Natural Plant Alkaloid (Emetine) Inhibits HIV-1 Replication by Interfering with Reverse Transcriptase Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
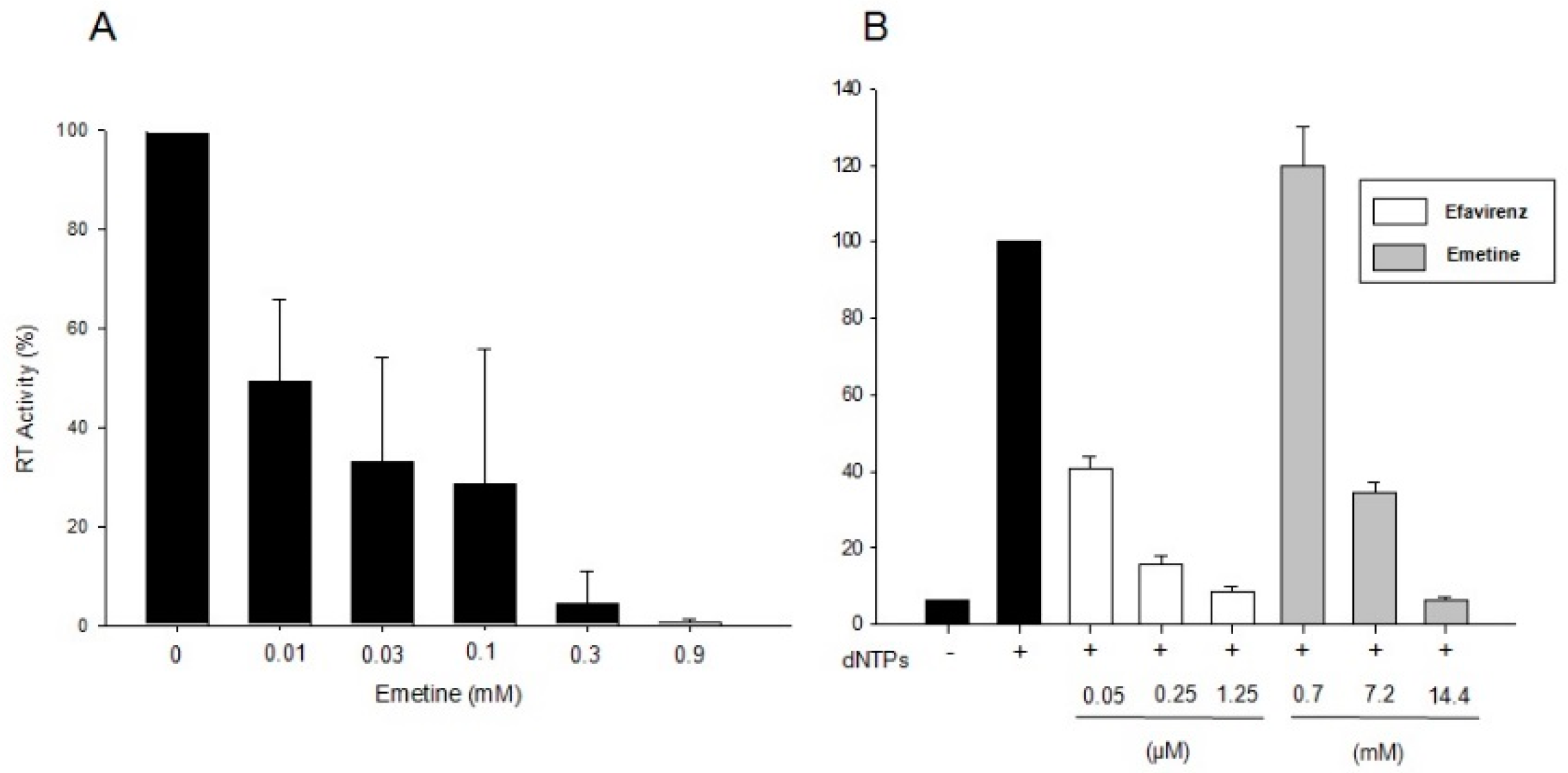
2.1.1. Emetine Impacts on RT Activity
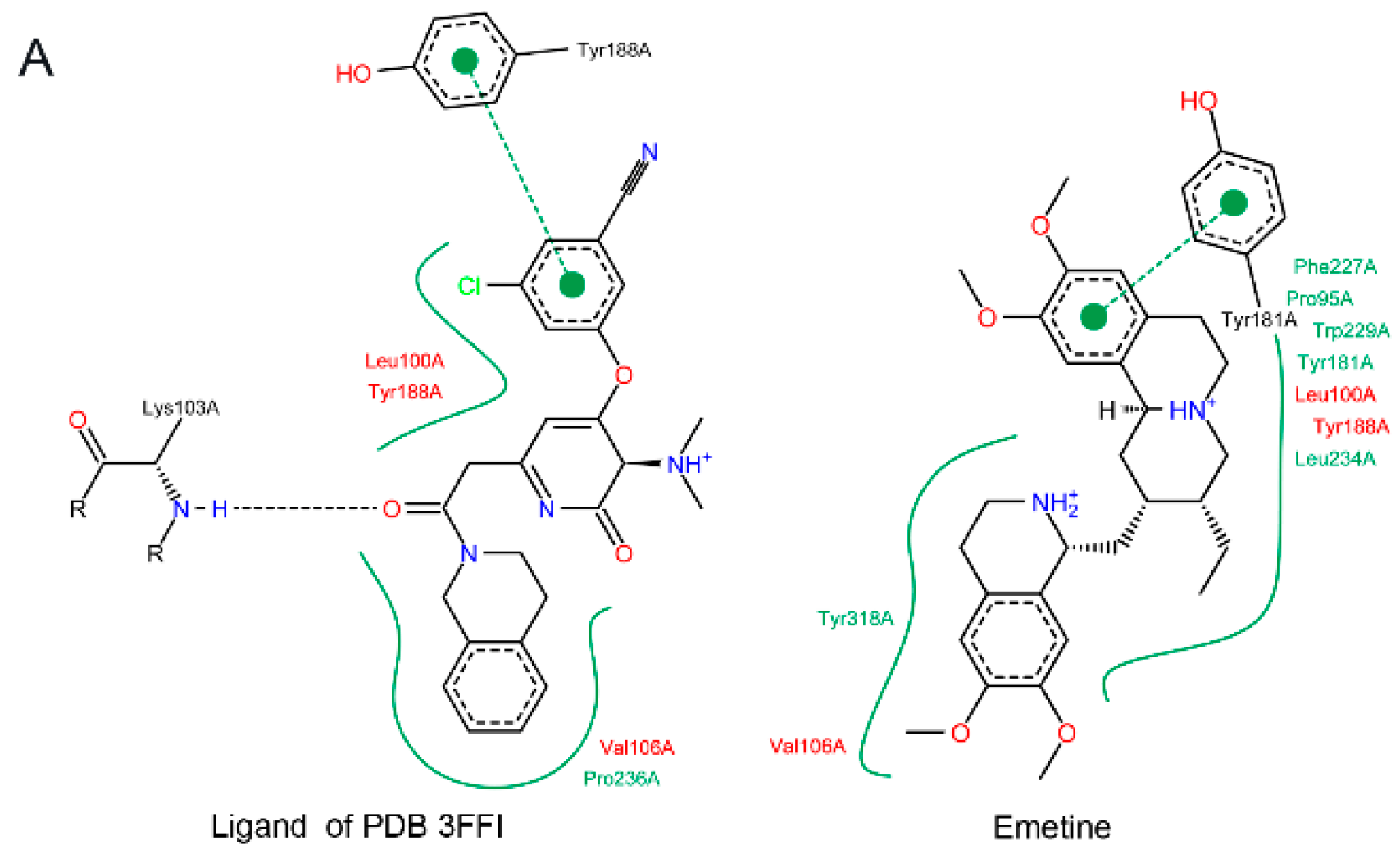
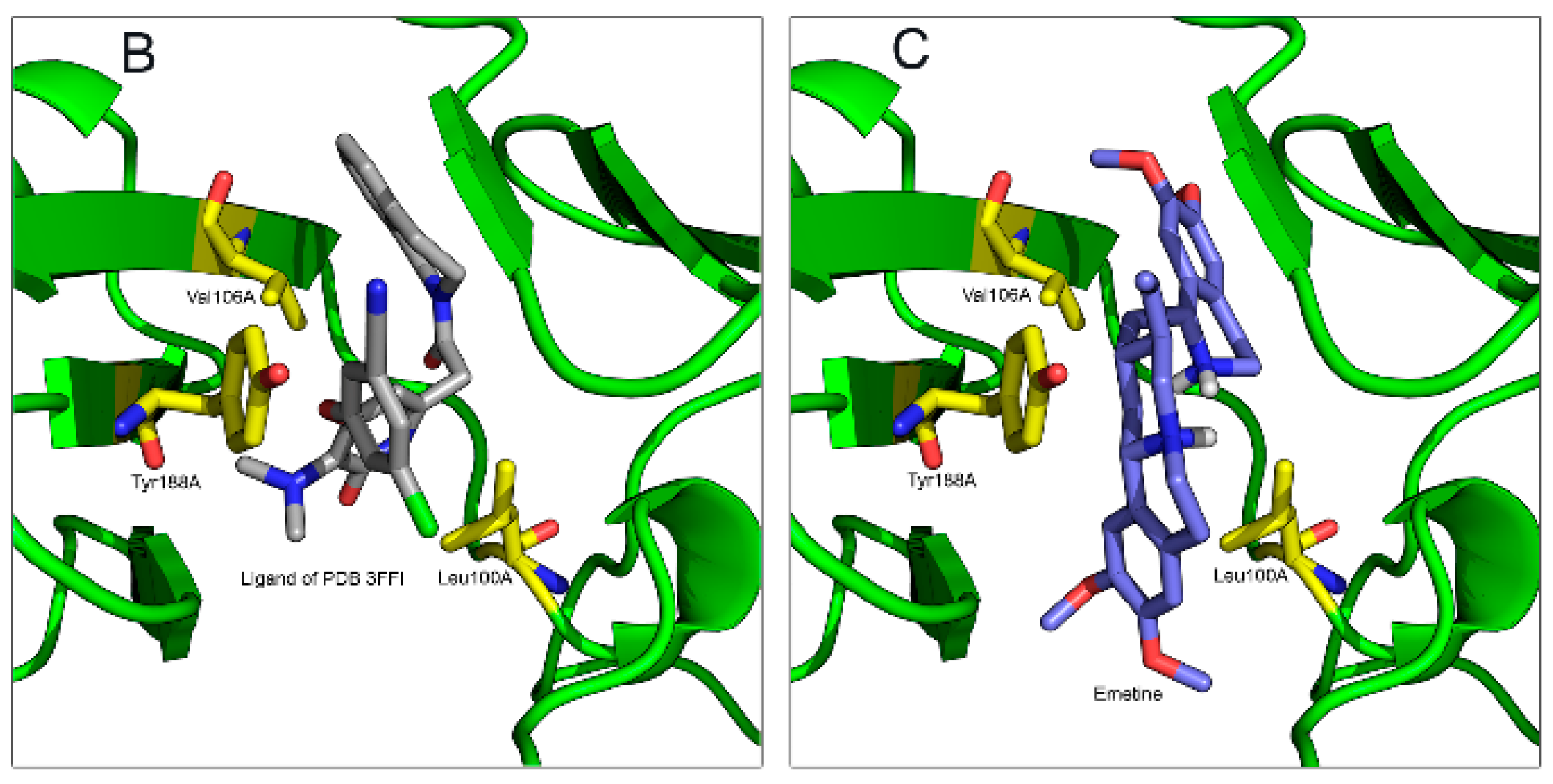
2.1.2. Emetine Complexation to RT
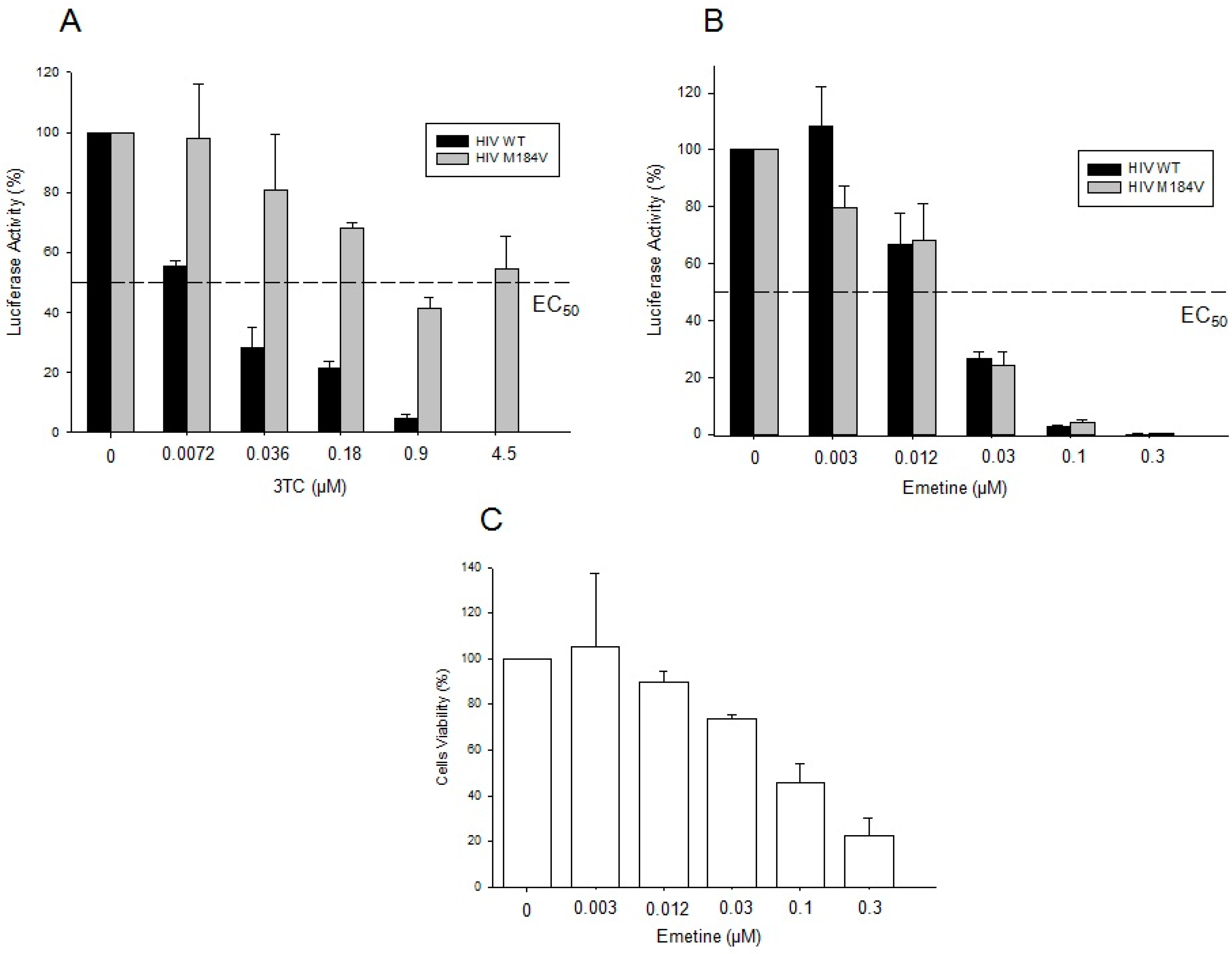
2.1.3. Emetine Blocks HIV Infectivity in GHOST Cells
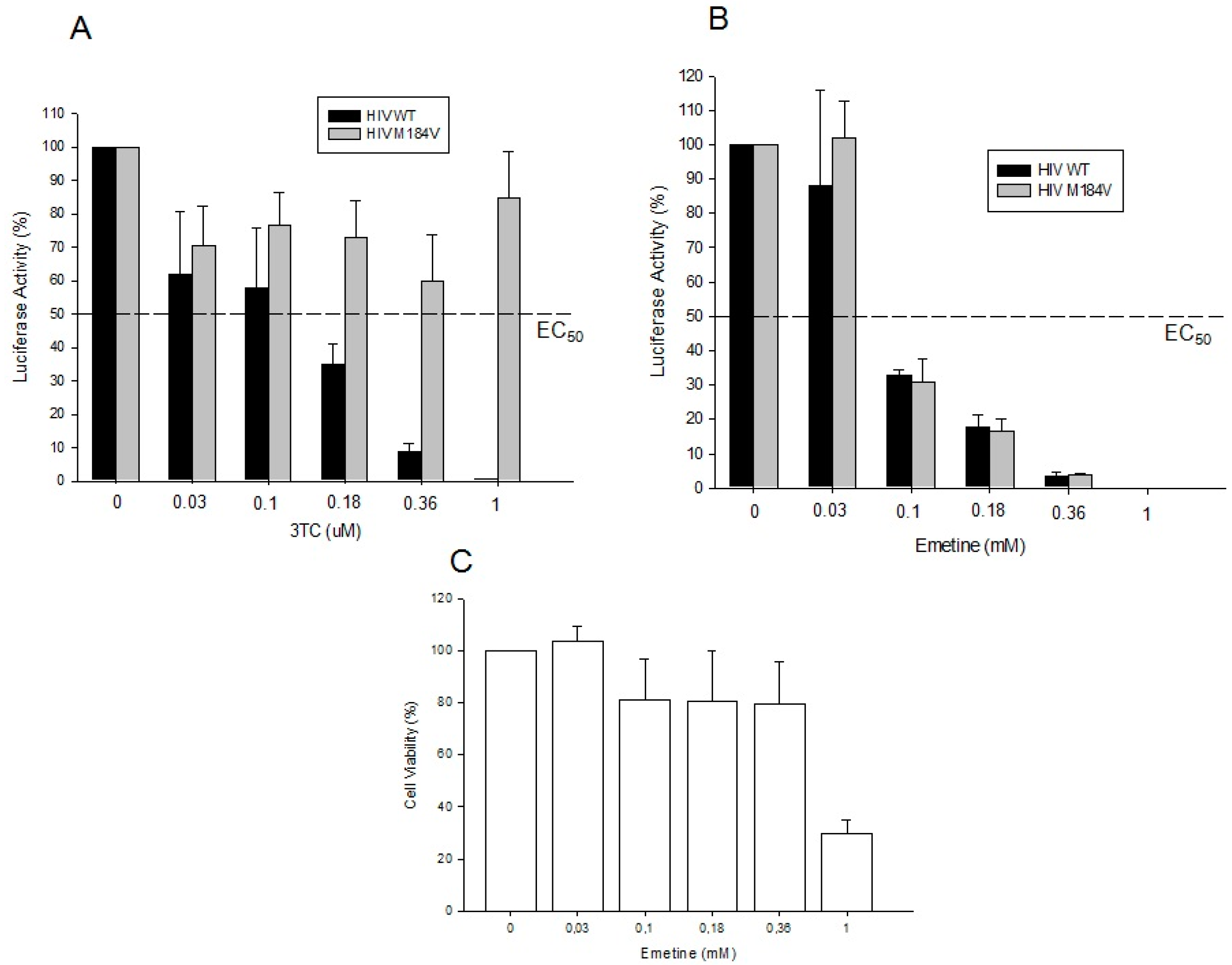
2.1.4. Emetine Inhibits HIV Infection in Primary Cells
2.2. Discussion
3. Experimental Section
3.1. Cell Culture and Reagents
3.2. Plasmids
3.3. Viral Production
3.4. Infectivity and Phenotypic Assay
3.5. Cell Viability
3.6. In Vitro HIV-1 RT Activity
3.7. Natural Endogenous Reverse Transcriptase Assay
3.8. Emetine Docking on HIV-1 RT
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The gap report 2014: People living with HIV. World Health Organization: Geneva, Switzerland, 2014.
- Berger, E.; Murphy, P.; Farber, J. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 1999, 17, 657–700. [Google Scholar] [CrossRef] [PubMed]
- Peterlin, B.; Trono, D. Hide, shield and strike back: How HIV-infected cells avoid immune eradication. Nat. Rev. Immunol. 2003, 3, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Götte, M.; Li, X.; Wainberg, M. HIV-1 reverse transcription: A brief overview focused on structure-function relationships among molecules involved in initiation of the reaction. Arch Biochem. Biophys. 1999, 365, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo Veronese, F.; Copeland, T.D.; DeVico, A.L.; Rahman, R.; Oroszlan, S.; Gallo, R.C.; Sarngadharan, M.G. Characterization of highly immunogenic p66/p51 as the reverse transcriptase of HTLV-III/LAV. Science 1986, 231, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Le Grice, S.F.; Naas, T.; Wohlgensinger, B.; Schatz, O. Subunit-selective mutagenesis indicates minimal polymerase activity in heterodimer-associated p51 HIV-1 reverse transcriptase. EMBO J. 1991, 10, 3905–3911. [Google Scholar] [PubMed]
- Hansen, J.; Schulze, T.; Mellert, W.; Moelling, K. Identification and characterization of HIV-specific RNase H by monoclonal antibody. EMBO J. 1988, 7, 239–243. [Google Scholar] [PubMed]
- Preston, B.; Poiesz, B.; Loeb, L. Fidelity of HIV-1 reverse transcriptase. Science 1988, 242, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Perelson, A.S.; Neumann, A.U.; Markowitz, M.; Leonard, J.M.; Ho, D.D. HIV-1 dynamics in vivo: Virion clearance rate, infected cell life-span, and viral generation time. Science 1996, 271, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E. Rapid evolution of viral RNA genomes. J. Nutr. 1997, 127, 958S–961S. [Google Scholar] [PubMed]
- Monini, P.; Sgadari, C.; Toschi, E.; Barillari, G.; Ensoli, B. Antitumour effects of antiretroviral therapy. Nat. Rev. Cancer 2004, 4, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Palella, F.J., Jr.; Delaney, K.M.; Moorman, A.C.; Loveless, M.O.; Fuhrer, J.; Satten, G.A.; Aschman, D.J.; Holmberg, S.D. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 1998, 338, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Ilina, T.; Parniak, M.A. Inhibitors of HIV-1 reverse transcriptase. Adv. Pharmacol. 2008, 56, 121–167. [Google Scholar] [PubMed]
- Sarafianos, S.; Marchand, B.; Das, K.; Himmel, D.; Parniak, M.; Hughes, S.; Arnold, E. Structure and function of HIV-1 reverse transcriptase: Molecular mechanisms of polymerization and inhibition. J. Mol. Biol. 2009, 385, 693–713. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.A.; Calvez, V.; Günthard, H.F.; Paredes, R.; Pillay, D.; Shafer, R.; Wensing, A.M.; Richman, D. 2011 update of the drug resistance mutations in HIV-1. Top. Antivir. Med. 2011, 19, 156–164. [Google Scholar] [PubMed]
- Esteves, A.; Nicolai, M.; Humanes, M.; Goncalves, J. Sulfated polysaccharides in marine sponges: Extraction methods and anti-HIV activity. Mar. Drugs 2011, 9, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Erik, D.C. Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med. Res. Rev. 2000, 20, 323–349. [Google Scholar]
- Nomura, T.; Kutchan, T.M. Is a metabolic enzyme complex involved in the efficient and accurate control of Ipecac alkaloid biosynthesis in Psychotria ipecacuanha? Plant Signal. Behav. 2010, 5, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Wiegrebe, W.; Kramer, W.J.; Shamma, M. The emetine alkaloids. J. Nat. Prod. 1984, 47, 397–408. [Google Scholar] [CrossRef]
- American Academy of Clinical Toxicology. Position paper: Ipecac syrup. J. Toxicol. Clin. Toxicol. 2004, 42, 133–143. [Google Scholar]
- Tan, G.T.; Kinghorn, A.D.; Hughes, S.H.; Pezzuto, J.M. Psychotrine and its O-methyl ether are selective inhibitors of human immunodeficiency virus-1 reverse transcriptase. J. Biol. Chem. 1991, 266, 23529–23536. [Google Scholar] [PubMed]
- Janot, M. The ipecac alkaloids. In The Alkaloids; Manske, R.H.F., Holmes, H.L., Eds.; Academic Press: San Diego, CA, USA, 1953; Volume 3, pp. 363–394. [Google Scholar]
- Panel, O.T.C. Vaginal contraceptive drug products for over-the-counter human use. Fed. Regist. 1980, 45, 82014–82019. [Google Scholar]
- Mera y Sierra, R.; Agramunt, V.H.; Cuervo, P.; Mas-Coma, S. Human fascioliasis in Argentina: Retrospective overview, critical analysis and baseline for future research. Parasit. Vectors 2011, 4. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.E.; Lövborg, H.; Rickardson, L.; Larsson, R.; Oberg, K.; Granberg, D. Identification and evaluation of potential anti-cancer drugs on human neuroendocrine tumor cell lines. Anticancer Res. 2006, 26, 4125–4129. [Google Scholar] [PubMed]
- Larsson, D.E.; Hassan, S.; Larsson, R.; Oberg, K.; Granberg, D. Combination analyses of anti-cancer drugs on human neuroendocrine tumor cell lines. Cancer Chemoth. Pharm. 2009, 65, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Herzer, K.; Wenger, T.; Herr, I.; Wink, M. The alkaloid emetine as a promising agent for the induction and enhancement of drug-induced apoptosis in leukemia cells. Oncol. Rep. 2007, 18, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P.K.; Kitchlu, S.; Dwivedi, A.; Agnihotri, P.K.; Srivastava, S.; Roy, R.; Bhaduri, A.P. Emetine ditartrate: A possible lead for emergency contraception. Contraception 2004, 69, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, R.S.; Costa, L.J.; Pereira, H.S.; Brindeiro, R.M.; Tanuri, A. Development of a new methodology for screening of human immunodeficiency virus type 1 microbicides based on real-time PCR quantification. Antimicrob. Agents Chemother. 2007, 51, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Dornadula, G.; Zhang, H.; Bagasra, O.; Pomerantz, R.J. Natural endogenous reverse transcription of simian immunodeficiency virus. Virology 1997, 227, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dornadula, G.; Alur, P.; Laughlin, M.A.; Pomerantz, R.J. Amphipathic domains in the C terminus of the transmembrane protein (gp41) permeabilize HIV-1 virions: a molecular mechanism underlying natural endogenous reverse transcription. Proc. Natl. Acad. Sci. 1996, 93, 12519–12524. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Tambwekar, K.R.; Vermani, K.; Kandarapu, R.; Garg, A.; Waller, D.P.; Zaneveld, L.J. Development pharmaceutics of microbicide formulations. Part II: formulation, evaluation, and challenges. AIDS Patient Care ST. 2003, 17, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Karim, Q.A.; Abdool Karim, S.S.; Frohlich, J.A.; Grobler, A.C.; Baxter, C.; Mansoor, L.E.; Kharsany, A.B.M.; Sibeko, S.; Mlisana, K.P.; Omar, Z.; et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010, 329, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Bunge, K.; Macio, I.; Meyn, L.; Noguchi, L.; Parniak, M.A.; Schwartz, J.L.; Moncla, B.; Hillier, S. The safety, persistence, and acceptability of an antiretroviral microbicide candidate UC781. J. Acquir. Immune Defic. Syndr. 2012, 60, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Parniak, M.A. Nonnuccleoside reverse transcriptase inhibitors as anti-HIV-1 microbicides. AIDS 2001, 15, S56. [Google Scholar] [CrossRef]
- Stone, A. Microbicides: A new approach to preventing HIV and other sexually transmitted infections. Nat. Rev. Drug Discovery 2002, 1, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Maass, G.; Immendoerfer, U.; Koenig, B.; Leser, U.; Mueller, B.; Goody, R.; Pfaff, E. Viral resistance to the thiazolo-iso-indolinones, a new class of nonnucleoside inhibitors of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 1993, 37, 2612–2617. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.F.; Schinazi, R.F. Combinations of 3'-azido-3'-deoxythymidine (zidovudine) and phosphonoformate (foscarnet) against human immunodeficiency virus type 1 and cytomegalovirus replication in vitro. Antimicrob Agents Chemother. 1989, 33, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.J. Therapy of amebiasis. B. New York Acad. Med. 1971, 47, 469–477. [Google Scholar]
- Deng, L.; Dai, P.; Ciro, A.; Smee, D.F.; Djaballah, H.; Shuman, S. Identification of novel antipoxviral agents: mitoxantrone inhibits vaccinia virus replication by blocking virion assembly. J. Virol. 2007, 81, 13392–13402. [Google Scholar] [CrossRef] [PubMed]
- Low, J.S.Y.; Chen, K.C.; Wu, K.X.; Ng, M.M.-L.; Chu, J.J.H. Antiviral activity of emetine dihydrochloride against dengue virus infection. J Antivir Antiretrovir. 2009, 1, 62–71. [Google Scholar]
- Boucher, C.A.; Cammack, N.; Schipper, P.; Schuurman, R.; Rouse, P.; Wainberg, M.A.; Cameron, J.M. High-level resistance to (−) enantiomeric 2′-deoxy-3′-thiacytidine in vitro is due to one amino acid substitution in the catalytic site of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 1993, 37, 2231–2234. [Google Scholar] [CrossRef] [PubMed]
- Harrigan, P. HIV drug resistance over the long haul. Clin. Infect. Dis. 2010, 50, 1286–1287. [Google Scholar] [CrossRef] [PubMed]
- HIV Drug Resistance Report 2012. World Health Organization: Geneva, Switzerland, 2012.
- Naswa, S.; Marfatia, Y.S. Pre-exposure prophylaxis of HIV. Indian J. Sex. Transm. Dis. 2011, 32, 1–8. [Google Scholar] [PubMed]
- Karim, Q.A.; Kharsany, A.B.M.; Naidoo, K.; Yende, N.; Gengiah, T.; Omar, Z.; Arulappan, N.; Mlisana, K.P.; Luthuli, L.R.; Abdool Karim, S.S. Co-enrollment in multiple HIV prevention trials—experiences from the CAPRISA 004 Tenofovir gel trial. Contemp. Clin. Trials. 2011, 32, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.M.; Lama, J.R.; Anderson, P.L.; McMahan, V.; Liu, A.Y.; Vargas, L.; Goicochea, P.; Casapia, M.; Guanira-Carranza, J.V.; Ramirez-Cardich, M.E.; et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010, 363, 2587–2599. [Google Scholar] [CrossRef] [PubMed]
- Karim, Q.A.; Kharsany, A.B.; Frohlich, J.A.; Baxter, C.; Yende, N.; Mansoor, L.E.; Mlisana, K.P.; Maarschalk, S.; Arulappan, N.; Grobler, A.; et al. Recruitment of high risk women for HIV prevention trials: baseline HIV prevalence and sexual behavior in the CAPRISA 004 tenofovir gel trial. Trials 2011, 12. [Google Scholar] [CrossRef] [PubMed]
- Thigpen, M.C.; Kebaabetswe, P.M.; Paxton, L.A.; Smith, D.K.; Rose, C.E.; Segolodi, T.M.; Henderson, F.L.; Pathak, S.R.; Soud, F.A.; Chillag, K.L.; et al. TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N. Engl. J. Med. 2012, 367, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Baeten, J.M.; Donnell, D.; Ndase, P.; Mugo, N.R.; Campbell, J.D.; Wangisi, J.; Tappero, J.W.; Bukusi, E.A.; Cohen, C.R.; Katabira, E.; et al. Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 2012, 367, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Celum, C.L. HIV preexposure prophylaxis: New data and potential use. Top. Antivir. Med. 2011, 19, 181–185. [Google Scholar] [PubMed]
- Tisdale, M.; Kemp, S.D.; Parry, N.R.; Larder, B.A. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc. Natl. Acad. Sci. 1993, 90, 5653–5656. [Google Scholar] [CrossRef] [PubMed]
- Beller, B.M. Observations on the mechanism of emetine poisoning of myocardial tissue. Circ. Res. 1968, 22, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Morner, A.; Bjorndal, A.; Albert, J.; Kewalramani, V.N.; Littman, D.R.; Inoue, R.; Thorstensson, R.; Fenyo, E.M.; Bjorling, E. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J. Virol. 1999, 73, 2343–2349. [Google Scholar] [PubMed]
- Aguiar, R.S.; Lovsin, N.; Tanuri, A.; Peterlin, B.M. Vpr.A3A chimera inhibits HIV replication. J. Biol. Chem. 2008, 283, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- O'Doherty, U.; Swiggard, W.J.; Malim, M.H. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 2000, 74, 10074–10080. [Google Scholar] [CrossRef] [PubMed]
- Lengruber, R.B.; Delviks-Frankenberry, K.A.; Nikolenko, G.N.; Baumann, J.; Santos, A.F.; Pathak, V.K.; Soares, M.A. Phenotypic characterization of drug resistance-associated mutations in HIV-1 RT connection and RNase H domains and their correlation with thymidine analogue mutations. J. Antimicrob. Chemother. 2011, 66, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Schaftenaar, G.; Noordik, J.H. Molden: A pre- and post-processing program for molecular and electronic structures. J. Comput.-Aided Mol. Des. 2000, 14, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.J.; et al. General atomic and molecular electronic structure system. J. Comp. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comp. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Goodsell, D.; Halliday, R.; Huey, R.; Hart, W.; Belew, R.; Olson, A. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comp. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Stierand, K.; Rarey, M. Drawing the PDB: Protein-ligand complexes in two dimensions. ACS Med. Chem. Lett. 2010, 1, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Delano, W.L. The PyMOL Molecular Graphics System. Available online: http://www.pymol.org. (accessed on 11 January 2013).
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valadão, A.L.C.; Abreu, C.M.; Dias, J.Z.; Arantes, P.; Verli, H.; Tanuri, A.; De Aguiar, R.S. Natural Plant Alkaloid (Emetine) Inhibits HIV-1 Replication by Interfering with Reverse Transcriptase Activity. Molecules 2015, 20, 11474-11489. https://doi.org/10.3390/molecules200611474
Valadão ALC, Abreu CM, Dias JZ, Arantes P, Verli H, Tanuri A, De Aguiar RS. Natural Plant Alkaloid (Emetine) Inhibits HIV-1 Replication by Interfering with Reverse Transcriptase Activity. Molecules. 2015; 20(6):11474-11489. https://doi.org/10.3390/molecules200611474
Chicago/Turabian StyleValadão, Ana Luiza Chaves, Celina Monteiro Abreu, Juliana Zanatta Dias, Pablo Arantes, Hugo Verli, Amilcar Tanuri, and Renato Santana De Aguiar. 2015. "Natural Plant Alkaloid (Emetine) Inhibits HIV-1 Replication by Interfering with Reverse Transcriptase Activity" Molecules 20, no. 6: 11474-11489. https://doi.org/10.3390/molecules200611474
APA StyleValadão, A. L. C., Abreu, C. M., Dias, J. Z., Arantes, P., Verli, H., Tanuri, A., & De Aguiar, R. S. (2015). Natural Plant Alkaloid (Emetine) Inhibits HIV-1 Replication by Interfering with Reverse Transcriptase Activity. Molecules, 20(6), 11474-11489. https://doi.org/10.3390/molecules200611474




