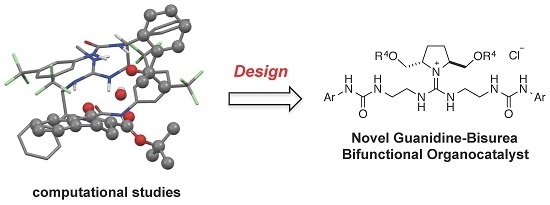
Development of Guanidine-Bisurea Bifunctional Organocatalysts with a Chiral Pyrrolidine Moiety and Application to α-Hydroxylation of Tetralone-Derived β-Keto Esters
Abstract
:1. Introduction
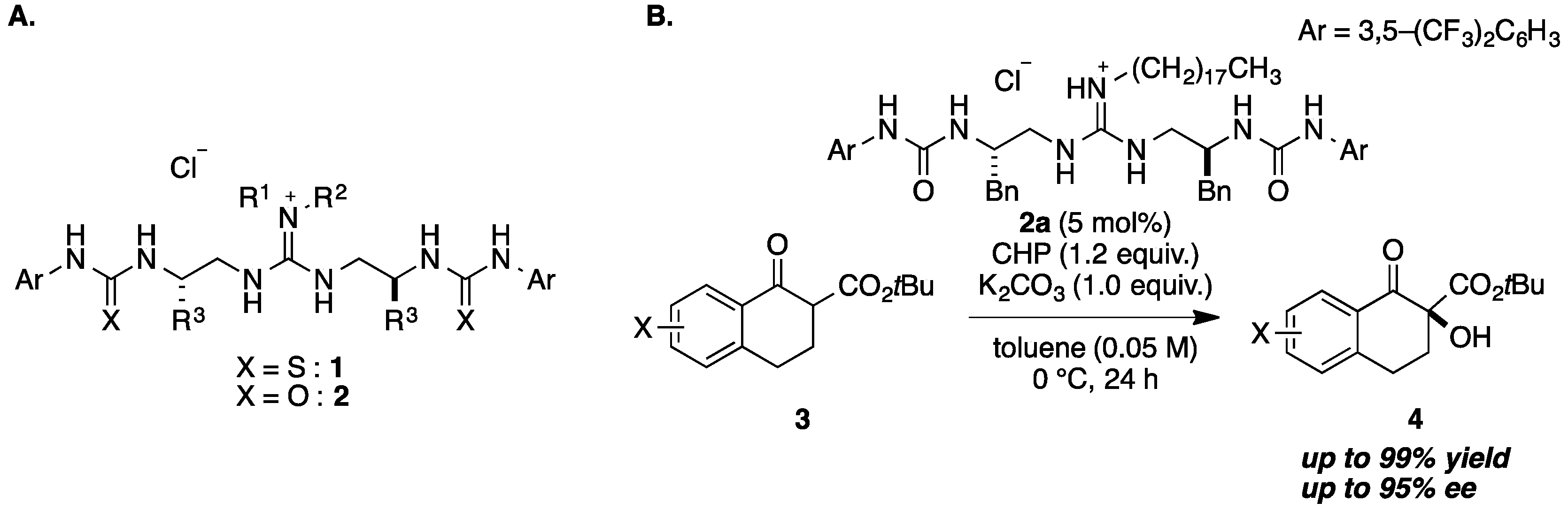
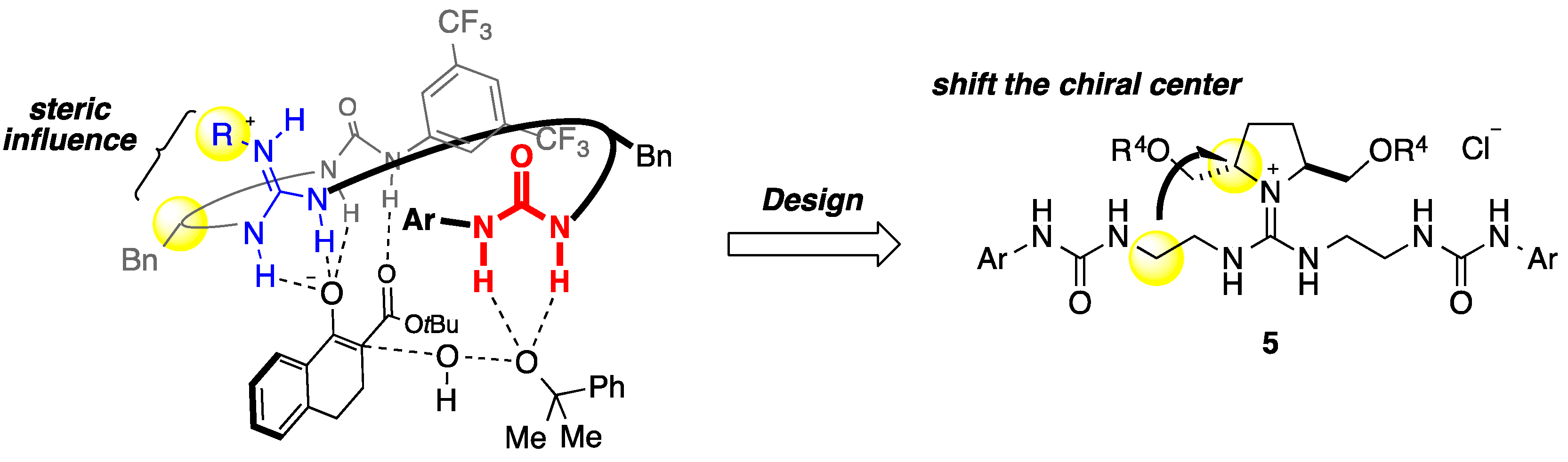
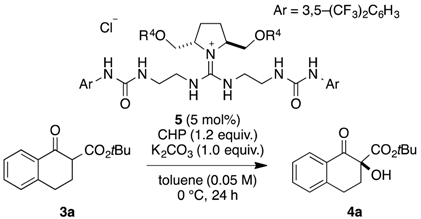
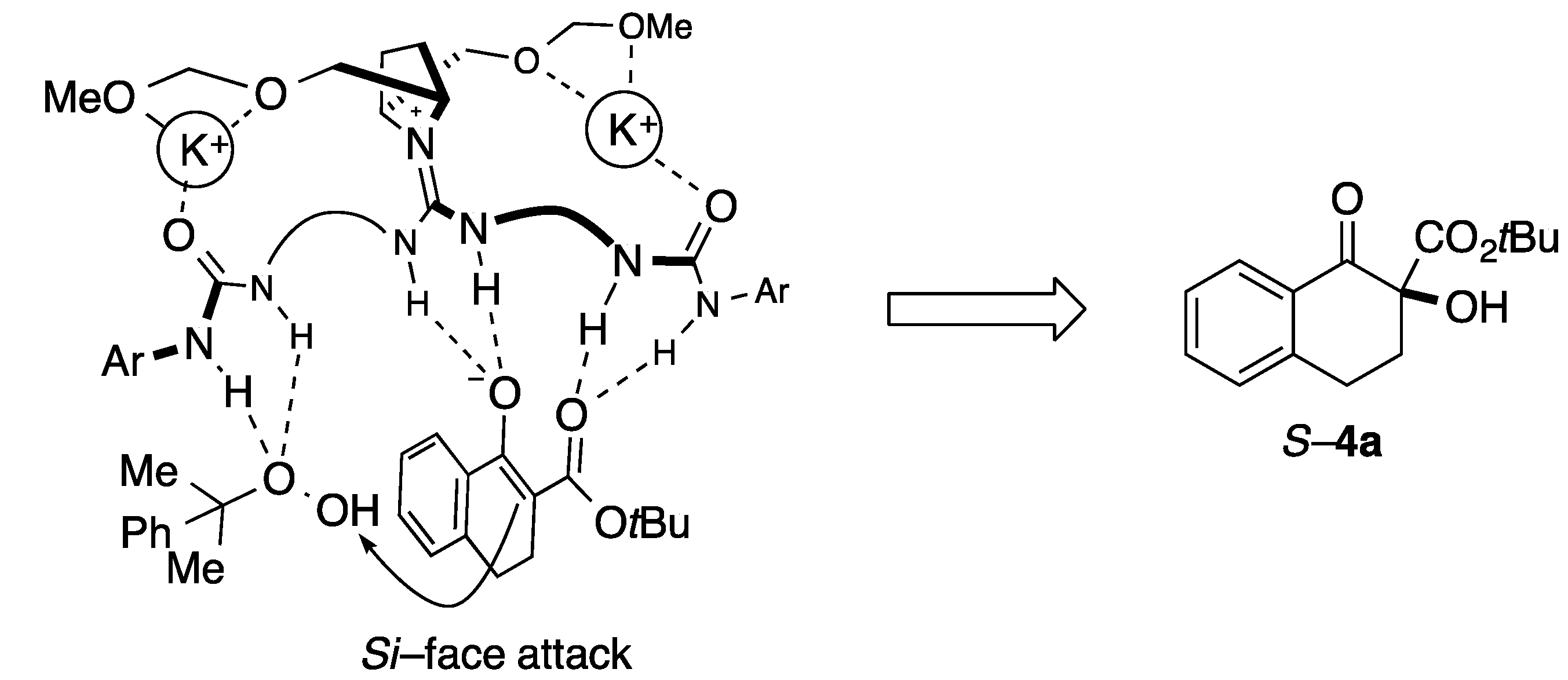
2. Results and Discussion


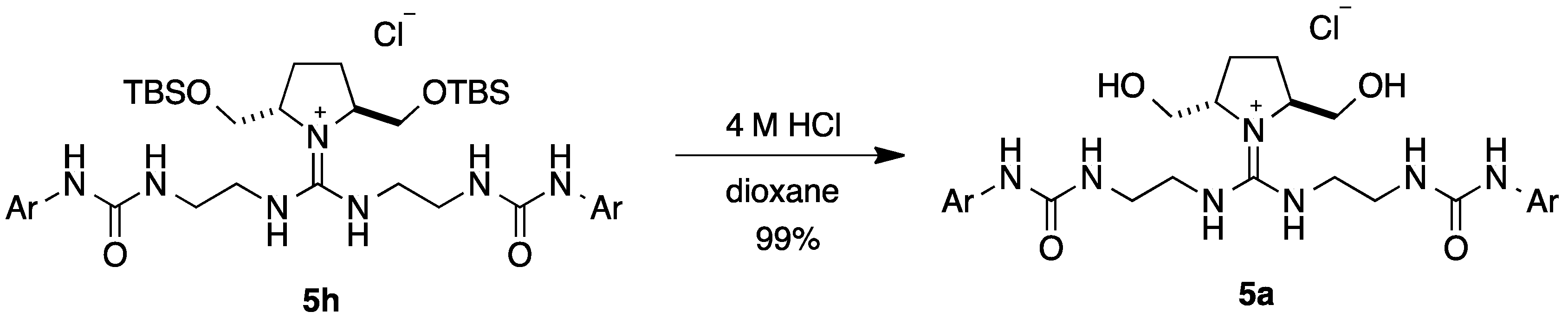
| Entry | 5 | 4a | ||
|---|---|---|---|---|
| R4 | Yield [%] b | ee [%] c | ||
| 1 | 5a | H | 5 | 30 |
| 2 | 5b | Me | 49 | 38 |
| 3 | 5c | n-Pr | 49 | 50 |
| 4 | 5d | n-Decyl | 47 | 47 |
| 5 | 5e | Bn | 36 | 30 |
| 6 | 5f | CH2(1-Naphtyl) | 58 | 40 |
| 7 | 5g | CH2(2-Naphtyl) | 55 | 32 |
| 8 | 5h | TBS | 55 | 49 |
| 9 | 5i | TIPS | 63 | 34 |
| 10 | 5j | MOM | 73 | 65 |
| 11 d | 5j | MOM | 73 | 48 |
3. Experimental Section
3.1. General Remarks
3.2. Typical Procedure for α-Hydroxylation of β-Keto Ester 3a Using 5
3.3. Characterizations of Novel Guanidine-Bisurea Bifunctional Organocatalysts 5a–j
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nagasawa, K.; Sohtome, Y. The Design of Chiral Double Hydrogen Bonding Networks and Their Applications to Catalytic Asymmetric Carbon-Carbon and Carbon-Oxygen Bond-Forming Reactions. Synlett 2009, 21, 1–22. [Google Scholar] [CrossRef]
- Sohtome, Y.; Nagasawa, K. Dynamic asymmetric organocatalysis: Cooperative effects of weak interactions and conformational flexibility in asymmetric organocatalysts. Chem. Commun. 2012, 48, 7777–7789. [Google Scholar] [CrossRef] [PubMed]
- Sohtome, Y.; Hashimoto, Y.; Nagasawa, K. Guanidine-Thiourea Bifunctional Organocatalyst for the Asymmetric Henry (Nitroaldol) Reaction. Adv. Synth. Catal. 2005, 347, 1643–1648. [Google Scholar] [CrossRef]
- Nagasawa, K.; Takada, K.; Tanaka, S. Asymmetric Mannich-Type Reaction of Aromatic α-Amido Sulfone with Malonate Using Guanidine-Thiourea Bifunctional Organocatalyst. Synlett 2009, 21, 1643–1646. [Google Scholar] [CrossRef]
- Sohtome, Y.; Tanaka, S.; Takada, K.; Yamaguchi, T.; Nagasawa, K. Solvent-dependent enantiodivergent Mannich-type reaction: Utilizing a conformationally flexible guanidine/bisthiourea organocatalyst. Angew. Chem. Int. Ed. 2010, 49, 9254–9257. [Google Scholar] [CrossRef] [PubMed]
- Sohtome, Y.; Horitsugi, N.; Takagi, R.; Nagasawa, K. Enantioselective Phospha-Michael Reaction of Diphenyl Phosphonate with Nitroolefins Utilizing Conformationally Flexible Guanidinium/Bisthiourea Organocatalyst: Assembly-State Tunability in Asymmetric Organocatalysis. Adv. Synth. Catal. 2011, 353, 2631–2636. [Google Scholar] [CrossRef]
- Horitsugi, N.; Kojima, K.; Yasui, K.; Sohtome, Y.; Nagasawa, K. Asymmetric Michael Reaction of Nitroolefins with β-Dicarbonyl Compounds Catalysed by 1,3-Diamine-Tethered Guanidine-Thiourea Bifunctional Organocatalysts. Asian J. Org. Chem. 2014, 3, 445–448. [Google Scholar] [CrossRef]
- Sohtome, Y.; Shin, B.; Horitsugi, N.; Takagi, R.; Noguchi, K.; Nagasawa, K. Entropy-controlled catalytic asymmetric 1,4-type Friedel-Crafts reaction of phenols using conformationally flexible guanidine/bisthiourea organocatalyst. Angew. Chem. Int. Ed. 2010, 49, 7299–7303. [Google Scholar] [CrossRef] [PubMed]
- Sohtome, Y.; Shin, B.; Horitsugi, N.; Noguchi, K.; Nagasawa, K. Linking conformational flexibility and kinetics: Catalytic 1,4-type Friedel-Crafts reactions of phenols utilizing 1,3-diamine-tethered guanidine/bisthiourea organocatalysts. Chem. Asian J. 2011, 6, 2463–2470. [Google Scholar] [CrossRef] [PubMed]
- Odagi, M.; Furukori, K.; Watanabe, T.; Nagasawa, K. Asymmetric α-hydroxylation of tetralone-derived β-ketoesters by using a guanidine-urea bifunctional organocatalyst in the presence of cumene hydroperoxide. Chem. Eur. J. 2013, 19, 16740–16745. [Google Scholar] [CrossRef] [PubMed]
- Odagi, M.; Furukori, K.; Yamamoto, Y.; Sato, M.; Iida, K.; Yamanaka, M.; Nagasawa, K. Origin of stereocontrol in guanidine-bisurea bifunctional organocatalyst that promotes α-hydroxylation of tetralone-derived β-ketoesters: Asymmetric synthesis of β- and γ-substituted tetralone derivatives via organocatalytic oxidative kinetic resolution. J. Am. Chem. Soc. 2015, 137, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Mirabdolbaghi, R.; Hassan, M.; Dudding, T. Design and synthesis of a chiral seven-membered ring guanidine organocatalyst applied to asymmetric vinylogous aldol reactions. Tetrahedron Asymmetry 2015, 26, 560–566. [Google Scholar] [CrossRef]
- Lian, M.; Li, Z.; Du, J.; Meng, Q.; Gao, Z. Asymmetric Direct α-Hydroxylation of β-Oxo Esters by Phase-Transfer Catalysis Using Chiral Quaternary Ammonium Salts. Eur. J. Org. Chem. 2010, 2010, 6525–6530. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Wang, Z.; Zhang, R.; Zhang, X.; Ding, K. Spiro-2,2ʹ-bichroman-based bisoxazoline (SPANbox) ligands for ZnII-catalyzed enantioselective hydroxylation of β-keto esters and 1,3-diester. Chem. Sci. 2011, 2, 1141–1144. [Google Scholar] [CrossRef]
- Yao, H.; Lian, M.; Li, Z.; Wang, Y.; Meng, Q. Asymmetric direct α-hydroxylation of β-oxo esters catalyzed by chiral quaternary ammonium salts derived from cinchona alkaloids. J. Org. Chem. 2012, 77, 9601–9608. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Cao, W.; Lin, L.; Liu, X.; Feng, X. N,Nʹ-Dioxide-Magnesium Ditriflate Complex-Catalyzed Asymmetric α-Hydroxylation of β-Keto Esters and β-Keto Amides. Adv. Synth. Catal. 2013, 355, 1924–1930. [Google Scholar] [CrossRef]
- Zou, L.; Wang, B.; Mu, H.; Zhang, H.; Song, Y.; Qu, J. Development of tartaric acid derived chiral guanidines and their application to catalytic enantioselective α-hydroxylation of β-dicarbonyl compounds. Org. Lett. 2013, 15, 3106–3109. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Hoshino, J.; Fujimoto, Y.; Ohtomo, J.; Sawada, S. A Convenient Synthesis of Enantiomeric Pairs of 2,5-Disubstituted Pyrrolidines of C2-Symmetry. Synthesis 1993, 3, 298–302. [Google Scholar] [CrossRef]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. [Google Scholar] [CrossRef]
- Yan, H.; Oh, J.S.; Lee, J.W.; Song, C.E. Scalable organocatalytic asymmetric Strecker reactions catalysed by a chiral cyanide generator. Nat. Commun. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odagi, M.; Takayama, K.; Sato, M.; Yamanaka, M.; Nagasawa, K. Development of Guanidine-Bisurea Bifunctional Organocatalysts with a Chiral Pyrrolidine Moiety and Application to α-Hydroxylation of Tetralone-Derived β-Keto Esters. Molecules 2015, 20, 12590-12598. https://doi.org/10.3390/molecules200712590
Odagi M, Takayama K, Sato M, Yamanaka M, Nagasawa K. Development of Guanidine-Bisurea Bifunctional Organocatalysts with a Chiral Pyrrolidine Moiety and Application to α-Hydroxylation of Tetralone-Derived β-Keto Esters. Molecules. 2015; 20(7):12590-12598. https://doi.org/10.3390/molecules200712590
Chicago/Turabian StyleOdagi, Minami, Kan Takayama, Makoto Sato, Masahiro Yamanaka, and Kazuo Nagasawa. 2015. "Development of Guanidine-Bisurea Bifunctional Organocatalysts with a Chiral Pyrrolidine Moiety and Application to α-Hydroxylation of Tetralone-Derived β-Keto Esters" Molecules 20, no. 7: 12590-12598. https://doi.org/10.3390/molecules200712590
APA StyleOdagi, M., Takayama, K., Sato, M., Yamanaka, M., & Nagasawa, K. (2015). Development of Guanidine-Bisurea Bifunctional Organocatalysts with a Chiral Pyrrolidine Moiety and Application to α-Hydroxylation of Tetralone-Derived β-Keto Esters. Molecules, 20(7), 12590-12598. https://doi.org/10.3390/molecules200712590