Abstract
The antioxidant features, polyphenolic composition and chromatographic fingerprints of the aerial parts from three Chilean endemic plants from the Paposo Valley located on the cost of the Atacama Desert were investigated for the first time using high pressure liquid chromatography coupled with photodiode array detector and electrospray ionization mass analysis (HPLC-PDA-ESI-MS) and spectroscopic methods. The phenolic fingerprints obtained for the plants were compared and correlated with the antioxidant capacities measured by the bleaching of the DPPH radical, the ferric reducing antioxidant power (FRAP) and quantification of the total content of phenolics and flavonoids measured by spectroscopic methods. Thirty phenolics were identified for the first time for these species, mostly phenolic acids, flavanones, flavonols and some of their glycoside derivatives, together with three saturated fatty acids (stearic, palmitic and arachidic acids). Nolana ramosissima showed the highest antioxidant activity (26.35 ± 1.02 μg/mL, 116.07 ± 3.42 μM Trolox equivalents/g dry weight and 81.23% ± 3.77% of inhibition in the DPPH, FRAP and scavenging activity (SA) assays, respectively), followed by N. aplocaryoides (85.19 ± 1.64 μg/mL, 65.87 ± 2.33 μM TE/g DW and 53.27% ± 3.07%) and N. leptophylla (124.71 ± 3.01, 44.23 ± 5.18 μM TE/g DW and 38.63% ± 1.85%).
Keywords:
Chilean plants; Atacama Desert; phenolics; antioxidant capacity; HPLC-MS; Paposo Valley; Nolana 1. Introduction
Polyphenols are one of the most important groups of compounds widely present in fruits and vegetables. High consumption of vegetables and fruits has been associated with a lowered incidence of degenerative, chronic and incurable diseases [1,2,3]. The protective effects of polyphenols against the induction of cellular and tissue impairment, mainly generated under oxidative stress conditions, can be attributed to direct scavenging activities against reactive species of oxygen (ROS), as well as to the induction of cell responses that activate the antioxidant effect at intracellular levels involved in cellular metabolism and cellular survival [1,4]. The analysis of polyphenol content in a complex organic matrix is a difficult task, and HPLC with photodiode array detectors is one of the most useful techniques [5,6,7,8]. Mass spectrometry with HPLC-PDA has undergone significant technological improvements in the last few years, especially with the development of interfaces, such as ElectroSpray (ESI) or Atmospheric Pressure Chemical Ionization (APCI) and several mass detectors, such as Time of Flight (TOF) OrbiTrap (OT) or Ion Trap (IT). Among all those techniques, several antioxidant phenolics in edible plants [9], fruits [5,7,10,11,12], nuts [13] and food byproducts [14] were analyzed using HPLC with Photodiode Array Detectors coupled to Ion Trap Mass analyzers thorough an ESI interface (HPLC-PDA-ESI-IT-MS). The genus Nolana comprises about 89 endemic species restricted to fog-dependent Lomas formations of coastal Peru and Chile [15]. So far, little is known about the chemistry of Nolana species. Nolana sedifolia was investigated for phenolic compounds and its activity against the fungus Botrytis cinerea [16]. However, mostly labdane diterpenoids were reported, to the best of our knowledge, from Nolana. From Nolana elegans, three new labdane diterpenoids were reported more than ten years ago [17], as well as two labdanes were reported from Nolana rostrata [18] and two more from N. filifolia [19]. Furthermore, from N. coelestis, four sesquiterpenoids were reported [20]. The aim of the present work is the chemical analysis of some important native Chilean Nolana plants (Figure 1) from a protected area of the Atacama Desert, in northern Chile (Paposo Valley), and the comparison of the antioxidant properties and total phenolics measured by spectroscopy. The polyphenolic fingerprints and polyphenolic content were compared and correlated with the antioxidant capacities measured by the bleaching of the DPPH radical, the ferric reducing antioxidant power (FRAP) and the superoxide anion scavenging activity assay (SA). The compounds in the plants were identified for the first time with the help of PDA analysis and mass spectrometry (HPLC-PDA-ESI-MS) plus a comparison with authentic standards.

Figure 1.
Pictures of herbarium specimens of (a) Nolana leptophylla, (b) N. aplocaryoides and (c) N. ramosissima collected in Paposo Valley, II region of Chile.
2. Results and Discussion
2.1. MS-PDA Identification of Polyphenolics in Three Nolana Species from Northern Chile
Several polyphenolics in Nolana species were detected and identified using HPLC with UV-visible data (PDA, Figure 2, Table 1) and ion trap electrospray mass spectrometry (IT-ESI-MS, Table 2). The 30 compounds identified in Nolana ramosissima, N. aplocaryoides and N. leptophylla were mainly flavones, flavanones, phenolic acids, fatty acids, some glycoside flavonoid conjugates and their derivatives. Twenty one compounds were detected in Nolana ramosissima, (Peaks 1, 2, 5, 6, 9–23 and 27–29), nine in N. leptophylla (Peaks 1, 6, 11, 16, 17, 20, 24, 25 and 26) and fourteen in N. aplocaryoides, (Peaks 3, 4, 6, 7, 8, 10–17, 20 and 21) (Figure 2, Table 2), respectively. Below is the detailed explanation and identification of the compounds using HPLC and PDA and MSn analysis plus co-elution with some of the authentic available standards. Figure 2 shows the HPLC fingerprints at 280 nm, Figure 3 the structures of the main compounds detected and Figure 4, Figure 5, Figure 6 and Figure 7 full MS and MSn spectra of several compounds detected.
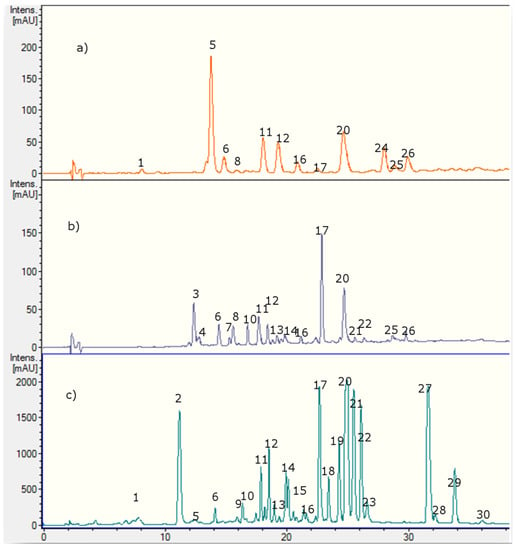
Figure 2.
HPLC-UV chromatograms at 280 nm of (a) Nolana leptophylla, (b) N. aplocaryoides and (c) N. ramosissima collected in Paposo Valley, II region of Chile.
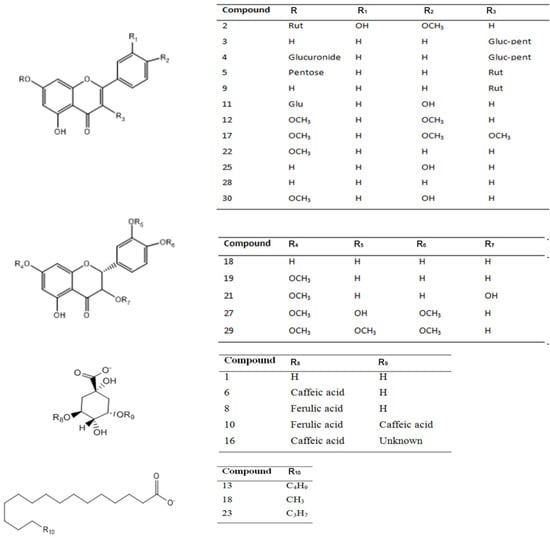
Figure 3.
Structures of the main compounds detected in Nolana leptophylla, N. aplocaryoides and N. ramosissima collected in Paposo Valley, II region of Chile.

Table 1.
HPLC-PDA-ESI-MSn data of Nolana leptophylla; N. aplocaryoides and N. ramosissima ethyl acetate extracts.
| Peak Number | Retention Time (min) | UV Max | M-Ion (ppm) | Other Ions (Aglycon Moiety) | Identification | Plant |
|---|---|---|---|---|---|---|
| 1 | 7.9 | 198 | 191 | 178, 173, 148, 110 | Quinic acid | Lepto, ramo |
| 2 | 11.3 | 350, 260 | 593 | 285 [M − H − rutinose]−, 253, 179, 151 | Luteolin-7-O-rutinose | ramo |
| 3 | 12.1 | 350, 254 | 595 | 463 (quercetin 3-O-glucoside), 301(quercetin-1H), 300 (quercetin-2H), 179, 151 | Quercetin-3-O-glucosyl-pentoside | aplo |
| 4 | 12.3 | 350, 254 | 771 | 595 (quercetin-3-O-hexosyl-hexoside), 463 (quercetin 3-O-glucoside), 301 (quercetin), 179, 151 | Quercetin-3-O-glucosyl-pentoside-7-O-glucuronide | aplo |
| 5 | 12.8 | 350, 254 | 741 | 609 ([M − H − xylose]−, 301 [M − H − rutinose − xylose]−,179, 151 | Quercetin-7-O-xyloside-3-O-rutinoside | ramo |
| 6 | 14.0 | 310, 246 | 353 | 707 [2M − H]−, 191 (quinic acid) | Chlorogenic acid * | Lepto, ramo, aplo |
| 7 | 14.7 | 324, 275 | 325 | 651 [2M − H]−, 163 [M − H − glucose]−, 119[M − H − glucose − CO2]− | p-Coumaric acid glucoside | aplo |
| 8 | 15.3 | 310, 247 | 367 | 191 (quinic acid) | Feruloyl-quinic acid | aplo |
| 9 | 16.0 | 350, 254 | 609 | 1219 [2M − H]−, 301 [M − H − rutinose]−, 179, 151 | Rutin * | ramo |
| 10 | 16.3 | 310, 246, | 529 | 367 ([M − H − caffeic acid moiety]− | Feruloyl-caffeoyl-quinic acid | Aplo, ramo |
| 11 | 18.0 | 431 | 269 (apigenin) | 7-O-glucosyl-apigenin | Lepto, ramo, aplo | |
| 12 | 18.7 | 350, 260 | 313.3 | 298, 282 | 4′,7′ -dimethoxyluteolin | Lepto, ramo, aplo |
| 13 | 19.0 | 207 | 311 | 267 [M − H − CO2]−, 223 [M − H − CO2 − H2O]− | Arachidic acid * | ramo, aplo |
| 14 | 20.0 | 275 | 441 | 305, 175, 147 (cinnamic acid moiety) | Cinnamic acid derivative | aplo |
| 15 | 20.2 | 310, 240 | 515 | 353, 141 | Dicaffeoyl-quinic acid | ramo |
| 16 | 21.8 | 310, 246 | 451 | 353 [chlorogenic acid − H]−, 191 [quinic acid]− | Chlorogenic acid derivative | Lepto, ramo, aplo |
| 17 | 22.9 | 334, 275 | 327 | 312 [M − H − CH3]−,297 314 [M − H − 2CH3]− | 5-hydroxy-3,4′7 trimethoxy-flavone * | Lepto, ramo, aplo |
| 18 | 23.5 | 207 | 255 | 212 [M − H − CO2]−, 182 [M − H − CO2 − H2O]− | Palmitic acid * | ramo |
| 19 | 24.6 | 292, 330 sh | 255 | 213, 183, 172 | Pinocembrin * | Ramo |
| 20 | 25.2 | 291, 330 sh | 269, 271 | 255[M − CH3]−, 213 | Pinostrobin * | Lepto, ramo, aplo |
| 21 | 25.8 | 292, 330 sh | 285, 287 | 267[M − H2O]−, 251 [M − H2O − CH3]− | 3,5-dihydroxy-7-methoxy-flavanone | ramo, aplo |
| 22 | 26.0 | 334, 270 | 267 | 253 [M − H − CH3]−, 231, 179, 151 | chrysin-7-methyl ether | ramo |
| 23 | 26.9 | 207 | 283 | 239 [M − H − CO2]− | Stearic acid * | ramo |
| 24 | 28.1 | 275, 215 29.1 sh | 417 | 255 [M − H − glucose]− | Liquiritin | lepto |
| 25 | 29.1 | 334, 269 | 269 | 240, 182, 179, 151 | Apigenin * | lepto |
| 26 | 29.6 | 310, 28 sh | 151 | 136 [M − CH3]− | Vanillin * | lepto |
| 27 | 31.9 | 285 | 315 | 300 [M − 2H − CH3]−, 284 [M − 2H − 2CH3]− | Hesperetin 7-O-methyl ester | ramo |
| 28 | 32.1 | 334, 270 | 253 | 179, 151 | Chrysin | ramo |
| 29 | 33.9 | 292, 330 sh | 329 | 659 [2M − H]−, 314 [M − H − CH3]−, 299 [M − H − 2CH3]− | 5-hydroxy-3′4′7 trimethoxy-flavanone * | ramo |
| 30 | 36.0 | 334, 269 | 283, 285 | 268, 238 | Apigenin-7-O-methyl ether | ramo |
Abbreviations: lepto, Nolana leptophylla; aplo, N. aplocaryoides; ramo, N. ramosissima EtOAc; * identified by spiking experiments with authentic compounds.
2.2. Flavonoids and Derivatives
MS profiling of Peak 2 produced a parent ion at m/z 593 (Figure 4) and daughter ions at m/z 285 [M − H − rutinose]−, 257, 179 and 151, corresponding to luteolin aglycone and its fragment ions, revealing the compound to be luteolin-7-O-rutinoside [21]. Peak 3 (Figure 4) was identified as quercetin-3-O-glucosyl-pentoside (595, and MSn ions at m/z 463, 301, 179 and 151) [22]. Peak 4 with a [M − H]− ion at m/z 771, producing daughter ions at 595 (loss of 176 mass units, a glucuronide moiety), 463 (quercetin-3-O-glucoside) and 301 (quercetin, with MS3 179 151), was identified tentatively as quercetin-3-O-glucosyl-pentoside-7-O-glucuronide (Figure 4). Likewise, Peak 5 with a [M − H]− at m/z 741 and MSn ions at m/z 609 (rutin, by loss of xylose or the rutinose moiety, 132 u), 591 (rutin−H2O), 301 (quercetin), 179 and 151 was tentatively identified as a rutin pentoside (Figure 4) [23]. Peak 9 was identified as rutin ([M − H]− ion at m/z 609, MS at 301, 179 and 151) by co-elution with an authentic standard. Peak 10 (Figure 5) with a [M − H]− parent ion at m/z 529, which yielded a feruloylquinic acid ion at m/z 365, was identified as a feruloyl-caffeoylquinic acid derivative. Peak 11 (Figure 5) presented a pseudomolecular ion at m/z 431, which experienced a hexoside loss (162 u) to produce an apigenin ion at m/z 269 and, thus, was identified as 8-O-glucosyl-apigenin. This compound was identified with a spiking experiment with the authentic compound. Peak 12 showed UV maxima at 268 and 346 nm, characteristic for 3′,4′ dimethoxyflavones [10]. This peak showed precursor ions at m/z 315 [M + H]+ and at m/z 313 [M − H]−. The MS/MS spectrum in negative ionization mode showed product ions at m/z 298 and at m/z 283, formed after elimination of methyl groups and probably indicating a dimethoxyluteolin. These data led to identification of this peak as 4′,7′ -dimethoxyluteolin (4′,5-dihydroxy-3′,7-dimethoxyflavone) [11,24], which was also compared with an authentic compound. Peak 20 with a [M − H]− ion at m/z 269 and a daughter fragment at m/z 255 ([M − H − CH3]−) was identified using co-elution with an authentic standard of pinostrobin, which was isolated from this plant previously by us, and its structure was elucidated by single-crystal X-ray analysis [25]. In the same manner, Peak 19 was identified as the related compound pinocembrin (5,7-dihydroxy-flavanone). A related compound with different UV-VIS data (λ max 292, 330), Peak 21 (Figure 5), showed a [M − H]− ion at m/z 285 and MS1 ion at m/z 267 ([M − H − H2O]−) and 251 ([M − H − H2O − CH3]−). This compound was identified as 3,5-dihydroxy-7-methoxyflavanone by spiking experiments with the authentic compound. Peak 22 (Figure 6) with a pseudomolecular ion at m/z 267 and daughter fragment at m/z 253 ([M − H − CH3]−, chrysin) was identified as 5′hydroxy-7-methoxyflavone or chrysin-7-methyl ether. Peak 23 (Figure 6) with a pseudomolecular ion at m/z 283 and an MS1 ion at m/z 268 was identified as 4,5-dihydroxy-7-methoxyflavone or apigenin 7-methyl-ether standard. Peak 24 with [M − H]− at m/z 417 and an MS1 ion at m/z 255 (liquiritigenin) was identified as the glucosylated flavanone liquiritin [26]. Peak 25 (Figure 6) showed [M − H]− ions at m/z 269 and produced daughter ions at m/z 240, 182 and 151 (substituted Rings A and B) and was identified as apigenin. Peak 27 (Figure 5) showed a [M − H]− ion at m/z 315 and MSn ions at m/z 300 and 284 produced by the loss of two methyl groups, which pointed to the presence of a dimethoxylated flavanone and, thus, was tentatively identified as 3′,5-dihydroxy-4′,7-dimethoxyflavanone, the identity being confirmed with the compound previously isolated from the plant. Peak 28 showed a [M − H]− ion at m/z 253 and fragments characteristic of Rings A and B of dihydroxyflavones and was identified as chrysin. Likewise, Peak 29 showed a [2M − H]− adduct ion at m/z 659 and [M − H]− ion at m/z 329, which produced daughter ions at m/z 314 and m/z 299, which evidenced the loss of two methyl groups from the parent 329 ion and, consequently, was tentatively identified as 5-hydroxy-3′,4′,7-trimethoxy-flavanone, the identity being confirmed with the compound previously isolated from the plant. In the same manner, a related flavone (UV max 254, 354 nm) identified with Peak 17 showed a pseudomolecular ion at m/z 327, which produced daughter ions at m/z 312 and m/z 297, which evidenced the loss of two methyl groups from the parent ion and was identified as 5-hydroxy-3,4′,7-trimethoxyflavone. Peak 30 showed a pseudomolecular ion at m/z 283 producing a fragment at 268 (M-methyl group) and was tentatively identified as apigenin methyl ester [27].
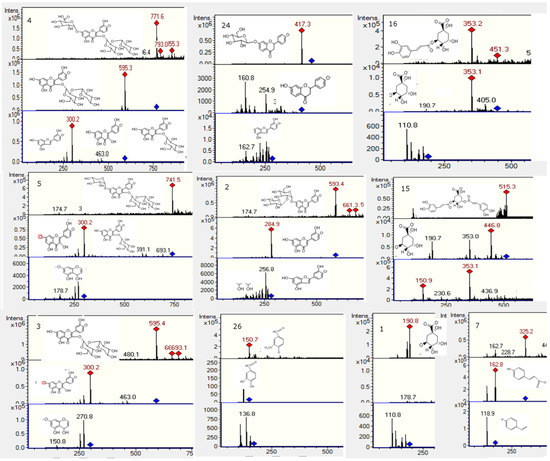
Figure 4.
Structures, full scan MS and MSn spectra of Peaks 1, 2, 3, 4, 5, 7, 15, 16, 24 and 26.
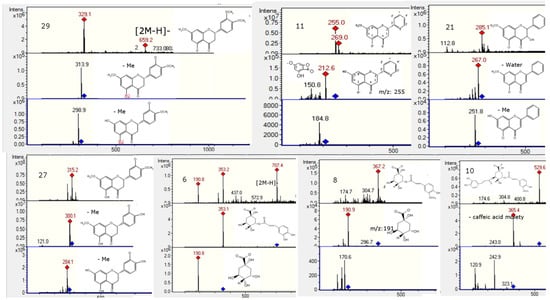
Figure 5.
Structures, full scan MS and MSn spectra of Peaks 6, 8, 10, 11, 21, 27 and 29.
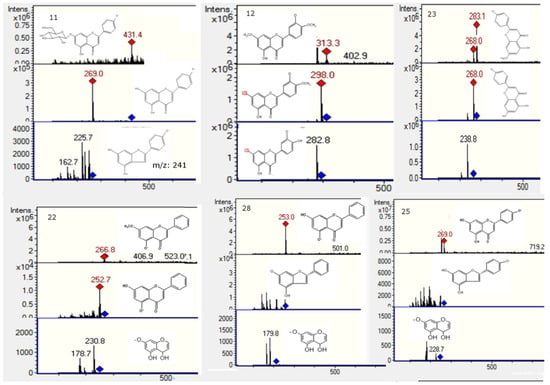
Figure 6.
Structures, full scan MS and MSn spectra of Peaks 11, 12, 22, 23, 25 and 28.
2.3. Fatty Acids
Peaks 13, 18 and 23 (Figure 7) were identified as saturated fatty acids. They showed [M − H]− ions at m/z 283, 255 and 311 [28] and neutral CO2 losses at m/z 238, 212 and 267, respectively, and were thus characterized as stearic, palmitic and arachidic acids. Further identification of the compounds was performed by TLC comparison with authentic samples (Silica gel F254 TLC plates, Merck, Darmstadt, Germany, with the solvent system n-hexane-ethyl acetate 7:3 v/v and developed with a solution of vanillin 1% in ethanol and 10% sulfuric acid and heating).
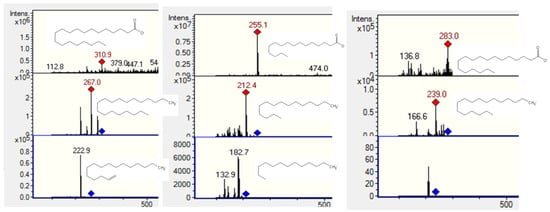
Figure 7.
Structures, full scan MS and MSn spectra of Peaks 13, 18 and 23.
2.4. Phenolic Acids and Related Compounds
Peak 1 with a [M − H]− ion at m/z 191 was identified as quinic acid (Figure 5). Peak 6 (Figure 5) was identified as one of the known chlorogenic acids, caffeoylquinic acid (molecular anion at m/z 353, MS2 at m/z 191 and 173 (191−H2O) by comparison with an authentic sample [29]). Peak 7 showed a [2M − H]− adduct ion at m/z 651 and [M − H]− ion at m/z 325, which produced daughter ions at m/z 163 (loss of glucose) and m/z 119 (decarboxylated coumaric acid) and, thus, was tentatively identified as coumaric acid glucoside [30,31]. Peak 8 (Figure 5) with a [M − H]− ion at m/z 367 yielding a quinic acid ion at m/z 191 by loss of a ferulic acid moiety was identified as feruloyl-quinic acid. Peak 15 (Figure 4) with a pseudomolecular ion at m/z 515 and MSn ions at m/z 353 (caffeoyl-quinic acid) and m/z 191 (quinic acid) was identified as dicaffeoylquinic acid [32]. Peak 26 with a pseudomolecular ion at m/z 151 was tentatively identified as vanillin. This compound was previously reported to occur in the related plant Nolana sedifolia [16].
2.5. Unknown Compounds
Peak 14 with a UV max corresponding to cinnamic acid (Table 1) with a parent ion at m/z 44,1 which yielded product ions at m/z 305, 175 and 147 (cinnamic acid), was identified as an unknown cinnamic acid derivative. Peak 16 with a [M − H]− ion at m/z 451 yielding an MS1 ion at m/z 353 (caffeoyl-quinic acid or chlorogenic acid) and MS2 ions at m/z 191 (quinic acid) and m/z 179 (caffeic acid) was identified as an unknown chlorogenic acid derivative.
2.6. Total Phenolics and Flavonoids Contents
The total phenolic content (TPC; Figure 8a) varied from 9.66 ± 0.34 for N. leptophylla to 70.50 ± 0.80 mg Gallic acid (GA) equivalents/g DW for N. ramosissima and showed linear correlation with the antioxidant assays (R2 = 0.990 and R2 = 0.999 for the TPC/DPPH and TPC/FRAP assays, respectively); the TPC of our sample of N. ramosissima showed values 1.5-times higher than a blueberry extract, but was close to that reported for an extract of Chilean Calafate Berberis microphylla [29] and was three times the value reported for an extract of strawberry leaves [8]. The total flavonoid content (TFC; Figure 8b) showed a similar trend, varying from 6.82 ± 0.20 for N. leptophylla to 50.50 ± 0.66 mg quercetin/g DW for N. ramosissima. The TFC showed a linear correlation with the antioxidant assays (R2 = 0.995 for TFC/DPPH and R2 = 0.997 for the TFC/FRAP assays, respectively). The total flavonoid content for our sample of N. ramosissima was five-times more than the Chilean Adesmia emarginata [33], but half of that presented by the aerial parts of Buddleja globosa [33].
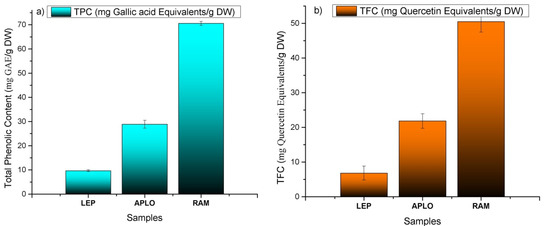
Figure 8.
(a) Total phenol content (TPC) and (b) total flavonoid content (TFC) of Nolana leptophylla (LEP), N. aplocaryoides (APLO) and N. ramosissima (RAM) collected in Paposo Valley, II region of Chile.
2.7. Antioxidant Features
The antioxidant capacity cannot be evaluated using a single test according to several authors [34]. Thus, three commonly-used assays, differing in their working principles (Figure 9), were employed in this work, using the DPPH method (Figure 9a), the ferric-reducing antioxidant power (FRAP; Figure 9b) and the superoxide anion scavenging activity assay (SA; Figure 9c). The DPPH assay is based on the hydrogen donating capacity to scavenge DPPH radicals, while the FRAP assay is an electron transfer-based test measuring the substance ability to reduce Fe3+ to Fe2+ [35]. The superoxide anion scavenging properties of a compound reduces the speed of the appearance of a formazan chromophore formed in a specific reaction using the xanthine-xanthine oxidase system [36]. In this work, the order of the antioxidant activity measured by the bleaching of the radical DPPH and the ferric reducing antioxidant power (FRAP) shown by the three Nolana plants was Nolana ramosissima > N. aplocaryoides > N. leptophylla. A similar trend was observed for superoxide anion scavenging activity. Indeed, N. ramosissima showed the highest antioxidant activity (26.35 ± 1.02 μg/mL, 116.07 ± 3.42 μM Trolox equivalents (TE)/g dry weight and 81.23% ± 3.77% of inhibition in the DPPH, FRAP and SA assays, respectively (Figure 9), followed by N. aplocaryoides (85.19 ± 1.64 μg/mL, 65.87 ± 2.33 μM TE/g DW and 53.27% ± 3.0%), and N. leptophylla (124.71 ± 3.01, 44.23 ± 5.18 μM TE/g DW and 38.63% ± 1.85%, Figure 9). The bleaching of the radical DPPH for N. ramosissima extract was close to that shown by the standard quercetin (28.53 ± 0.89 μg/mL, respectively). The antioxidant activities showed a positive correlation with polyphenolic content assays (0.990 ≥ R2 ≥ 0.999). Superoxide anion scavenging activity showed the best activity for N. ramosissima (81.23% ± 3.77% tested at 100 μg/mL), which was close to that presented by quercetin (83.77% ± 5.15% at 100 μg/mL). SA activity showed also good correlations with the DPPH (R2 0.990) and FRAP assays (R2 0.998).
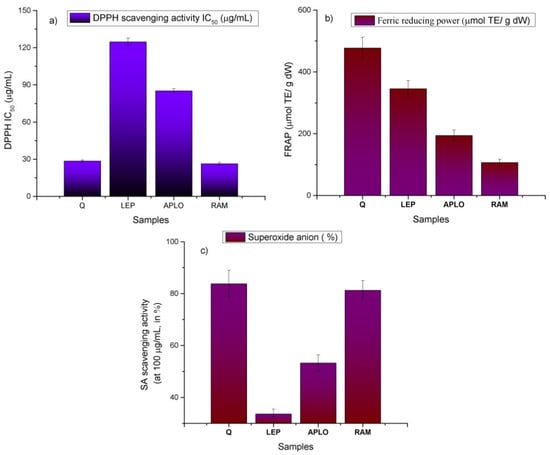
Figure 9.
(a) DPPH scavenging activity, (b) ferric reducing antioxidant power and (c) Superoxide anion scavenging activity of N. leptophylla (LEP), N. aplocaryoides (APLO) and N. ramosissima (RAM) from the II region of Chile.
3. Experimental Section
3.1. Chemicals and Plant Material
Folin-Ciocalteu phenol reagent (2 N), reagent grade Na2CO3, AlCl3,HCl, FeCl3,NaNO2, NaOH, quercetin, trichloroacetic acid, sodium acetate, HPLC-grade water, HPLC-grade acetonitrile, reagent grade MeOH and formic acid were obtained from Merck (Darmstadt, Germany); several fatty acids, quercetin, pinocembrin (all standards with purity higher than 95% by HPLC) were purchased either from ChromaDex (Santa Ana, CA, USA), Extrasynthèse (Genay, France) or Wuxi apptec Co. Ltd. (Shangai, China). Gallic acid, TPTZ (2, 4, 6-Tris(2-pyridyl)-s-triazine), Trolox, tert-butyl-hydroperoxide, nitro blue tetrazolium, xanthine oxidase and DPPH (1,1-diphenyl-2-picrylhydrazyl radical) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Nolana leptophylla (Miers) I. M. Johnst. sp. leptophylla, Nolana aplocaryoides (Gaudich.) I.M. Johnst and Nolana ramosissima I.M. Johnst were collected at Paposo Valley II region, northern Chile in April 2011. Voucher herbarium specimens were deposited at the Laboratorio de Productos Naturales, Universidad de Antofagasta, Antofagasta, Chile, with the numbers Nl-111004-1, Na-111004-1, Nr-111004-1, respectively. Sampling was performed using sterile disposable gloves and rigid plastic sample containers, and each sample was submitted individually by overnight courier to our laboratory in Antofagasta to prevent deterioration, dried under dark and milled at ambient temperature in our laboratory in Antofagasta. This sampling methodology was previously used for other Chilean samples [5,7,37].
3.2. Sample Preparation
Ten grams of each dried plant were finally pulverized in a mortar, defatted thrice with 100 mL of n-hexane and then extracted with 100 mL of ethyl acetate in the dark in an ultrasonic bath for one hour each. The extracts were combined, filtered and evaporated in vacuo in the dark (40 °C). The solutions were combined and evaporated to dryness under reduced pressure (40 °C) to give 416.51, 467.30 and 499.93 mg of Nolana leptophylla, N. aplocaryoides and N. ramosissima extracts, respectively.
3.3. Liquid Chromatography Analysis
A portion of each extract (approximately 2.5 mg) obtained as explained above was dissolved in 2 mL 0.1% HCl in MeOH, filtered through a 0.45-μm micropore membrane (PTFE, Waters) before use and was injected (20 μL) into the HPLC-PDA and ESI-MS equipment.
3.4. Mass Spectrometric Conditions
An Esquire 4000 Ion Trap mass spectrometer (Bruker Daltoniks, Bremen, Germany) was connected to an Agilent 1100 HPLC instrument via an ESI interface for HPLC-ESI-MS analysis. Full scan mass spectra were measured between m/z 150 and 2000 μ in positive ion mode and negative ion mode for all compounds. High purity nitrogen was used as the nebulizer gas at 27.5 psi, 350 °C and at a flow rate of 8 L/min. The mass spectrometric conditions for negative ion mode were: electrospray needle, 4000 V; end plate offset, −500 V; skimmer 1, −56.0 V; skimmer 2, −6.0 V; capillary exit offset, −84.6 V; and the operating conditions for positive ion mode were: electrospray needle, 4000 V; end plate offset, −500 V; skimmer 1, 56.0 V; skimmer 2, 6.0 V; capillary exit offset, 84.6 V; capillary exit, 140.6 V. Collisionally-induced dissociation (CID) spectra were obtained with a fragmentation amplitude of 1.00 V (MS/MS) using ultrahigh pure helium as the collision gas.
3.5. Antioxidant Assays
3.5.1. Free Radical Scavenging Capacity
The free radical scavenging capacity of the extracts was determined by the DPPH .assay as previously described with some modifications [8,34]. Briefly, 50 μL of extract or pure compound prepared at different concentrations were added to 2 mL of a fresh 0.1 mM solution of DPPH in methanol and allowed to react at 37 °C in the dark. After thirty minutes, the absorbance was measured at 517 nm. The DPPH scavenging ability as a percentage was then calculated as: DPPH scavenging ability = (Acontrol − Asample/Acontrol) × 100. Afterwards, a curve of % DPPH bleaching activity versus concentration was plotted, and IC50 values were calculated. IC50 denotes the concentration of sample required to scavenge 50% of DPPH free radicals. The lower the IC50 value, the more powerful the antioxidant activity. Quercetin (from 15.0 to 250.0 μg/mL, R2 = 0.999) was used as the standard antioxidant compound.
3.5.2. Ferric Reducing Antioxidant Power
The determination of ferric reducing antioxidant power or ferric reducing ability (FRAP assay) of the extracts was performed as previously described [29,38]. Quantification was performed using a standard calibration curve of antioxidant Trolox (from 0.2 to 2.5 μmol/mL, R2: 0.995). Samples were analyzed in triplicate, and results are expressed in μmol TE/gram dry mass.
3.5.3. Superoxide Anion Scavenging Activity
The enzyme xanthine oxidase is able to generate superoxide anion radical (O2•−) ‘in vivo’ by oxidation of reduced products from intracellular ATP metabolism. In the reaction, the superoxide anion generated by the enzyme reduces nitro blue tetrazolium (NBT) to a blue formazan. The absorbance of the formazan produced was determined at 560 nm. Superoxide anion scavengers reduce the speed of generation of the chromophore. The superoxide anion scavenging activities of isolated compounds and fractions were measured spectrophotometrically in a microplate reader as reported previously, and the absorbance at 560 nm was recorded for 60 s (formation of blue formazan) [36,37,39]. The standard flavonoid and the EtOAc extracts were evaluated at 100 μg/mL. Values are presented as the mean ± the standard deviation of three determinations. The percentage of superoxide anion scavenging effect was calculated as follows:
where A is the optical density of the control; B is the optical density of the control blank; C is the optical density of the sample; and D is the optical density of the sample blank.
% of scavenging activity: (E − S)/E × 100, where E = A − B and S = C − (B + D)
3.5.4. Polyphenol and Flavonoids Contents
The total polyphenolic contents (TPC) of Nolana extracts were determined by the Folin-Ciocalteu method [6,7,40] with some modifications. An aliquot of each crude EtOAc extract (200 μL, approximately 2 mg/mL) was added to the Folin-Ciocalteu reagent (2 mL, 1:10 v/v in purified water), and after 5 min of reaction at room temperature (25 °C), 2 mL of a 100 g/L solution of Na2CO3 were added. Sixty min later, the absorbance was measured at 710 nm. The calibration curve was performed with gallic acid (concentrations ranging from 16–500 μg/mL, R2 = 0.999), and the results were expressed as mg gallic acid equivalents/g dry mass. Determination of total flavonoid content (TFC) of the extracts was performed as reported previously [41] using the AlCl3 colorimetric method. Quantification was expressed by reporting the absorbance in the calibration graph of quercetin, which was used as a standard (from 0.1–65.0 μg/mL, R2 = 0.994). Results are expressed as mg quercetin equivalents/g dry weight. All spectrometric measurements were performed using a Unico 2800 UV-VIS spectrophotometer (Shangai, Unico instruments, Co, Ltd, Shangai, China) or a Pharospectroquant 300 (Merck, Darmstadt, Germany).
3.6. Statistical Analysis
The statistical analysis was carried out using the originPro 9.0 software packages (Originlab Corporation, Northampton, MA, USA). The determination was repeated at least three times for each sample solution. Analysis of variance was performed using ANOVA. Significant differences between means were determined by a Tukey comparison test (p-values <0.05 were regarded as significant).
4. Conclusions
Thirty compounds, including three fatty acids (Peaks 13, 18 and 23), six phenolic acids or derivatives (Peaks 6–8, 10, 14 and 16), six flavones (Peaks 12, 17, 22, 24, 25, 28 and 30) and five flavanones (Peaks 19–21, 27 and 29) were identified in three Nolana species from the Chilean Desert in the II region of Chile using ESI-MS for the first time. Among the 30 compounds identified in the three plants under study, twenty three compounds were detected in N. aplocaryoides, fourteen in N. leptophylla and nine in N. ramosissima. Indeed, N. ramosissima showed the most complex polyphenol profile, while the other Nolana species showed a simpler pattern. However, N. ramosissima presented the highest antioxidant features and polyphenolic content followed by N. aplocaryoides, which makes this plant the better candidate for industrial crop production and potential use in functional foods and nutraceuticals.
Acknowledgments
This work was financially supported by the National Fund of Scientific and Technological Development of Chile (Fondecyt No. 1140178). We also acknowledge to FONDEQUIP for the equipment acquired thorough the grant: Equipo de HPLC-DAD-espectrometría de masas-TOF-EQM14002.
Author Contributions
Carlos Areche made the MS analysis, Beatriz Sepúlveda performed the biological assays and Mario Simirgiotis and Julio Benites wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Berries: Anti-inflammatory Effects in Humans. J. Agric. Food Chem. 2014, 62, 3886–3903. [Google Scholar] [CrossRef] [PubMed]
- Urquiaga, I.; Strobel, P.; Perez, D.; Martinez, C.; Cuevas, A.; Castillo, O.; Marshall, G.; Rozowski, J.; Leighton, F. Mediterranean diet and red wine protect against oxidative damage in young volunteers. Atherosclerosis 2010, 211, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Pennington, J.A.T.; Fisher, R.A. Food component profiles for fruit and vegetable subgroups. J. Food Compos. Anal. 2010, 23, 411–418. [Google Scholar] [CrossRef]
- Cavazza, A.; Corradini, C.; Musci, M.; Salvadeo, P. High-performance liquid chromatographic phenolic compound fingerprint for authenticity assessment of honey. J. Sci. Food Agric. 2013, 93, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Borquez, J.; Schmeda-Hirschmann, G. Antioxidant capacity, polyphenolic content and tandem HPLC-DAD-ESI/MS profiling of phenolic compounds from the South American berries Luma apiculata and L. chequen. Food Chem. 2013, 139, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Caligari, P.D.S.; Schmeda-Hirschmann, G. Identification of phenolic compounds from the fruits of the mountain papaya Vasconcellea pubescens A. DC. grown in Chile by liquid chromatography-UV detection-mass spectrometry. Food Chem. 2009, 115, 775–784. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Ramirez, J.E.; Schmeda Hirschmann, G.; Kennelly, E.J. Bioactive coumarins and HPLC-PDA-ESI-ToF-MS metabolic profiling of edible queule fruits (Gomortega keule), an endangered endemic Chilean species. Food Res. Int. 2013, 54, 532–543. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G. Determination of phenolic composition and antioxidant activity in fruits, rhizomes and leaves of the white strawberry (Fragaria chiloensis spp. chiloensis form chiloensis) using HPLC-DAD-ESI-MS and free radical quenching techniques. J. Food Compos. Anal. 2010, 23, 545–553. [Google Scholar] [CrossRef]
- Bórquez, J.; Kennelly, E.J.; Simirgiotis, M.J. Activity guided isolation of isoflavones and hyphenated HPLC-PDA-ESI-ToF-MS metabolome profiling of Azorella madreporica Clos. from northern Chile. Food Res. Int. 2013, 52, 288–297. [Google Scholar] [CrossRef]
- Wu, S.-B.; Wu, J.; Yin, Z.; Zhang, J.; Long, C.; Kennelly, E.J.; Zheng, S. Bioactive and Marker Compounds from Two Edible Dark-Colored Myrciaria Fruits and the Synthesis of Jaboticabin. J. Agric. Food Chem. 2013, 61, 4035–4043. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-B.; Dastmalchi, K.; Long, C.; Kennelly, E.J. Metabolite Profiling of Jaboticaba (Myrciaria cauliflora) and Other Dark-Colored Fruit Juices. J. Agric. Food Chem. 2012, 60, 7513–7525. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; von Baer, D. Polyphenols and antioxidant activity of Calafate (Berberis microphylla) fruits and other native berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef] [PubMed]
- Verardo, V.; Arráez-Román, D.; Segura-Carretero, A.; Marconi, E.; Fernández-Gutiérrez, A.; Caboni, M.F. Identification of buckwheat phenolic compounds by reverse phase high performance liquid chromatography-electrospray ionization-time of flight-mass spectrometry (RP-HPLC–ESI-TOF-MS). J. Cereal Sci. 2010, 52, 170–176. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, L.; Zhu, Q.; Wang, D.; Wang, W.; Sun, X.; Liu, X.; Du, F. Screening natural antioxidants in peanut shell using DPPH–HPLC–DAD–TOF/MS methods. Food Chem. 2012, 135, 2366–2371. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.O.; Tu, T.; Xie, L.; Silvestre, V.; Wen, J. Biogeographic diversification in Nolana (Solanaceae), a ubiquitous member of the Atacama and Peruvian Deserts along the western coast of South America. J. Syst. Evol. 2009, 47, 457–476. [Google Scholar] [CrossRef]
- Vio-Michaelis, S.; Apablaza-Hidalgo, G.; Gómez, M.; Peña-Vera, R.; Montenegro, G. Antifungal activity of three Chilean Plant extracts on Botritis cinerea. Bot. Sci. 2012, 90, 179–183. [Google Scholar]
- Chamy, M.C.; Piovano, M.; Garbarino, J.A. Diterpenoids from Nolana elegans. Bol. Soc. Chil. Quim. 2002, 47, 367–370. [Google Scholar] [CrossRef]
- Chamy, M.C.; Garbarino, J.A.; Piovano, E.; López Pérez, J.L.; Nicoletti, M.; Gandolfo, R.; Feliciano, A. 9-epi-labdane diterpenoids from Nolana rostrata var. rostrata. Phytochemistry 1997, 45, 797–800. [Google Scholar] [CrossRef]
- Garbarino, J.A.; Chamy, M.C.; Piovano, M.; Gambaro, V. Labdane diterpenoids from Nolana filifolia. Phytochemistry 1988, 27, 1795–1796. [Google Scholar] [CrossRef]
- Garbarino, J.A.; Chamy, M.C.; Montagna, M.P.; Gambaro, V. Sesquiterpenoids in Nolana coelestis. Phytochemistry 1993, 32, 987–989. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Zeng, X.; Huang, S.; Hou, S.; Lai, X. Characterization of phenolic constituents in Lithocarpus polystachyus. Anal. Methods. 2014, 6, 1359–1363. [Google Scholar] [CrossRef]
- Dahia, M.; Siracusa, L.; Laouer, H.; Ruberto, G. Constituents of the Polar Extracts from Algerian Pituranthos scoparius. Nat. Prod. Commun. 2009, 12, 1691–1692. [Google Scholar]
- Engels, C.; Gräter, D.; Esquivel, P.; Jiménez, V.M.; Gänzle, M.G.; Schieber, A. Characterization of phenolic compounds in jocote (Spondias purpurea L.) peels by ultra high-performance liquid chromatography/electrospray ionization mass spectrometry. Food Res. Int. 2012, 46, 557–562. [Google Scholar] [CrossRef]
- Markham, K.R. Techniques of Flavonoid Identification; Academic Press: London, UK, 1982. [Google Scholar]
- Brito, I.; Simirgiotis, M.J.; Rodríguez Werner, M.; Bórquez, J.; Winterhalter, P.; Cárdenas, A.; Brito, A. A non-centrosymmetric polymorph of 5-hydroxy-7-methoxy-2-phenylchroman-4-one. J. Chil. Chem. Soc. 2015, 60, 2864–2866. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Yu, J.; Liu, C.; Li, L.; Zhang, Y. Isolation of Liquiritigenin-4′-Apiosyl-Glucoside and Liquiritin from the Root of Glycyrrhiza uralensis by High-Performance Centrifugal Partition Chromatography. J.Chromatogr. Sci. 2014, 52, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, C.; Sun, Q.; Li, F.; Liu, J.; Zheng, C. Isolation and purification of four flavonoid constituents from the flowers of Paeonia suffruticosa by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1075, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Villalobos Solis, M.I.; Patel, A.; Orsat, V.; Singh, J.; Lefsrud, M. Fatty acid profiling of the seed oils of some varieties of field peas (Pisum sativum) by RP-LC/ESI-MS/MS: Towards the development of an oilseed pea. Food Chem. 2013, 139, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.E.; Zambrano, R.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanins and antioxidant capacities of six Chilean berries by HPLC–HR-ESI-ToF-MS. Food Chem. 2015, 176, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martínez, E.J.; Spínola, V.; Gouveia, S.; Castilho, P.C. HPLC-ESI-MSn characterization of phenolic compounds, terpenoid saponins, and other minor compounds in Bituminaria bituminosa. Ind. Crops Prod. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Pinela, J.; Carvalho, A.M.; Buelga, C.; Ferreira, I.C.F.R. Characterization and Quantification of Phenolic Compounds in Four Tomato (Lycopersicon esculentum L.) Farmers’ Varieties in Northeastern Portugal Homegardens. Plant Foods Hum. Nutr. 2012, 67, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Kuhnert, N. Identification and characterization of five new classes of chlorogenic acids in burdock (Arctium lappa L.) roots by liquid chromatography/tandem mass spectrometry. Food Funct. 2011, 2, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Berti, M.; Wilckens, R.; Baeza, M.; Pastene, E.; Inostroza, L.; Tramón, C.; Gonzalez, W. Characterization and propagation of some medicinal plants in the central-south region of Chile. Ind. Crops Prod. 2011, 34, 1313–1321. [Google Scholar]
- Žugić, A.; Đorđević, S.; Arsić, I.; Marković, G.; Živković, J.; Jovanović, S.; Tadić, V. Antioxidant activity and phenolic compounds in 10 selected herbs from Vrujci Spa, Serbia. Ind. Crops Prod. 2014, 52, 519–527. [Google Scholar] [CrossRef]
- Fernández-Arroyo, S.; Rodríguez-Medina, I.C.; Beltrán-Debón, R.; Pasini, F.; Joven, J.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Quantification of the polyphenolic fraction and in vitro antioxidant and in vivo anti-hyperlipemic activities of Hibiscus sabdariffa aqueous extract. Food Res. Int. 2011, 44, 1490–1495. [Google Scholar] [CrossRef]
- Masuoka, N.; Nihei, K.-I.; Maeta, A.; Yamagiwa, Y.; Kubo, I. Inhibitory effects of cardols and related compounds on superoxide anion generation by xanthine oxidase. Food Chem. 2015, 166, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G.; Bórquez, J.; Kennelly, E.J. The Passiflora tripartita (Banana Passion) Fruit: A source of bioactive flavonoid C-glycosides isolated by HSCCC and characterized by HPLC-DAD-ESI/MS/MS. Molecules 2013, 18, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘Antioxidant Power’’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS Profiles of Phenolic Compounds and Antioxidant Activity of Fruits From Three Citrus Species Consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J. Antioxidant Capacity and HPLC-DAD-MS Profiling of Chilean Peumo (Cryptocarya alba) Fruits and Comparison with German Peumo (Crataegus monogyna) from Southern Chile. Molecules 2013, 18, 2061–2080. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Adachi, S.; To, S.; Yang, H.; Reynertson, K.A.; Weinstein, I.B.; Kennelly, E.J.; Basile, M.J.; Gil, R.R. Cytotoxic chalcones and antioxidants from the fruits of Syzygium samarangense (Wax Jambu). Food Chem. 2008, 107, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the plants and extracts are available from authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).