Topical Anti-inflammatory Activity of New Hybrid Molecules of Terpenes and Synthetic Drugs
Abstract
:1. Introduction
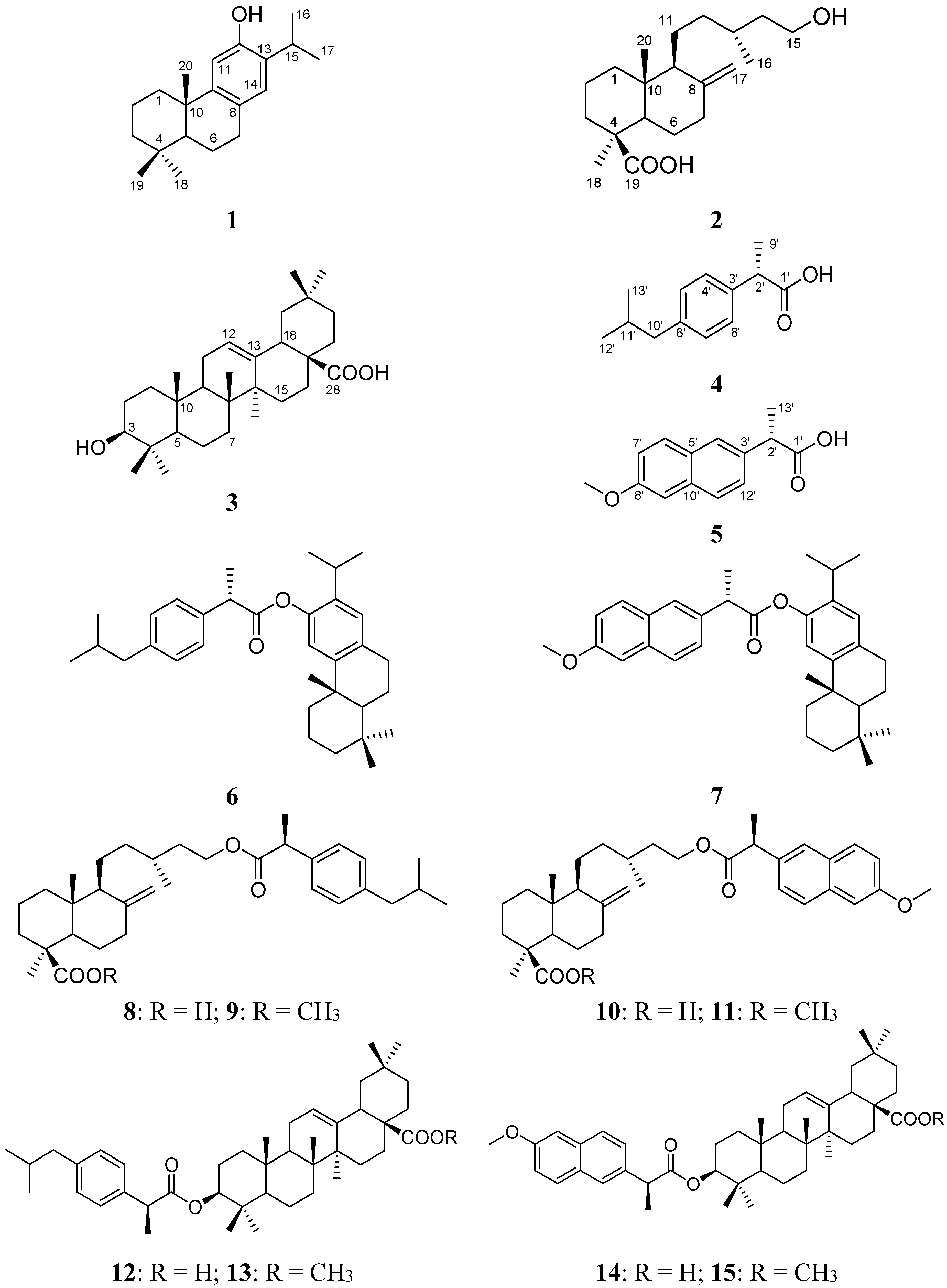
2. Results and Discussion
| Compounds | % TAAA ± SEM | % TATPA ± SEM |
|---|---|---|
| Terpenes | ||
| Ferruginol (1) | 21.0 ± 2.1 *,a | 20.4 ± 3.1 *,a |
| Imbricatolic acid (2) | 5.0 ± 3.0 | 10.1 ± 4.0 a |
| Oleanolic acid (3) | 2.7 ± 7.5 | 70.2 ± 8.3 *,a |
| Synthetic anti-inflammatory compounds | ||
| Ibuprofen (4) | 7.2 ± 5.5 | 56.2 ± 3.4 *,a |
| Naproxen (5) | 1.8 ± 7.5 a | 29.9 ± 6.2 *,a |
| Esters | ||
| Ferruginyl ibuprofenate (6) | 14.9 ± 7.1 *,a | 47.9 ± 4.6 *,a |
| Ferruginyl naproxenate (7) | 26.6 ± 4.1 *,a | 26.8 ± 3.8 *,a |
| Imbricatol-15-yl ibuprofenate (8) | 10.0 ± 6.0 a | 40.3 ± 2.4 *,a |
| Imbricatol-15-yl ibuprofenate methyl ester (9) | 14.9 ± 7.4 * | 41.2 ± 6.2 *,a |
| Imbricatol-15-yl naproxenate (10) | 16.3 ± 3.8 *,a | 37.7 ± 3.3 *,a |
| Imbricatol-15-yl naproxenate methyl ester (11) | 20.0 ± 9.4 *,a | 42.7 ± 8.3 *,a |
| Oleanoyl ibuprofenate (12) | 56.8 ± 7.4 * | 79.9 ± 10.6 * |
| Oleanoyl ibuprofenate methyl ester (13) | 19.5 ± 9.2 *,a | 47.4 ± 2.6 *,a |
| Oleanoyl naproxenate (14) | 9.0 ± 11.3 a | 55.5 ± 5.1 *,a |
| Oleanoyl naproxenate methyl ester (15) | 2.7 ± 8.1 a | 80.0 ± 11.0 * |
| Reference compounds | ||
| Nimesulide | ↑48.9 ± 5.5 * | n.d. b |
| Indomethacin | n.d. b | ↑92.9 ± 13.2 * |
| Compounds | (IC50 ± SD, µM) a | ||
|---|---|---|---|
| MRC-5 | AGS | HepG2 | |
| Terpenes | |||
| Ferruginol (1) | 31 ± 2 | 29 ± 1 | 39 ± 3 |
| Imbricatolic acid (2) | 134 ± 7 | 148 ± 9 | 111 ± 7 |
| Oleanolic acid (3) | 186 ± 9 | 234 ± 16 | 170 ± 5 |
| Synthetic anti-inflammatory compounds | |||
| Ibuprofen (4) | >1000 | >1000 | >1000 |
| Naproxen (5) | >1000 | >1000 | >1000 |
| Esters | |||
| Ferruginyl ibuprofenate (6) | >1000 | >1000 | 874 ± 34 |
| Ferruginyl naproxenate (7) | 945 ± 56 | 874 ± 49 | >1000 |
| Imbricatol-15-yl ibuprofenate (8) | 34 ± 2 | 23 ± 1 | 79 ± 8 |
| Imbricatol-15-yl ibuprofenate methyl ester (9) | 706 ± 35 | 623 ± 39 | 746 ± 38 |
| Imbricatol-15-yl naproxenate (10) | 37 ± 2 | 29 ± 2 | 69 ± 4 |
| Imbricatol-15-yl naproxenate methyl ester (11) | 907 ± 54 | 601 ± 25 | >1000 |
| Oleanoyl ibuprofenate (12) | 616 ± 32 | 526 ± 19 | >1000 |
| Oleanoyl ibuprofenate methyl ester (13) | 402 ± 20 | 454 ± 21 | 742 ± 48 |
| Oleanoyl naproxenate (14) | 554 ± 22 | 486 ± 26 | >1000 |
| Oleanoyl naproxenate methyl ester (15) | 816 ± 42 | 593 ± 31 | >1000 |
| Etoposide b | 3.9 ± 0.1 | 0.36 ± 0.02 | 2.4 ± 0.1 |
3. Experimental Section
3.1. Equipment and General Procedures
3.2. Preparation of Derivatives
3.2.1. Starting Compounds 1–5
3.2.2. Synthesis of Compounds 6–15
3.3. Topical Anti-Inflammatory Effect
3.4. Cytotoxicity Assay
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schmeda-Hirschmann, G.; Astudillo, L.; Rodríguez, J.A.; Theoduloz, C.; Yáñez, T. Gastroprotective effect of the Mapuche crude drug Araucaria araucana resin and its main constituents. J. Ethnopharmacol. 2005, 101, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Mösbach, E.W. Botanica Indigena de Chile; Aldunate, C., Villagrán, C., Eds.; Museo Chileno de Arte Precolombino. Fundación Andes and Editorial Andrés Bello: Santiago de Chile, Chile, 1992. [Google Scholar]
- Girón, N.; Través, P.G.; Rodríguez, B.; López-Fontal, R.; Boscá, L.; Hortelano, S.; de las Heras, B. Supression of inflammatory responses by labdane-type diterpenoids. Toxicol. Appl. Pharmacol. 2008, 228, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, I.; Cidre, F.; Herranz, S.; Estevez-Braun, A.; de las Heras, B.; Hortelano, S. Labdanolic methyl ester (LAME) exerts anti-inflammatory effects through inhibition of TAK-1 activation. Toxicol. Appl. Pharmacol. 2012, 258, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Díaz, M.; Areche, C.; Delporte, C. Anti-inflammatory activity of ferruginol from Prumnopitys andina. J. Life Sci. 2013, 7, 1165–1168. [Google Scholar]
- Rodríguez, J.; Theoduloz, C.; Yáñez, T.; Becerra, J.; Schmeda-Hirschmann, G. Gastroprotective effect of the diterpene ferruginol in mice: Protection against membrane lipid peroxidation and involvement of prostaglandins. Life Sci. 2006, 78, 2503–2509. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Lin, K.W.; Gan, K.H.; Wang, J.P.; Won, S.J.; Lin, C.N. New diterpenoids and cytotoxic and anti-inflammatory diterpenoids from Amentotaxus formosana. Fitoterapia 2011, 82, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Cordoba, C.; San Román, J.A.; Gutierrez, B.; Cachofeiro, V.; Nieto, M.L. Oleanolic acid modulates the immune-inflammatory response in mice with experimental autoimmune myocarditis and protects from cardiac injury. Therapeutic implications for the human disease. J. Mol. Cell Cardiol. 2014, 72, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.Y.; Kang, S.W.; Kim, J.L.; Li, J.; Lee, E.S.; Gong, J.H.; Han, S.J.; Kang, Y.H. Oleanolic acid reduces markers of differentiation in 3T3-L1 adipocytes. Nutr. Res. 2010, 30, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk-Cwynar, B.; Zaprutko, L.; Marciniak, J.; Lewandowski, G.; Szulc, M.; Kaminska, E.; Wachowiak, N.; Mikolajczak, P. The analgesic and anti-inflammatory effect of new oleanolic acid acyloxyimino derivative. Eur. J. Pharm. Sci. 2012, 47, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.C.; Delporte, C.; Backhouse, N.; Erazo, S.; Letelier, M.E.; Cassels, B.K.; Silva, X.; Alegría, S.; Negrete, R. Topical anti-inflammatory activity of 2α-hydroxy pentacyclic triterpene acids from the leaves of Ugni molinae. Bioorg. Med. Chem. 2006, 14, 5673–5677. [Google Scholar] [CrossRef] [PubMed]
- Theoduloz, C.; Bravo, C.I.; Pertino, M.W.; Valenzuela, D.; Schmeda-Hirschmann, G. Potential gastroprotective effect of novel cyperenoic acid/quinone derivatives in human cell cultures. Planta Med. 2012, 78, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Theoduloz, C.; Bravo, I.; Pertino, M.W.; Schmeda-Hirschmann, G. Diterpenylquinone hybrids: Synthesis and assessment of gastroprotective mechanisms of action in human cells. Molecules 2013, 18, 11044–11066. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.S.; Currie, J.L.; Shaffer, A.F.; Isakson, P.C. Comparative evaluation of arachidonic acid (AA)-and tetradecanoylphorbol acetate (TPA)-induced dermal inflammation. Inflammation 1993, 17, 723–741. [Google Scholar] [CrossRef] [PubMed]
- Recio, M.C.; Giner, R.M.; Mánez, S.; Ríos, J.L. Structural requirements for the anti-inflammatory activity of natural triterpenoids. Planta Med. 1995, 61, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.O.; Oh, J.H.; Kim, T.M.; Kim, D.J.; Jeong, H.S.; Han, S.B.; Hong, J.T. Anti-inflammatory and arthritic effects of thiacremonone, a novel sulfur compound isolated from garlic via inhibition of NF-κB. Arthritis Res. Ther. 2009, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, K.; Ahn, H.S.; Kim, D. Importance of structural information in predicting human acute toxicity from in vitro cytotoxicity data. Toxicol. Appl. Pharmacol. 2010, 246, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Benavides, T.; Mitjans, M.; Martı́nez, V.; Clapés, P.; Infante, M.R.; Clothier, R.H.; Vinardell, M.P. Assessment of primary eye and skin irritants by in vitro cytotoxicity and phototoxicity models: An in vitro approach of new arginine-based surfactant-induced irritation. Toxicology 2004, 197, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Gábor, M. Models of acute inflammation in the ear. Methods Mol. Biol. 2003, 225, 129–137. [Google Scholar] [PubMed]
- Carlson, R.P.; O’Neill-Davis, L.; Chang, J.; Lewis, A.J. Modulation of mouse ear edema by cyclooxygenase and lipoxygenase inhibitors and other pharmacologic agents. Agents Action 1985, 17, 197–204. [Google Scholar] [CrossRef]
- Bustos, G.; Ferrándiz, M.L.; Sanz, M.J.; Payá, M.; Alcaraz, M.J. A study of the novel anti-inflammatory agent florifenine topical anti-inflammatory activity and influence on arachidonic acid metabolism and neutrophil functions. Naunyn Schmiedebergs Arch. Pharmacol. 1995, 351, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Goodman, L.; Gilman, A.; Hardman, J.; Limbird, L. Goodman & Gilman’s the pharmacological Basis of Therapeutics, 9th ed.; McGraw-Hill: New York, NY, USA, 1996. [Google Scholar]
- Bucci, E.; Mignogna, M.D.; Bucci, P. Aulin: A new modern treatment for inflammatory disorders in dentistry. Min. Stomatol. 1987, 36, 101–103. [Google Scholar]
- Rodríguez-Díaz, M.; Delporte, C.; Cartagena, C.; Cassels, B.K.; González, P.; Silva, X.; León, F.; Wessjohann, L.A. Topical anti-inflammatory activity of quillaic acid from Quillaja saponaria Mol. and some derivatives. J. Pharm. Pharmacol. 2011, 63, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Estrada, M.; Reyes, R.; Morales, B.A.; Reyes, S.M.A.; de la Parra, M.G.; Aumelas, A.; Jankowski, C.K. Synthesis and preliminary biological evaluation of two new cacalol esters of naproxen and ibuprofen. Rev. Latinoam. Quím. 2012, 40, 106–111. [Google Scholar]
- Cocco, M.T.; Congiu, C.; Onnis, V.; Morelli, M.; Cauli, O. Synthesis of ibuprofen heterocyclic amides and investigation of their analgesic and toxicological properties. Eur. J. Med. Chem. 2003, 38, 513–518. [Google Scholar] [CrossRef]
- Zhao, X.; Tao, X.; Wei, D.; Song, Q. Pharmacological activity and hydrolysis behavior of novel ibuprofen glucopyranoside conjugates. Eur. J. Med. Chem. 2006, 41, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Sarigol, D.; Uzgoren-Baran, A.; Cahide, B.T.; Somuncuoglu, E.I.; Kazkayasi, I.; Ozadali-Sari, K.; Unsal-Tan, O.; Okay, G.; Ertan, M.; Tozkoparan, B. Novel thiazolo[3,2-b]-1,2,4-triazoles derived from naproxen with analgesic/anti-inflammatory properties: Synthesis, biological evaluation and molecular modeling studies. Bioorg. Med. Chem. 2015, 23, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Areche, C.; Rodríguez, J.A.; Razmilic, I.; Yañez, T.; Theoduloz, C.; Schmeda-Hirschmann, G. Gastroprotective and cytotoxic effect of semisynthetic ferruginol derivatives. Pharm. Pharmacol. 2007, 59, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Theoduloz, C.; Schmeda-Hirschmann, G.; Razmilic, I.; Yañez, T.; Rodriguez, J.A. Gastroprotective and ulcer-healing activity of oleanolic acid derivatives: In vitro-in vivo relationships. Life Sci. 2006, 79, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Lloret, S.; Moreno, J.J. Effects of an anti-inflammatory peptide (antiflammin 2) on cell influx, eicosanoid biosynthesis and oedema formation by arachidonic acid and tetradecanoyl phorbol dermal application. Biochem. Pharmacol. 1995, 50, 347–353. [Google Scholar] [CrossRef]
- Delporte, C.; Backhouse, N.; Salinas, P.; San-Martín, A.; Bohórquez, J.; Loyola, A. Pharmaco-toxicological study of new diterpenoids. Bioorg. Med. Chem. 2003, 11, 1187–1190. [Google Scholar] [CrossRef]
- Rodríguez, J.A.; Haun, M. Cytotoxicity of trans-dehydrocrotonin from Croton cajucara (Euphorbiaceae) on V79 cells and rat hepatocytes. Planta Med. 1999, 65, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Samples Availability: Samples of the parent compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theoduloz, C.; Delporte, C.; Valenzuela-Barra, G.; Silva, X.; Cádiz, S.; Bustamante, F.; Pertino, M.W.; Schmeda-Hirschmann, G. Topical Anti-inflammatory Activity of New Hybrid Molecules of Terpenes and Synthetic Drugs. Molecules 2015, 20, 11219-11235. https://doi.org/10.3390/molecules200611219
Theoduloz C, Delporte C, Valenzuela-Barra G, Silva X, Cádiz S, Bustamante F, Pertino MW, Schmeda-Hirschmann G. Topical Anti-inflammatory Activity of New Hybrid Molecules of Terpenes and Synthetic Drugs. Molecules. 2015; 20(6):11219-11235. https://doi.org/10.3390/molecules200611219
Chicago/Turabian StyleTheoduloz, Cristina, Carla Delporte, Gabriela Valenzuela-Barra, Ximena Silva, Solange Cádiz, Fernanda Bustamante, Mariano Walter Pertino, and Guillermo Schmeda-Hirschmann. 2015. "Topical Anti-inflammatory Activity of New Hybrid Molecules of Terpenes and Synthetic Drugs" Molecules 20, no. 6: 11219-11235. https://doi.org/10.3390/molecules200611219
APA StyleTheoduloz, C., Delporte, C., Valenzuela-Barra, G., Silva, X., Cádiz, S., Bustamante, F., Pertino, M. W., & Schmeda-Hirschmann, G. (2015). Topical Anti-inflammatory Activity of New Hybrid Molecules of Terpenes and Synthetic Drugs. Molecules, 20(6), 11219-11235. https://doi.org/10.3390/molecules200611219






