Pharm. 2014, 11, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Šimeček, J.; Zemek, O.; Hermann, P.; Notni, J.; Wester, H.J. Tailored gallium(III) chelator NOPO: Synthesis, characterization, bioconjugation, and application in preclinical Ga-68-PET imaging. Mol. Pharm. 2014, 11, 3893–3903. [Google Scholar] [CrossRef] [PubMed]
- Van Haveren, J.; DeLeon, L.; Ramasamy, R.; van Westrenen, J.; Sherry, A.D. The design of macrocyclic ligands for monitoring magnesium in tissue by 31P-NMR. NMR Biomed. 1995, 8, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Huskens, J.; Sherry, A.D. Synthesis and characterization of 1,4,7-triazacyclononane derivatives with methylphosphinate and acetate side chains for monitoring free MgII by 31P- and 1H-NMR spectroscopy. J. Am. Chem. Soc. 1996, 118, 4396–4404. [Google Scholar] [CrossRef]
- Atkins, T.J. Tricyclic trisaminomethanes. J. Am. Chem. Soc. 1980, 102, 6364–6365. [Google Scholar] [CrossRef]
- Schulz, D.; Weyhermüller, T.; Wieghard, K.; Nuber, B. The monofunctionalized 1,4,7-triazacyclononane derivatives 1,4,7-triazacyclononane-N-acetate (L1) and N-(2-hydroxybenzyl-1,4,7-triazacyclononane (HL2) and their complexes with vanadium(IV)/(V). Localized and delocalized electronic structures in compounds containing the mixed valent [OVIV-O-VVO]3+ core. Inorg. Chim. Acta 1995, 240, 217–229. [Google Scholar]
- Warden, A.C.; Spiccia, L.; Hearn, M.T.W.; Boas, J.F.; Pilbrow, J.R. The synthesis, structure and properties of copper(II) complexes of asymmetrically functionalized derivatives of 1,4,7-triazacyclononane. Dalton Trans. 2005, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Studer, M.; Kaden, T.A. Metal complexes with macrocyclic ligands. Part XXV. One-step synthesis of mono-N-substituted azamacrocycles with a carboxylic group in the side-chain and their complexes with Cu2+ and Ni2+. Helv. Chim. Acta 1986, 69, 2081–2086. [Google Scholar] [CrossRef]
- Warden, A.; Graham, B.; Hearn, M.T.W.; Spiccia, L. Synthesis of novel derivatives of 1,4,7-triazacyclononane. Org. Lett. 2001, 3, 2855–2858. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Z.; Sherry, A.D. A general synthesis of mono- and disubstituted 1,4,7-triazacyclononanes. Tetrahedron Lett. 1995, 36, 9269–9272. [Google Scholar] [CrossRef]
- Moedritzer, K.; Irani, R.R. The direct synthesis of α-aminomethylphosphonic acids. Mannich-type reactions with orthophosphorous acid. J. Org. Chem. 1966, 31, 1603–1607. [Google Scholar] [CrossRef]
- Remore, D. Chemistry of phosphorous acid: new routes to phosphonic acids and phosphate esters. J. Org. Chem. 1978, 43, 992–996. [Google Scholar] [CrossRef]
- Lukeš, I.; Kotek, J.; Vojtíšek, J.; Hermann, P. Complexes of tetraazacycles bearing methylphosphinic/phosphonic acid pendant arms with copper(II), zinc(II) and lanthanides(III). A comparison with their acetic acid analogues. Coord. Chem. Rev. 2001, 216–217, 287–312. [Google Scholar] [CrossRef]
- Drahoš, B.; Kubíček, V.; Bonnet, C.S.; Hermann, P.; Lukeš, I.; Tóth, É. Dissociation kinetics of Mn2+ complexes of NOTA and DOTA. Dalton Trans. 2011, 40, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Holub, J.; Meckel, M.; Kubíček, V.; Rösch, F.; Hermann, P. Gallium(III) complexes of NOTA-bis(phosphonate) conjugates as PET radiotracers for bone imaging. Contrast Media Mol. Imaging 2015, 10, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Gröβ, S.; Elias, H. Kinetics and mechanism of complex formation: The reaction of nickel(II) with 1,4,7-triazacyclononane-N,N',N"-triacetic acid. Inorg. Chim. Acta 1996, 251, 347–354. [Google Scholar]
- Řezanka, P.; Kubíček, V.; Hermann, P.; Lukeš, I. Synthesis of a bifunctional monophosphinate DOTA derivative having free carboxylate group in the phosphorus side chain. Synthesis 2008, 1431–1435. [Google Scholar] [CrossRef]
- Kubíček, V.; Havlíčková, J.; Kotek, J.; Tircsó, G.; Hermann, P.; Tóth, É.; Lukeš, I. Gallium(iii) complexes of DOTA and DOTA-monoamide: Kinetic and thermodynamic studies. Inorg. Chem. 2010, 49, 10960–10969. [Google Scholar] [CrossRef] [PubMed]
- Kývala, M.; Lubal, P.; Lukeš, I. Determination of equilibrium constants with the OPIUM computer program. In Proceedings of the IX. Spanish-Italian and Mediterranean Congress on Thermodynamics of Metal Complexes (SIMEC 98), Girona, Spain, 2–5 June 1998; p. 94. The Full Version of the OPIUM Program is Available (Free of Charge) on http://web.natur.cuni.cz/~kyvala/opium.html (accessed on 23 September 2014).
- NIST Standard Reference Database 46 (Critically Selected Stability Constants of Metal Complexes); Version 7.0; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2003.
- Baes, C.F., Jr.; Mesmer, R.E. The Hydrolysis of Cations; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Sample Availability: Samples are not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Máté, G.; Šimeček, J.; Pniok, M.; Kertész, I.; Notni, J.; Wester, H.-J.; Galuska, L.; Hermann, P. The Influence of the Combination of Carboxylate and Phosphinate Pendant Arms in 1,4,7-Triazacyclononane-Based Chelators on Their 68Ga Labelling Properties. Molecules 2015, 20, 13112-13126. https://doi.org/10.3390/molecules200713112
Máté G, Šimeček J, Pniok M, Kertész I, Notni J, Wester H-J, Galuska L, Hermann P. The Influence of the Combination of Carboxylate and Phosphinate Pendant Arms in 1,4,7-Triazacyclononane-Based Chelators on Their 68Ga Labelling Properties. Molecules. 2015; 20(7):13112-13126. https://doi.org/10.3390/molecules200713112
Chicago/Turabian StyleMáté, Gábor, Jakub Šimeček, Miroslav Pniok, István Kertész, Johannes Notni, Hans-Jürgen Wester, László Galuska, and Petr Hermann. 2015. "The Influence of the Combination of Carboxylate and Phosphinate Pendant Arms in 1,4,7-Triazacyclononane-Based Chelators on Their 68Ga Labelling Properties" Molecules 20, no. 7: 13112-13126. https://doi.org/10.3390/molecules200713112
APA StyleMáté, G., Šimeček, J., Pniok, M., Kertész, I., Notni, J., Wester, H.-J., Galuska, L., & Hermann, P. (2015). The Influence of the Combination of Carboxylate and Phosphinate Pendant Arms in 1,4,7-Triazacyclononane-Based Chelators on Their 68Ga Labelling Properties. Molecules, 20(7), 13112-13126. https://doi.org/10.3390/molecules200713112





