Abstract
BINAM-prolinamides are very efficient catalyst for the synthesis of non-protected and N-benzyl isatin derivatives by using an aldol reaction between ketones and isatins under solvent-free conditions. The results in terms of diastereo- and enantioselectivities are good, up to 99% de and 97% ee, and higher to those previously reported in the literature under similar reaction conditions. A high variation of the results is observed depending on the structure of the isatin and the ketone used in the process. While 90% of ee and 97% ee, respectively, is obtained by using (Ra)-BINAM-l-(bis)prolinamide as catalyst in the addition of cyclohexanone and α-methoxyacetone to free isatin, 90% ee is achieved for the reaction between N-benzyl isatin and acetone using N-tosyl BINAM-l-prolinamide as catalyst. This reaction is also carried out using a silica BINAM-l-prolinamide supported catalyst under solvent-free conditions, which can be reused up to five times giving similar results.
1. Introduction
Isatin (1H-indole-2,3-dione) is a natural product found in different plants, as well as in the parotid gland secretions of Bufo frogs and also in humans metabolism as an endogenous compound [1,2]. This molecule and its derivatives have attracted the interest of the scientific community due to their relevant properties as drug candidates and interesting biological activities [3,4,5]. Chiral 3-substituted-3-hydroxyindolin-2-ones, having a quaternary stereocenter, are found as a core structures in several natural products and pharmaceutically active compounds [6], with its biological activity being determined by the nature of the alkyl group at the C3 position and its absolute configuration. The synthesis of this type of chiral derivatives can be efficiently accomplished by an enantioselective organocatalyzed reaction of free or N-protected isatin with enolizable compounds [7]. Following this strategy, the application of the organocatalyzed aldol reaction [8,9,10,11,12,13] has played a major role in the synthesis of isatin derivatives. The use of proline [14,15] and its derivatives [16,17,18,19,20,21,22,23,24] has been described for this transformation achieving typically high yields and enantioselectivities ranging from 75% to 95% ee. In most cases, the nucleophilic ketone acted as nucleophile and also as solvent. However, recently the use of two prolinamides derivatives (1 and 2) have been reported to promote this aldol reaction under solvent free conditions [25] (Figure 1). While prolinamide 1 (5 mol %) [26] was used in combination with TFA (7.5 mol %) in the addition of 2 equiv. of acetone or cyclohexanone to free isatin at 0 °C using conventional stirring, thiodipeptide 2 (10 mol %) [27] required the use of 3 equiv. of nucleophiles and ball-mill stirring (2760 rpm) at −20 °C, both affording the corresponding aldol products in moderate enantioselectivities (53%–86% ee).
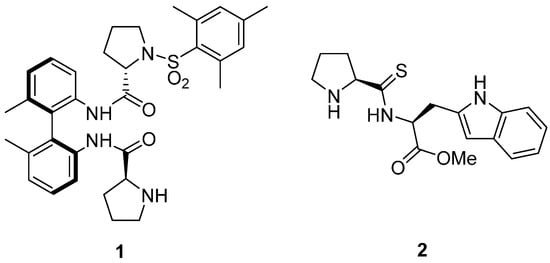
Figure 1.
Prolinamides used in the solvent-free conditions aldol reaction of isatins.
Figure 1.
Prolinamides used in the solvent-free conditions aldol reaction of isatins.
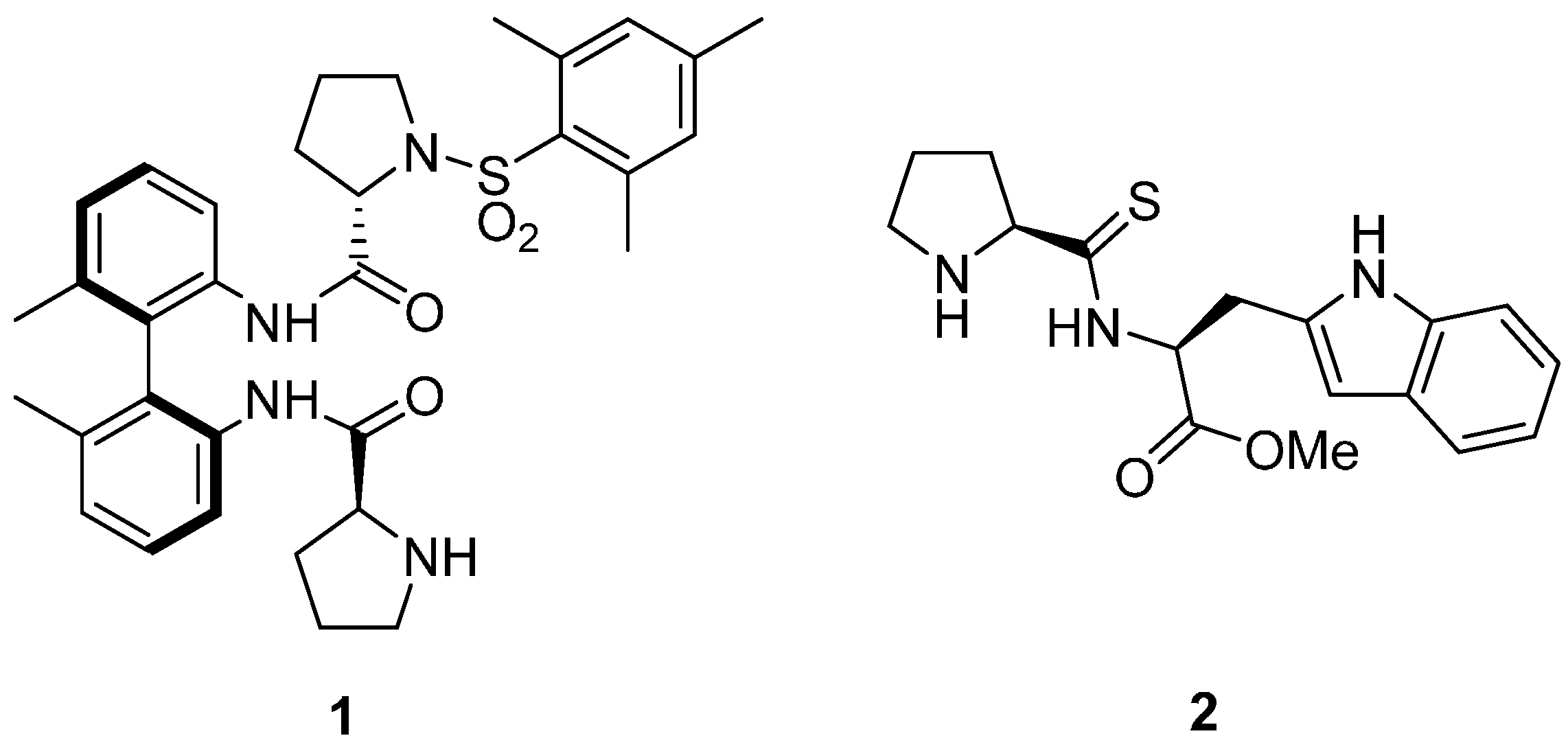
Figure 2.
BINAM-prolinamides used under solvent-free conditions for the aldol reaction.
Figure 2.
BINAM-prolinamides used under solvent-free conditions for the aldol reaction.
On the other hand, different 1,1′-binaphthyl-2,2′-diamine (BINAM) prolinamide derivatives (Figure 2) has been tested as catalysts for the intra- and intermolecular aldol reaction between cyclic, acyclic, alkyl and α-functionalized ketones and aldehydes under different reaction conditions [28,29,30,31,32,33,34,35,36,37,38,39,40,41], including solvent-free conditions. Thus, BINAM-l-(bis)prolinamide 3 [42,43,44] and N-tosyl BINAM-l-prolinamide derivative 4 [45,46,47,48,49,50] and even their supported analogues [51,52,53,54,55], such as the silica derivative 5, have shown to be very effective catalysts for the aldol reaction. Therefore, we decided to evaluate the use of these binam-prolinamide derivatives in the aldol reaction of ketones with isatins under solvent-free conditions.
2. Results and Discussion
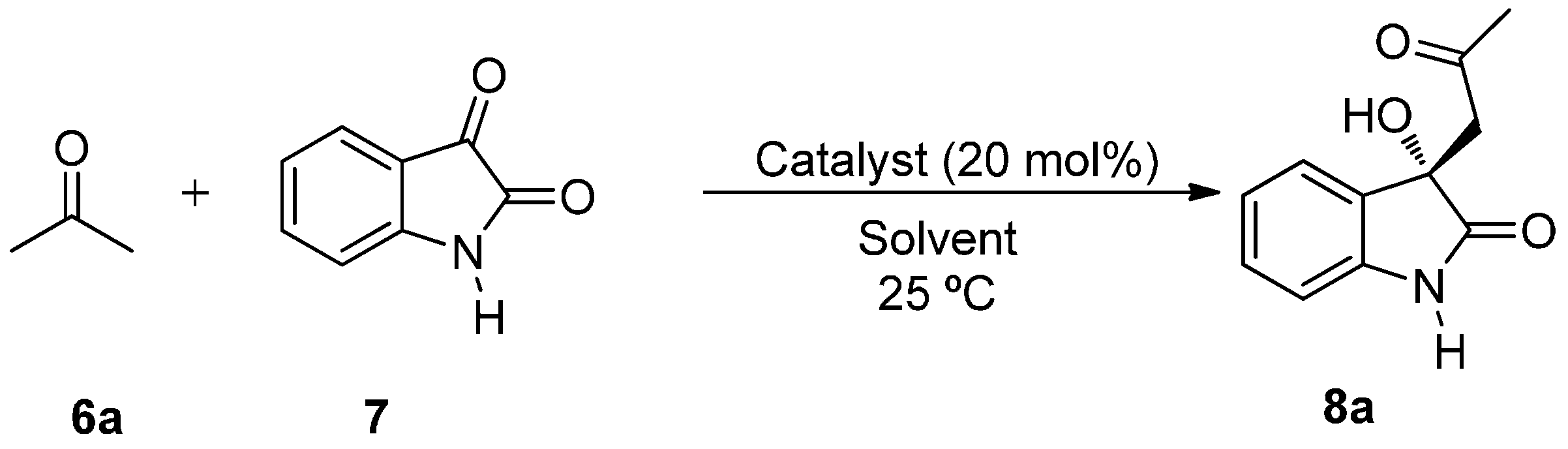
The aldol reaction between acetone 6a (3 equiv.) and non-protected isatin 7 with a catalyst loading of 20 mol % at room temperature was chosen as benchmark to test the efficiency of catalyst 3 under different reaction media (Scheme 1).
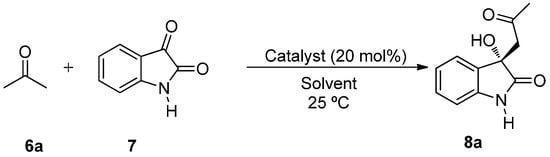
Scheme 1.
BINAM-prolinamide catalyzed reaction between acetone and isatin.
Scheme 1.
BINAM-prolinamide catalyzed reaction between acetone and isatin.
The reaction proceeded very slowly using different type of polar and apolar solvents and even in water (entries 1–6, Table 1), with low conversions and enantioselectivities being achieved. However, when the process was carried out under solvent-free conditions, the reaction rate increased tremendously, achieving the expected product 8a after only 6h with almost full conversion, but unfortunately in low ee (entry 7, Table 1). In order to improve the enantioselectivity, the reaction temperature was decreased to 0, −20, −50 and −70 °C (entries 8–11, Table 1), just an increase of the reaction time was observed without enantioselectivity enhancement. We have observed in previous aldol processes that the addition of a small amount of benzoic acid [30] and water provoked an increase on the reaction rate. Therefore, the use of these two additives was evaluated (entries 12–14, Table 1), but again a deleterious effect on the enantioselectivities was observed. The effect of the decrease in the amount of catalyst was checked (entries 15–16, Table 1), affording with only a 5 mol % of catalyst, product 8a in similar results than those obtained by using 20 mol %, with more time being required for the reaction completion. The reduction of the amount of the used ketone to 2 or 1 equiv. gave similar results in terms of enantioselectivities, but longer reaction times and lower conversion was found when only 1 equiv. of ketone (entries 17–18, Table 1) was used. The use of acetone as reaction media didn’t led to any improvement of the result (entry 19, Table 1). Thus, using only 2 equiv. of acetone and performing the reaction at room temperature and just 5 mol % of catalyst 3, the performance of the diastereoisomer of compound 3, (Sa)-binam-l-prolinamide (diast-3), N-tosyl-(Sa)-binam-l-prolinamide 4 and its diastereoisomer N-tosyl-(Ra)-binam-l-prolinamide (diast-4) was studied. While (Sa)-binam-l-prolinamide (diast-3) gave product 8a with similar results to those encountered with catalyst 3 (entry 20, Table 1), N-tosyl-(Sa)-binam-l-prolinamide 4 and its diastereoisomer led to lower conversions in longer reaction times (entries 21–22, Table 1).

Table 1.
Optimization of reaction conditions.
| Entry | Solvent a | Cat. (mol %) | Ketone (equiv.) | T a (°C) | t | Conv (%) b | ee (%) c |
|---|---|---|---|---|---|---|---|
| 1 | DMSO | 3(20) | 3 | 25 | 3 days | <50 | 12 |
| 2 | THF | 3 (20) | 3 | 25 | 3 days | <30 | 21 |
| 3 | Hexane | 3 (20) | 3 | 25 | 3 days | <20 | 14 |
| 4 | H2O:DMF | 3 (20) | 3 | 25 | 3 days | <20 | 0 |
| 5 | DMF | 3 (20) | 3 | 25 | 3 days | <20 | 0 |
| 6 | H2O | 3 (20) | 3 | 25 | 3 days | <30 | 11 |
| 7 | – | 3 (20) | 3 | 25 | 6 h | >99 | 29 |
| 8 | – | 3 (20) | 3 | 0 | 1 days | 83 | 27 |
| 9 | – | 3 (20) | 3 | −20 | 3 days | 74 | 31 |
| 10 | – | 3 (20) | 3 | −50 | 3 days | <50 | 33 |
| 11 | – | 3 (20) | 3 | −70 | 3 days | <30 | 30 |
| 12 d | – | 3 (20) | 3 | 25 | 6 h | >99 | 22 |
| 13 d,e | – | 3 (20) | 3 | 25 | 4 h | >99 | 17 |
| 14 e | – | 3 (20) | 3 | 25 | 4 h | >99 | 20 |
| 15 | – | 3 (5) | 3 | 25 | 1 days | >99 | 31 |
| 16 | – | 3 (10) | 3 | 25 | 1 days | >99 | 28 |
| 17 | – | 3 (5) | 2 | 25 | 1 days | >99 | 30 |
| 18 | – | 3 (5) | 1 | 25 | 1 days | 82 | 27 |
| 19 | Acetone | 3 (5) | excess | 25 | 1 days | >99 | 22 |
| 20 | – | diast-3 (5) | 2 | 25 | 1 days | >99 | 20 |
| 21 | – | 4 (5) | 2 | 25 | 3 days | <30 | 24 |
| 22 | – | diast-4 (5) | 2 | 25 | 3 days | <30 | 29 |
a: 0.15 mL for 0.3 mmol of 7 was used; b: Conversion based on the amount of the unreacted isatin; c: Determined by chiral phase HPLC analysis; d: 5 mol % PhCO2H was used; e: 10 equiv. of water was added.
The best reaction conditions, 5 mol % of catalyst 3 at room temperature and under solvent-free reaction conditions were applied in the reaction between different ketones 6 (2 equiv.) and free isatins 7 (Scheme 2). Good yields were achieved for all the assayed ketones, with good diastereo- and enantioselectivities when cyclohexanone and α-methoxyacetone were used as nucleophiles, although a longer reaction time was required for the reaction completion. Product 8c was obtained mainly as its anti-isomer in high diastereoselectivity.
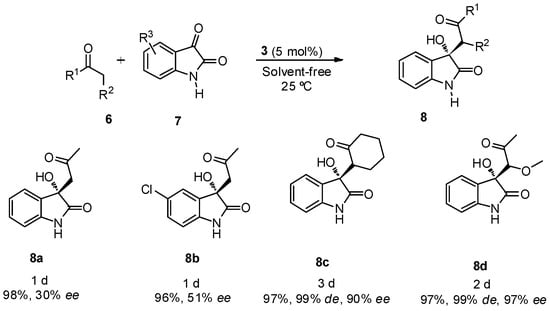
Scheme 2.
BINAM-prolinamide 3 catalyzed reaction between ketone and free isatins.
Scheme 2.
BINAM-prolinamide 3 catalyzed reaction between ketone and free isatins.
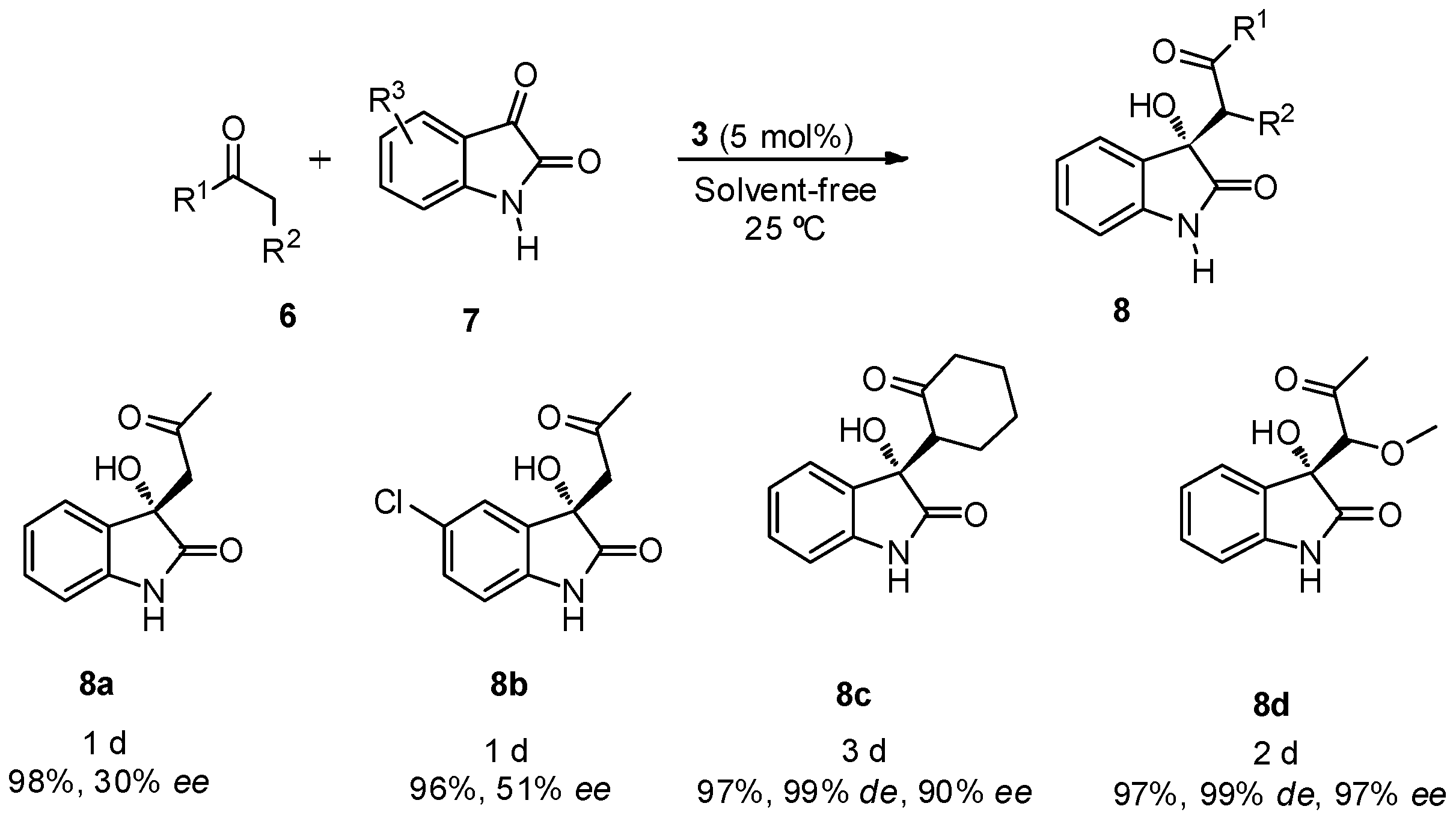
When α-methoxyacetone was used as nucleophile, the reaction took place through the methylene group, being the main diastereoisomer achieved the anti-product 8d. The same regioselectivity was observed in the reaction with other ketones, such as butanone, α-methylsulfanylacetone or α-chloroacetone, but unfortunately these products were achieved as racemic mixtures. Also, attempts to use aldehydes, as nucleophiles, for this kind of transformation led to the formation of racemic mixtures.
As compounds 8 and free isatins 7 are rather insoluble in most solvents, a more soluble N-benzyl isatin 9 was tested in the aldol reaction with acetone (Scheme 3).
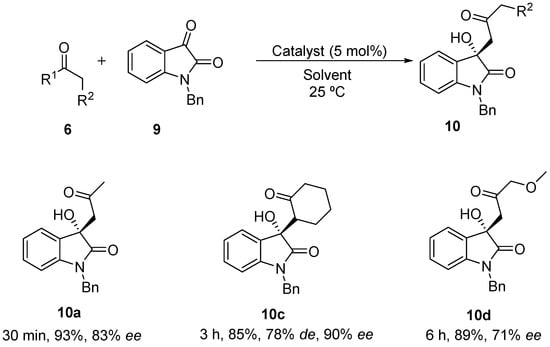
Scheme 3.
Solvent-free BINAM-prolinamide catalyzed reaction between acetone and N-benzyl isatin.
Scheme 3.
Solvent-free BINAM-prolinamide catalyzed reaction between acetone and N-benzyl isatin.
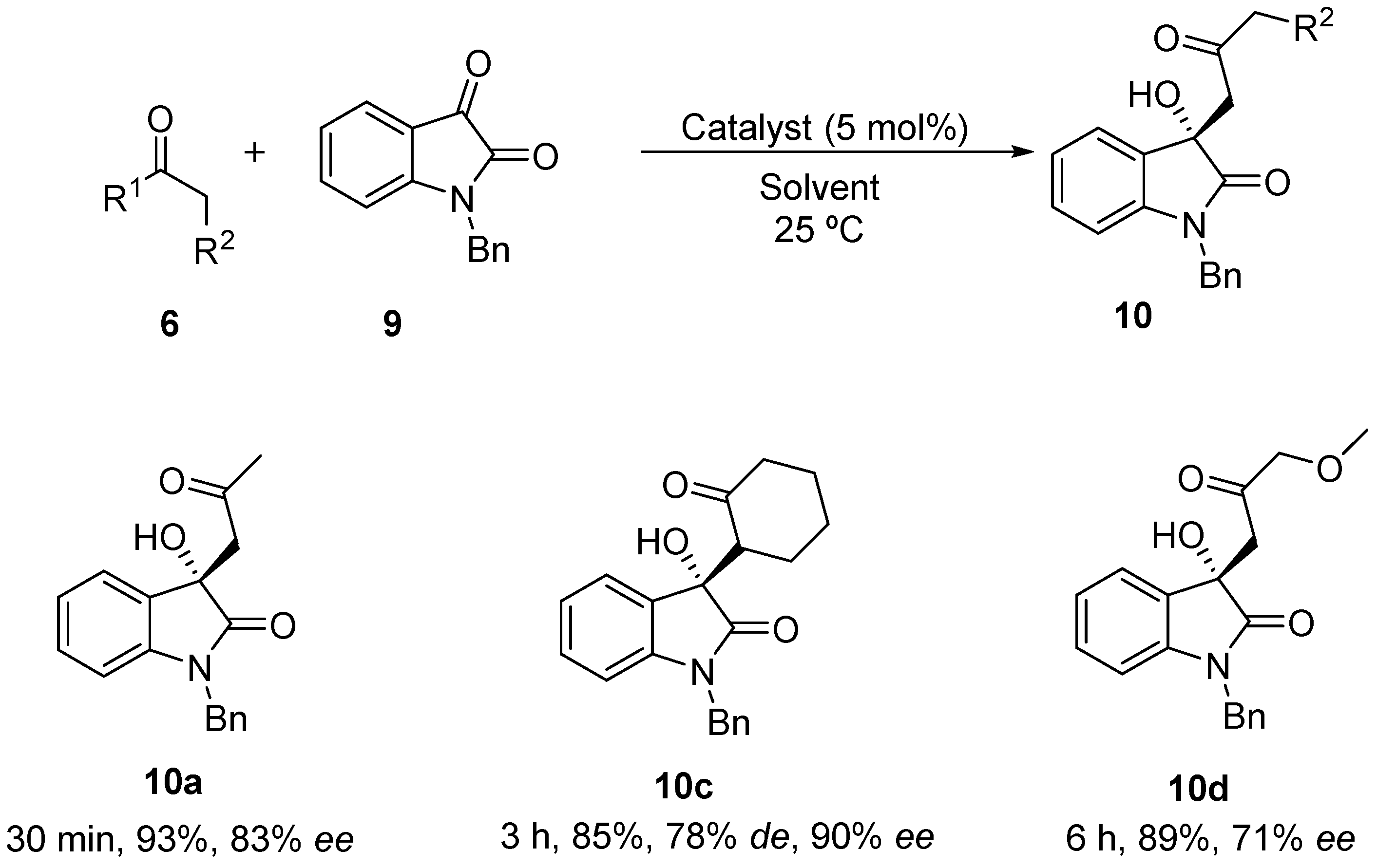
First, the performance of the different catalysts under solvent-free conditions was evaluated, using just 5 mol % of catalyst loading (Table 2). As expected, the higher solubility of the electrophile decreases the heterogeneousity of the reaction, with the expected product being formed in only minutes. Surprisingly, catalyst 3 and its diastereoisomer led to the racemic product in the reaction with acetone (entries 1 and 2, Table 2), while catalyst 4 led to product 10 with up to 83% ee (entry 3, Table 2). When N-tosyl-(Ra)-binam-l-prolinamide (diast-4) was used as catalyst, product 10 was obtained with lower enantioselectivity (entry 4, Table 2), showing that in catalyst 4 as synergistic effect between the axial chirality of the binaphthyl framework and the proline takes place. Attempts to improve the catalytic efficiency of 4 by adding water, benzoic acid or both failed (entries 5–7, Table 2). When cyclohexanone was used as nucleophile in this reaction, only catalyst 3 showed to be active. Using this catalyst, product 10c was achieved as anti-isomer, but in lower diastereoselectivity compared to the one achieved in the reaction with the free isatin 7. Also, an unexpected result was achieved when α-methoxyacetone was used as nucleophile with a reverse regioselectivity being found. The regioisomer 10d was the only product formed in a 71% ee.
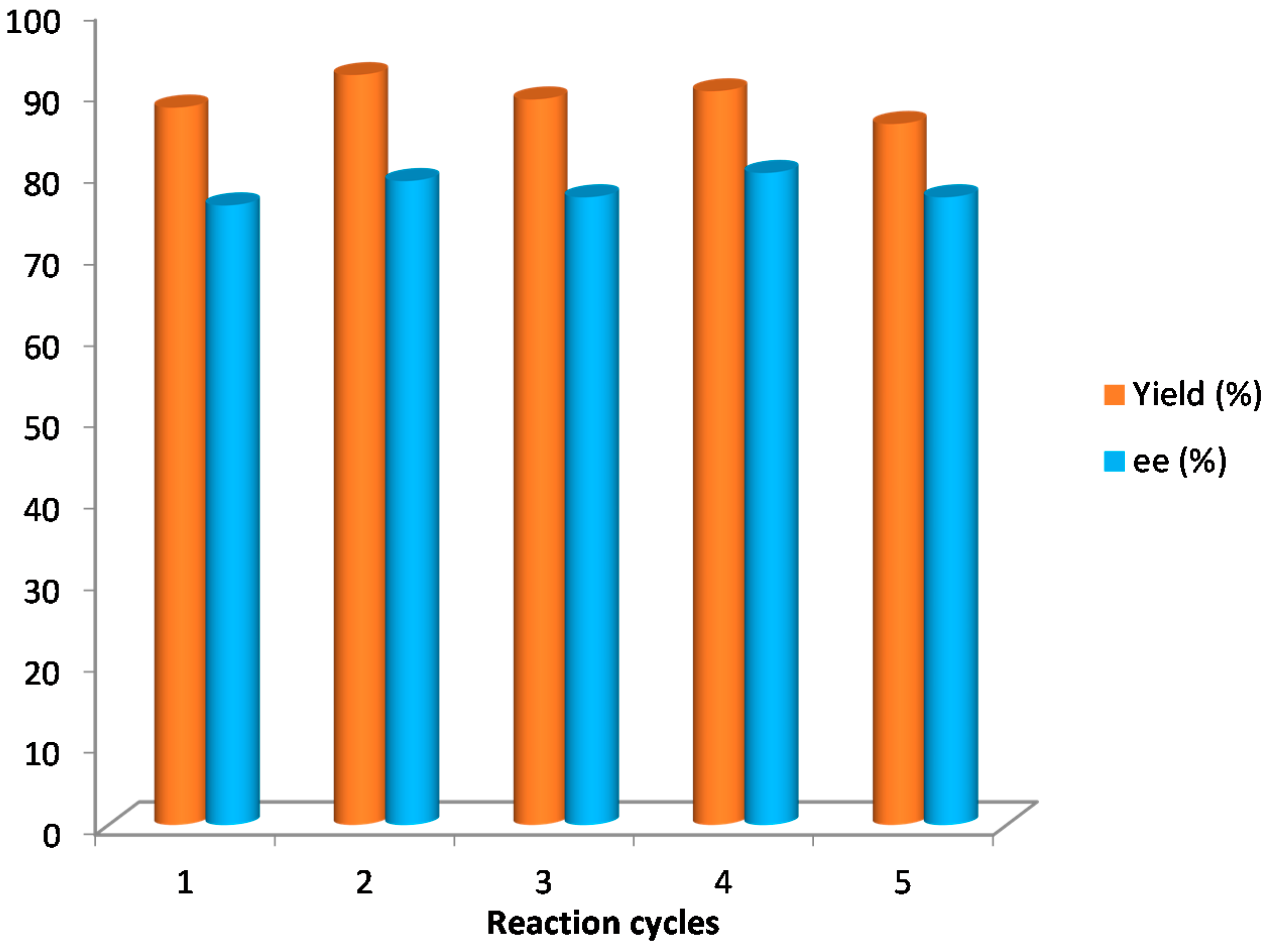
Finally, the silica-supported catalyst 5 (10 mol %) was applied under solvent-free conditions for the synthesis of compound 10a, its recovery and recyclability being studied. The results achieved were similar in terms of yields and enantioselectivities to those achieved by using catalyst 4. The catalyst was recovered by filtration after reaction completion and reused five times without observing any changes in the results (Figure 3).

Table 2.
Optimization of reaction conditions.
| Entry | R1,R2 | Cat. (mol %) | Product | Ketone (equiv.) | T a (°C) | t (min) | Conv (%) a,b | de (%) c | ee (%) d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Me,H | 3 | 10a | 2 | 25 | 5 | >99 | - | 0 |
| 2 | Me,H | diast-3 | 10a | 2 | 25 | 5 | >99 | - | 0 |
| 3 | Me,H | 4 | 10a | 2 | 25 | 30 | >99 (93) | - | 83 |
| 4 | Me,H | diast-4 | 10a | 2 | 25 | 90 | >99 | - | 75 |
| 5 e | Me,H | 4 | 10a | 2 | 25 | 30 | >99 | - | 70 |
| 6 f | Me,H | 4 | 10a | 2 | 25 | 30 | >99 | - | 59 |
| 7 e,f | Me,H | 4 | 10a | 2 | 25 | 30 | >99 | - | 63 |
| 8 | (CH2)4 | 3 | 10c | 2 | 25 | 90 | 90 (85) | 78 | 90 |
| 9 | Me,OMe | 3 | 10d | 2 | 25 | 360 | 92 (89) | - | 71 |
a: Conversion based on the amount of the unreacted isatin; b: In parenthesis, yield after column chromatography purification; c: Determined by 1H-NMR; d: Determined by chiral phase HPLC analysis; e: 5 mol % PhCO2H was used; f: 10 equiv. of water was added.
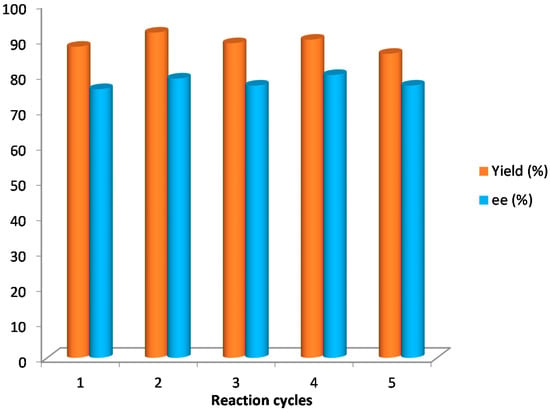
Figure 3.
Recycling study of catalyst 5 in the synthesis of 10a.
Figure 3.
Recycling study of catalyst 5 in the synthesis of 10a.
3. Experimental Section
3.1. General Information
All reactions for catalyst preparation were carried out under argon. Dry DMF, dry toluene, dry DCM, dry THF, pyridine, and triethylamine and all others reagents were commercially available and used without further purification. 1H-NMR (300 or 400 MHz) and 13C-NMR (75 or 100 MHz) spectra were obtained at 25 °C with CDCl3 as solvent and TMS as internal standard, unless otherwise stated. HPLC analyses were performed with a chiral column (detailed for each compound below), with mixtures of n-hexane/isopropyl alcohol as mobile phase, at 25 °C. Analytical thin-layer chromatography (TLC) was performed on silica gel plates and the spots were visualized under UV light (λ = 254 nm). For flash chromatography silica gel 60 (0.063–0.2 mm) was employed.
Catalysts 3, diast-3, 4 and diast-4 were prepared as previously described [43,48].
3.2. Procedure for the Addition of Ketones to Isatins
To the mixture of the corresponding isatin (0.3 mmol), and catalyst 3 or 4 (5 mol %), ketone (0.6 mmol) was added. The reaction was stirred using magnetic stirring until the isatin was consumed (monitored by TLC), then the crude product was purified by a silica gel chromatography.
3.3. Procedure for Catalysis Recover and Reuse
After each cycle, the reaction was quenched by filtration and the resin was washed with a 0.5 M solution of NaOH, to remove the benzoic acid occluded in the polymeric matrix. Then, the resin was washed several times either with ethyl acetate, ethanol or acetone and dried under vacuum. The same resin was used in the following reaction cycle.
3.4. Physical, Analytical and Spectal Data
(R)-3-Hydroxy-3-(2-oxopropyl)indolin-2-one (8a) [56]. Yield 98%; 1H-NMR (400 MHz, CDCl3,): δ 7.63 (d, J = 7.8 Hz, 1H), 7.37 (d, J = 7.6 Hz, 1H), 7.07 (t, J = 7.4 Hz, 1H), 6.89 (d, J = 7.6 Hz, 1H), 3.20 (d, J = 17.2 Hz, 1H), 2.98 (d, J = 17.2 Hz, 1H), 2.22 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 207.4, 179.8, 137.0, 132.3, 128.2, 125.3, 123.0, 112.7, 76.2, 49.5, 31.6, 49.5, 76.2, 112.7. HPLC Chiralcel OJ column, n-hexane/2-propanol = 80:20, flow rate 0.8 mL/min, λ = 254 nm; tR 15.34 min (major) and 19.22 min (minor).
(S)-5-Chloro-3-hydroxy-3-(2-oxopropyl)indolin-2-one (8b) [56]. Yield 96%; 1H-NMR (300 MHz, MeOD) δ 7.37–7.29 (m, 1H), 7.24 (dd, J = 8.3, 2.2 Hz, 1H), 6.91–6.80 (m, 1H), 3.41 (d, J = 17.2 Hz, 1H), 3.20 (d, J = 17.3 Hz, 1H), 2.08 (s, 3H). 13C-NMR (75 MHz, MeOD) δ 207.2, 180.7, 142.4, 134.3, 130.4, 128.5, 125.2, 112.3, 74.6, 50.9, 30.4. HPLC Chiralcel OJ column, n-hexane/2-propanol = 80:20, flow rate 0.8 mL/min, λ = 254 nm; tR 23.12 min (major) and 35.44 min (minor).
(S)-3-Hydroxy-3-((R)-2-oxocyclohexyl)indolin-2-one (8c). Yield 97%; 1H-NMR (400 MHz, DMSO) δ 10.18 (s, 1H), 7.17 (ddd, J = 11.2, 8.8, 4.3 Hz, 2H), 6.83 (ddd, J = 22.7, 14.6, 4.3 Hz, 2H), 5.80 (s, 1H), 3.07 (dd, J = 13.1, 5.2 Hz, 1H), 2.59 (ddd, J = 12.4, 5.1, 2.6 Hz, 1H), 2.40–2.25 (m, 1H), 2.10–1.99 (m, 1H), 1.94 (dt, J = 22.7, 8.8 Hz, 2H), 1.86–1.76 (m, 1H), 1.67 (dt, J = 12.6, 3.2 Hz, 1H), 1.55–1.37 (m, 1H). 13C-NMR (101 MHz, DMSO) δ 209.1, 178.6, 143.4, 130.8, 128.5, 126.3, 124.7, 120.8, 109.3, 73.8, 57.3, 41.4, 26.6, 24.4. Elemental analysis C14H15NO3: C 68.56, H 6.16, N 5.71. Found C 69.19, H 6.32, N 5.65. HPLC Chiralpak AS-H column, n-hexane/2-propanol = 70:30, flow rate 1.0 mL/min, λ = 254 nm; tR 27.34 min (major) and 43.12 min (minor).
(R)-3-Hydroxy-3-((R)-1-methoxy-2-oxopropyl)indolin-2-one (8d). Yield 85%; 1H-NMR (300 MHz, MeOD) δ 7.53–7.46 (m, 1H), 7.25 (td, J = 7.7, 1.3 Hz, 1H), 7.02 (td, J = 7.6, 0.9 Hz, 1H), 6.83 (d, J = 7.7 Hz, 1H), 4.03 (s, 1H), 3.54 (s, 3H), 1.99 (s, 3H). 13C-NMR (75 MHz, MeOD) δ 210.5, 179.0, 143.5, 131.0, 130.3, 126.3, 123.3, 111.1, 91.3, 78.6, 61.4, 27.4. Elemental analysis C12H13NO4: C 61.27, H 5.57, N 5.95. Found C 62.02, H 5.69, N 5.87. HPLC Chiralpak AS-H column, n-hexane/2-propanol = 80:20, flow rate 1.0 mL/min, λ = 254 nm; tR 34.46 min (major) and 38.23 min (minor).
(S)-1-Benzyl-3-hydroxy-3-(2-oxopropyl)indolin-2-one (10a) [56]. Yield 97%; 1H-NMR (300 MHz, CDCl3) δ 7.39–7.22 (m, 13H), 7.19 (td, J = 7.8, 1.3 Hz, 2H), 7.02 (td, J = 7.6, 1.0 Hz, 2H), 6.69 (d, J = 7.8 Hz, 2H), 4.95 (d, J = 15.8 Hz, 2H), 4.83 (d, J = 15.8 Hz, 2H), 4.54 (s, 2H), 3.27 (d, J = 17.0 Hz, 2H), 3.06 (d, J = 17.1 Hz, 2H), 2.16 (s, 6H). 13C-NMR (75 MHz, CDCl3) δ 207.0, 176.4, 142.7, 135.3, 129.7, 128.7, 127.6, 127.1, 123.7, 123.1, 109.6, 77.4, 77.0, 76.5, 74.1, 49.0, 43.8, 31.2. HPLC Chiralpak AD-H column, n-hexane/2-propanol = 90:10, flow rate 1.0 mL/min, λ = 280 nm; tR 27.34 min (minor) and 33.12 min (major).
(R)-1-Benzyl-3-hydroxy-3-((S)-20-oxocyclohexyl)indolin-2-one (10c) (Product CAS number 881081-60-3). Yield 93%; 1H-NMR (300 MHz, CDCl3): δ 1.54–2.00 (m, 6H) 2.28–2.47 (m, 2H), 2.97–3.06 (m, 1H,) 3.20–3.27 (m, 1H), 4.73–4.81 (m, 1H), 4.88–5.00 (m, 1H), 6.62–6.69 (m, 1H), 6.95–7.01 (m, 1H), 7.17–7.46 (m, 7H); 13C-NMR (75 MHz, CDCl3): δ 24.1, 25.7, 27.1, 42.0, 43.5, 55.3, 76.3, 109.2, 122.8, 124.0, 125.1, 126.8, 127.5, 128.5, 128.6, 128.8, 129.6, 135.3, 143.4, 176.6, 211.3. Elemental analysis C21H21NO3: C 75.20, H 6.31, N 4.18. Found C 75.45, H 6.25, N 4.07. HPLC Chiralpak OD-H column, n-hexane/2-propanol = 95:05, flow rate 1.0 mL/min, λ = 254 nm; tR 19.25 min (major) and 45.23 min (minor).
(S)-1-Benzyl-3-hydroxy-3-(3-methoxy-2-oxopropyl)indolin-2-one (10d). Yield 89%; 1H-NMR (300 MHz, CDCl3) δ 7.44–7.29 (m, 12H), 7.22 (td, J = 7.8, 1.3 Hz, 3H), 7.10–7.00 (m, 2H), 6.72 (d, J = 7.8 Hz, 2H), 4.92 (q, J = 15.7 Hz, 4H), 4.31 (s, 2H), 4.03 (s, 4H), 3.42 (s, 6H), 3.35 (d, J = 16.5 Hz, 3H), 3.03 (s, 2H). 13C-NMR (75 MHz, CDCl3) δ 40.1, 49.8, 50.9, 77.7, 78.3, 110.5, 123.0, 123.8, 126.9, 127.3, 128.5, 129.0, 129.8, 136.0, 144.5, 176.2, 210.0. Elemental analysis C19H19NO4: C 70.14, H 5.89, N 4.31. Found C 71.00, H 6.14, N 4.19. HPLC Chiralpak OD-H column, n-hexane/2-propanol = 90:10, flow rate 0.7 mL/min, λ = 254 nm; tR 35.03 min (major) and 40.30 min (minor).
4. Conclusions
In conclusion, BINAM-prolinamides have shown to be efficient catalysts under solvent-free conditions, in terms of diastereo- and enantioselectivities, for the synthesis of non-protected and N-benzyl isatin derivatives. The achieved enantioselectivities are superior to those reported under solvent-free conditions using other prolinamide systems. However, these results are highly dependent on the structure of the isatin and the ketone used. The use of (Ra)-BINAM-l-(bis)prolinamide as catalyst in the addition of cyclohexanone and α-methoxyacetone to free isatin led to 90% of ee and 97% ee, respectively. On the other hand, using acetone as nucleophile, good enantioselectivities were obtained with N-tosyl BINAM-l-prolinamide 4 as catalyst and N-benzyl isatin as electrophile with up to 90% ee being achieved. This good result has allowed the application of a silica-supported catalyst (5) that can be recovered and reused for at least five times without being detrimental on the results.
Acknowledgments
This work was financially supported by the Ministerio de Economia y Competitividad (MINECO: Projects: CTQ2010-20387 and Consolider INGENIO CSD2007-0006), FEDER, the Generalitat Valenciana (Prometeo/2009/039, the University of Alicante and the EU (ORCA action CM0905). A.B.-C. thanks the Spanish MICINN for a predoctoral fellowship (FPU AP2009-3601). We thank to Rosa M. Ortiz for the synthesis of both enantiomers of [1,1′-binaphthalene]-2,2′-diamine. We are grateful to O. C. Townley and A. Ramón for English polishing and corrections.
Author Contributions
Abraham Bañón-Caballero and Jesús Flores-Ferrándiz have performed the experiments and analyzed the data; Gabriela Guillena has conceived the experiments and wrote the article; Carmen Nájera has contributed to the reagents/materials/analysis tools.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Da Silva, J.F.M.; Garden, S.J.; Pinto, A.C. The Chemistry of Isatins: A Review from 1975 to 1999. J. Braz. Chem. Soc. 2001, 12, 273–324. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zhang, R.; Wu, Q.Y.; Chen, Q.; Yang, G.F. Recent Developments in the Synthesis and Applications of Isatins. Org. Prep. Proc. Int. 2014, 46, 317–362. [Google Scholar] [CrossRef]
- Grewal, A.S. Isatin derivatives with several biological activities. Int. J. Pharm. Res. 2014, 6, 1–7. [Google Scholar]
- Khan, N.; Khan, Z.; Ahmed, W.; Khan, N.; Hameed, Z. Recent Pharmacological Advancements in Isatin Chemistry. Int. J. Chem. Sci. 2014, 12, 1596–1606. [Google Scholar]
- Phogat, P.; Singh, P. A Mini Review on Central Nervous System Potential of Isatin Derivatives. Cent. Nerv. Syst. Agents Med. Chem. 2015, 15, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Heiran, R.; Herrera, R.P.; Marqués-López, E. Isatin as a Strategic Motif for Asymmetric Catalysis. ChemCatChem 2013, 5, 2131–3148. [Google Scholar] [CrossRef]
- Flores, M.; Peña, J.; García-García, P.; Garrido, N.M.; Díez, D. Enantioselective Organocatalytic Reactions of Isatin. Curr. Org. Chem. 2013, 17, 1957–1985. [Google Scholar] [CrossRef]
- Guillena, G.; Nájera, C.; Ramón, D.J. Enantioselective Direct Aldol Reaction: The Blossoming of Modern Organocatalysis. Tetrahedron Asymmetry 2007, 18, 2249–2293. [Google Scholar] [CrossRef]
- Geary, L.M.; Hultin, P.G. The State of the Art in Asymmetric Induction: The Aldol Reaction as a Case Study. Tetrahedron Asymmetry 2009, 20, 131–173. [Google Scholar] [CrossRef]
- Zlotin, S.G.; Kucherenko, A.S.; Beletskaya, I.P. Organocatalysis of Asymmetric Aldol Reaction. Catalysts and Reagents. Russ. Chem. Rev. 2009, 78, 737–784. [Google Scholar] [CrossRef]
- Trost, B.; Brindle, C.S. The Direct Catalytic Asymmetric Aldol Reaction. Chem. Soc. Rev. 2010, 39, 1600–1632. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Asadi, S. Recent applications of organocatalysts in asymmetric aldol reactions. Tetrahedron Asymmetry 2012, 23, 1431–1465. [Google Scholar] [CrossRef]
- Guillena, G. Modern Methods in Stereoselective Aldol Reactions; Mahrwald, R., Ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 155–268. [Google Scholar]
- Chen, G.; Wang, Y.; He, H.; Gao, S.; Yang, X.; Hao, X. l-Proline-Catalyzed Asymmetric Aldol Condensation of N-Substituted Isatins with Acetone. Heterocycles 2006, 68, 2327–2333. [Google Scholar] [CrossRef]
- Corrêa, R.J.; Garden, S.J.; Angelici, G.; Tomasini, C. A DFT and AIM Study of the Proline-Catalyzed Asymmetric Cross-Aldol Addition of Acetone to Isatins: A Rationalization for the Reversal of Chirality. Eur. J. Org. Chem. 2008, 2008, 736–744. [Google Scholar] [CrossRef]
- Luppi, G.; Cozzi, P.G.; Monari, M.; Kaptein, B.; Broxterman, Q.B.; Tomasini, C. Dipeptide-Catalyzed Asymmetric Aldol Condensation of Acetone with (N-Alkylated) Isatins. J. Org. Chem. 2005, 70, 7418–7421. [Google Scholar] [CrossRef] [PubMed]
- Angelici, G.; Corrêa, R.J.; Garden, S.J.; Tomasini, C. Water influences the enantioselectivity in the proline or prolinamide-catalyzed aldol addition of acetone to isatins. Tetrahedron Lett. 2009, 50, 814–817. [Google Scholar] [CrossRef]
- Chen, J.R.; Liu, X.P.; Zhu, X.Y.; Li, L.; Qiao, Y.F.; Zhang, J.M.; Xiao, W.J. Organocatalytic asymmetric aldol reaction of ketones with isatins: Straightforward stereoselective synthesis of 3-alkyl-3-hydroxyindolin-2-ones. Tetrahedron 2007, 63, 10437–10444. [Google Scholar] [CrossRef]
- Nakamura, S.; Hara, N.; Nakashima, H.; Kubo, K.; Shibata, N.; Toru, T. Enantioselective Synthesis of (R)-Convolutamydine A with New N-Heteroarylsulfonylprolinamides. Chem. Eur. J. 2008, 14, 8079–8081. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Zhang, S.; Liu, L.; Duan, W.; Wang, W. Organocatalytic Enantioselective Cross-Aldol Reactions of Aldehydes with Isatins: Formation of Two Contiguous Quaternary Centered 3-Substituted 3-Hydroxyindol-2-ones Chem. Asian J. 2009, 4, 1664–1667. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.B.; Du, X.L.; Cun, L.F.; Zhang, X.M.; Yuan, W.C. Highly enantioselective aldol reaction of acetaldehyde and isatins only with 4-hydroxydiarylprolinol as catalyst: Concise stereoselective synthesis of (R)-convolutamydines B and E, (−)-donaxaridine and (R)-chimonamidine. Tetrahedron 2010, 66, 1441–1446. [Google Scholar] [CrossRef]
- Pearson, A.J.; Panda, S. N-Prolinylanthranilamide Pseudopeptides as Bifunctional Organocatalysts for Asymmetric Aldol Reactions. Org. Lett. 2011, 13, 5548–5551. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, M.; Duggan, P.G.; Lennon, C.M. Screening of simple N-aryl and N-heteroaryl pyrrolidine amide organocatalysts for the enantioselective aldol reaction of acetone with isatin. Tetrahedron Asymmetry 2011, 22, 1423–1433. [Google Scholar] [CrossRef]
- Pearson, A.J.; Panda, S.; Bunge, S.D. Synthesis of a Potential Intermediate for TMC-95A via an Organocatalyzed Aldol Reaction. J. Org. Chem. 2013, 78, 9921–9928. [Google Scholar] [CrossRef] [PubMed]
- Bañón-Caballero, A.; Guillena, G.; Najera, C. Solvent-Free Enantioselective Organocatalyzed Aldol Reactions. Mini-Rev. Org. Chem. 2014, 11, 118–128. [Google Scholar] [CrossRef]
- Zhang, F.; Li, C.; Qi, C. Highly diastereo- and enantioselective direct aldol reaction under solvent-free conditions. Tetrahedron Asymmetry 2013, 24, 380–388. [Google Scholar] [CrossRef]
- Hernández, J.G.; García-López, V.; Juaristi, E. Solvent-free asymmetric aldol reaction organocatalyzed by (S)-proline-containing thiodipeptides under ball-milling conditions. Tetrahedron 2012, 68, 92–97. [Google Scholar] [CrossRef]
- Guillena, G.; Hita, M.C.; Nájera, C. BINAM-prolinamides as recoverable catalysts in the direct aldol condensation. Tetrahedron Asymmetry 2006, 17, 729–733. [Google Scholar] [CrossRef]
- Gryko, D.; Kowalczyk, B.; Zawadzki, L. Bisprolinediamides with the binaphthyl backbone as organocatalysts for the direct asymmetric aldol reaction. Synlett 2006, 1059–1062. [Google Scholar] [CrossRef]
- Guillena, G.; Hita, M.C.; Nájera, C. High acceleration of the direct aldol reaction cocatalyzed by BINAM-prolinamides and benzoic acid in aqueous media. Tetrahedron Asymmetry 2006, 17, 1493–1497, (Corrigendum: Tetrahedron Asymmetry 2007, 18, 1031). [Google Scholar] [CrossRef]
- Guizzetti, S.; Benaglia, M.; Pignataro, L.; Puglisi, A. A multifunctional proline-based organic catalyst for enantioselective aldol reactions. Tetrahedron Asymmetry 2006, 17, 2754–2760. [Google Scholar] [CrossRef]
- Ma, G.N.; Zhang, Y.P.; Shi, M. l-Proline diamides with an axially chiral binaphthylene backbone as efficient- organocatalysts for direct asymmetric aldol reactions: The effect of acetic acid. Synthesis 2007, 197–208. [Google Scholar] [CrossRef]
- Guizzetti, S.; Benaglia, M.; Raimondi, L.; Celentano, G. Enantioselective direct aldol reaction “on water” promoted by chiral organic catalysts. Org. Lett. 2007, 9, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Guillena, G.; Hita, M.C.; Nájera, C. Organocatalyzed direct aldol condensation using l-proline and BINAM-prolinamides: Regio-, diastereo-, and enantioselective controlled synthesis of 1,2-diols. Tetrahedron Asymmetry 2006, 17, 1027–1031, (Corrigendum: Tetrahedron Asymmetry 2007, 18, 1030). [Google Scholar] [CrossRef]
- Guillena, G.; Hita, M.C.; Nájera, C. Highly selective direct aldol reaction organocatalyzed by (S)-BINAM-l-prolinamide and benzoic acid using α-chalcogen-substituted ketones as donors. ARKIVOC 2007, iv, 260–269, (Corrigendum: ARKIVOC 2007, i, 146–147). [Google Scholar] [CrossRef]
- Guillena, G.; Hita, M.C.; Nájera, C. α-Chloroacetone as a donor in the BINAM-l-prolinamide organocatalyzed aldol reaction: Application to the enantioselective synthesis of α,β-epoxy ketones. Tetrahedron Asymmetry 2007, 18, 1272–1277. [Google Scholar] [CrossRef]
- Kucherenko, A.S.; Syutkin, D.E.; Zlotinivat, S.G. Asymmetric aldol condensation in an ionic liquid-water system catalyzed by (S)-prolinamide der ives. Russ. Chem. Bull. 2008, 57, 591–594. [Google Scholar] [CrossRef]
- Moles, F.J.N.; Guillena, G.; Nájera, C. Aqueous organocatalyzed aldol reaction of glyoxylic acid for the enantioselective synthesis of α-hydroxy-γ-keto acids. RSC Adv. 2014, 4, 9963–9966. [Google Scholar] [CrossRef]
- Moles, F.J.N.; Bañón-Caballero, A.; Guillena, G.; Nájera, C. Enantioselective aldol reactions with aqueous 2,2-dimethoxyacetaldehyde organocatalyzed by binam-prolinamides under solvent-free conditions. Tetrahedron Asymmetry 2014, 25, 1323–1330. [Google Scholar] [CrossRef]
- Moles, F.J.N.; Guillena, G.; Nájera, C. Glyoxylic Acid versus Ethyl Glyoxylate for the Aqueous EnantioselectiveSynthesis of α-Hydroxy-β-Keto Acids and Esters by the N-Tosyl-(Sa)-binam-prolinamide-Organocatalyzed Aldol Reaction. Synthesis 2015. [Google Scholar] [CrossRef]
- Moles, F.J.N.; Guillena, G.; Nájera, C. Aqueous enantioselective aldol reaction of methyl- and phenylglyoxal organocatalyzed by N-Tosyl-(Sa)-binam-l-prolinamide. Synlett 2015, 26, 656–660. [Google Scholar] [CrossRef]
- Guillena, G.; Hita, M.C.; Nájera, C.; Viózquez, S.F. Solvent-free asymmetric direct aldol reactions organocatalysed by recoverable (Sa)-binam-l-prolinamide. Tetrahedron Asymmetry 2007, 18, 2300–2304. [Google Scholar] [CrossRef]
- Guillena, G.; Hita, M.C.; Nájera, C.; Viózquez, S.F. A highly efficient solvent-free asymmetric direct aldol reaction organocatalyzed by recoverable (S)-binam-l-prolinamides. ESI-MS evidence of the enamine-iminium formation. J. Org. Chem. 2008, 73, 5933–5943. [Google Scholar] [CrossRef] [PubMed]
- Bañón-Caballero, A.; Guillena, G.; Nájera, C. Solvent-Free Enantioselective Friedlander Condensation with Wet 1,1′-Binaphthalene-2,2′-diamine-Derived Prolinamides as Organocatalysts. J. Org. Chem. 2013, 78, 5349–5356. [Google Scholar] [CrossRef] [PubMed]
- Viózquez, S.F.; Bañón- Caballero, A.; Guillena, G.; Nájera, C; Gómez-Bengoa, E. Enantioselective direct aldol reaction of α-keto esters catalyzed by (Sa)-binam-d-prolinamide under quasi solvent-free conditions. Org. Biomol. Chem. 2012, 10, 4029–4035. [Google Scholar] [CrossRef] [PubMed]
- Guillena, G.; Nájera, C.; Viózquez, S.F. N-Tosyl-(Sa)-binam-l-prolinamide as highly efficient bifunctional organocatalyst for the general enantioselective solvent-free aldol reaction. Synlett 2008, 3031–3035. [Google Scholar] [CrossRef]
- Bradshaw, B.; Etxebarria-Jardí, G.; Bonjoch, J.; Viózquez, S.F.; Guillena, G.; Nájera, C. Efficient solvent-free Robinson annulation protocols for the highly enantioselective synthesis of the Wieland-Miescher ketone and analogues. Adv. Synth. Catal. 2009, 351, 2482–2490. [Google Scholar] [CrossRef]
- Viózquez, S.F.; Guillena, G.; Nájera, C.; Bradshaw, B.; Etxebarria-Jardí, G.; Bonjoch, J. (Sa,S)-N-[2′-(4-Methylphenylsulfonamido)-1,1′-binaphthyl-2-yl]pyrrolidine-2-carboxamide: An organocatalyst for the direct aldol reaction. Org. Synth. 2011, 88, 317–329. [Google Scholar]
- Bradshaw, B.; Etxebarria-Jardí, G.; Bonjoch, J.; Viózquez, S.F.; Guillena, G.; Nájera, C. Synthesis of (S)-8a-methyl-3,4,8,8a-tetrahydro-1,6-(2H,7H)-naphthalenedione via N-tosyl-(Sa)-binam-l-prolinamide organocatalysis. Org. Synth. 2011, 88, 330–341. [Google Scholar]
- Bradshaw, B.; Etxebarria-Jardí, G.; Bonjoch, J. Total synthesis of (−)-anominine. J. Am. Chem. Soc. 2010, 132, 5966–5967. [Google Scholar] [CrossRef] [PubMed]
- Bañón-Caballero, A.; Guillena, G.; Nájera, C. Solvent-free direct enantioselective aldol reaction using polystyrene-supported N-sulfonyl-(Ra)-binam-d-prolinamide as a catalyst. Green Chem. 2010, 12, 1599–1606. [Google Scholar] [CrossRef]
- Bañón-Caballero, A.; Guillena, G.; Nájera, C. Cross-linked-polymer-supported N-{2′-[(Arylsulfonyl)amino][1,1′-binaphthalen]-2-yl}prolinamide as organocatalyst for the direct aldol intermolecular reaction under solvent-free conditions. Helv. Chim. Acta 2012, 95, 1831–1841. [Google Scholar] [CrossRef]
- Uozumi, Y.; Sakurai, F. Asymmetric aldol reaction with BINAM-sulfonyl polymeric organocatalyst. Synfacts 2013, 9, 114. [Google Scholar] [CrossRef]
- Bañón-Caballero, A.; Guillena, G.; Nájera, C.; Faggi, E.; Sebastián, R.M.; Vallribera, A. Recoverable silica-gel supported binam-prolinamides as organocatalysts for the enantioselective solvent-free intra- and intermolecular aldol reaction. Tetrahedron 2013, 69, 1307–1315. [Google Scholar] [CrossRef]
- Uozumi, Y.; Sakurai, F. Silica-supported prolinamide for solvent-free asymmetric aldol reaction. Synfacts 2013, 9. [Google Scholar] [CrossRef]
- Li, L.; Gou, S.; Liu, F. Highly stereoselective direct aldol reactions catalyzed by a bifunctional chiral diamine. Tetrahedron Asymmetry 2014, 25, 193–197. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
