2.1. Construction, Cloning and Sequence Determination of Sclerotium rolfsii Lectin Variants
Considering the significance of SRL to recognize cancer-associated TF antigen binding properties, full length synthetic genes (SSR1 and SSR2 ), which encode variant forms of SRL were chemically constructed based on the amino acid sequence data of the X-ray crystal structures and MALDI MS/MS analysis of SRL [
7,
14]. Since SRL had poor solubility and aggregated with loss of activity upon storage changes in amino acid sequence were introduced to enhance the solubility by altering the surface charge.
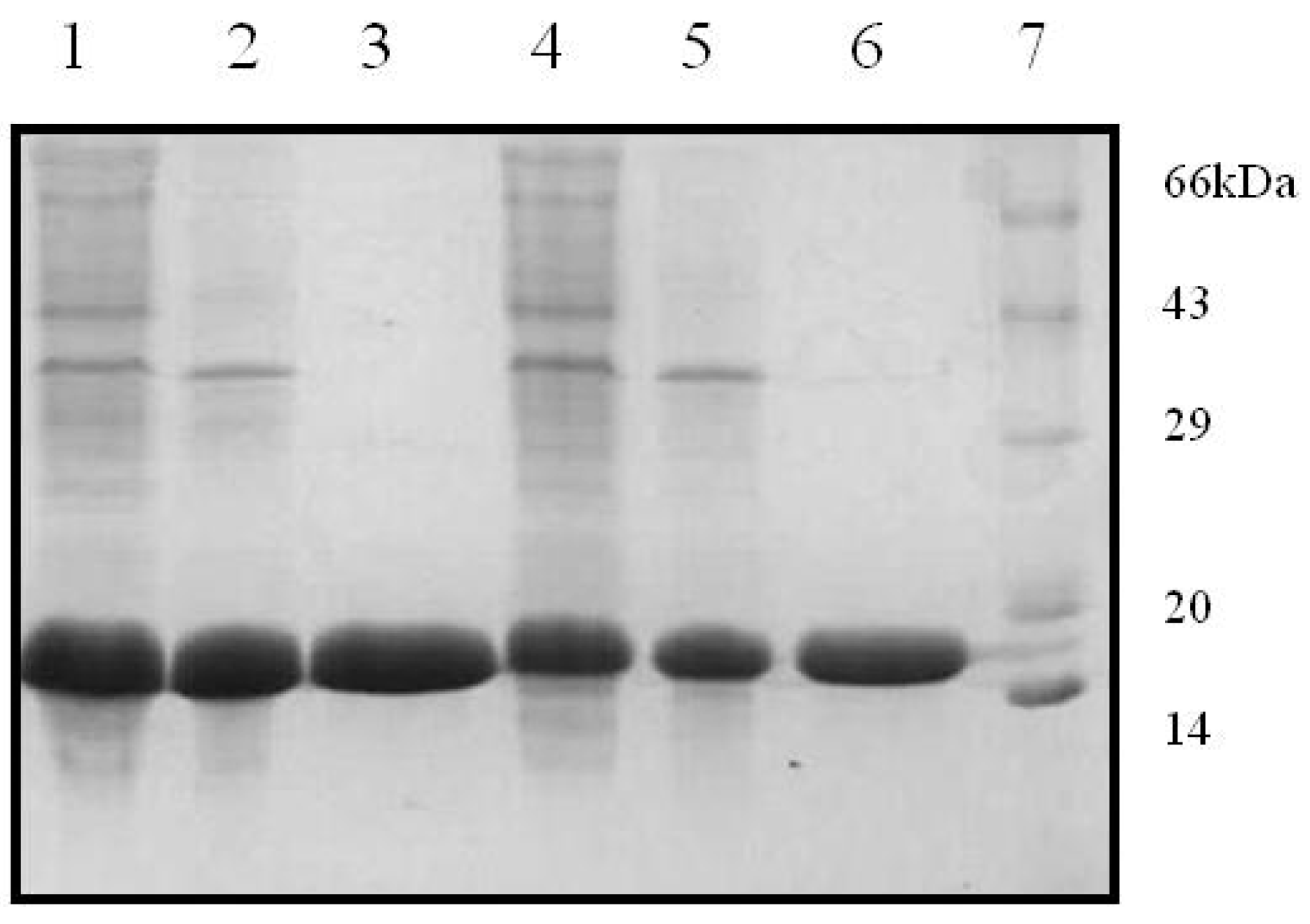
The two recombinant variants of SRL, SSR1 and SSR2 obtained encode for 141 and 143 amino acids, respectively. Restriction digestion analysis using NdeI and BamHI demonstrated that the two variants corresponded to gene lengths of 423 and 426 bps, respectively. The gene sequencing data confirmed the incorporation of the modified amino acids at the desired position in the primary sequence of SRL. Further the expression analysis of the recombinant proteins suggested that the lectins had monomeric molecular masses of ~16 kDa as determined by the SDS-PAGE analysis (
Figure 1). In the current study attempts were made to incorporate the changes in the amino acid sequence of TF antigen binding lectin SRL were successful. The recombinant variants of SRL were expressed in a prokaryotic host, and purified to homogeneity, by the combination of ion exchange and gel filtration chromatographic methods. The hemagglutination assay of recombinant variants revealed that SSR1 and SSR2 agglutinated trypsinized human erythrocytes, irrespective of the blood groups, like SRL. The sequence based phylogenetic analysis has demonstrated that during the evolution, all the lectins derived from common ancestors and SRL and its variants maintains close relationship with ABL, a TF antigen binding lectin. Whereas other fungal lectin XCL which emerged much later than SRL during evolution also shares some similar properties with respect to structure as well as sugar binding ability. The common features of SRL and its variants with other fungal lectins observed with respect to their sugar binding ability and structural design are because of the conserved amino acids which contribute towards the specificity of the lectin.
Figure 1.
Purification profiles of SSR1 and SSR2 in 15% SDS-PAGE. Lane 1: SSR1 lysate, lane 2: DEAE flow through, Lane 3: Gel filtration elute, lane 4: SSR2 lysate, lane 5: DEAE flow through, lane 6: Gel filtration elute, and lane 7: Standard protein marker.
Figure 1.
Purification profiles of SSR1 and SSR2 in 15% SDS-PAGE. Lane 1: SSR1 lysate, lane 2: DEAE flow through, Lane 3: Gel filtration elute, lane 4: SSR2 lysate, lane 5: DEAE flow through, lane 6: Gel filtration elute, and lane 7: Standard protein marker.
2.4. Carbohydrate Binding Specificities of SSR1 and SSR2 as Determined by Glycan Array Analysis.
The glycan array analysis was carried out at the Consortium for Functional Glycomics (CFG), using mammalian printed array ver. 5.0 (
Supplementary files S1 and S2 for SSR1 and SSR2, respectively) to determine the relative carbohydrate binding affinity of SSR1 and SSR2. The glycan array analysis has revealed that SSR1 recognizes Neu5Acα2-3GalNAcα-Sp8 (G#237), a sialylated-Tn antigen with highest affinity of RFU, 60561. It is interesting to note that these binding differences were observed only at the highest concentration of the protein used (200 μg/mL) and not at lower concentrations of SSR1 (20, 2 and 0.2 μg/mL). Additionally, SSR1 has also shown considerable affinity ranging from 36016 to 42247 RFU for GlcNAc/GalNAc linked to Tn (GalNAcα) antigen through α 1-3, β 1-3, or β 1-6 linkages (
Table 2), signifying the importance of non-reducing GalNAcα moiety in SSR1’s specificity. The affinity of SSR1 towards TF (Galβ1-3GalNAcα) (βα) antigen is moderate compared to Tn with an affinity of RFU 37094 and slightly higher (39767) for β anomer (ββ) while it decreases significantly towards Galα1-3GalNAcα (αα) (
Table 1). In contrast, SSR1 did not recognize Galα1-3GalNAcβ (αβ) (
Table 3), showing its similarity with SRL in recognizing different anomers of TF disaccharide [
9]. Although different techniques are essential to provide exclusive specificity, the relative binding affinity of SSR1 among different TF anomers can be assigned as TFββ > TFβα > TFαα >> TFαβ. The affinity of SSR1 drastically increases for TF disaccharide if C3 of galactose is substituted with sialic acid or GlcNAc or SO
4 but decreases completely if the sugar is fucose (
Supplementary file S1). Interestingly, SSR1 is also capable of recognizing N-linked glycans with high affinity (
Supplementary file S3) which make this variant different from SRL which has been shown to recognize exclusively O-linked glycans [
9].
Table 1.
Relative binding of SSR1 to different glycans.
Table 1.
Relative binding of SSR1 to different glycans.
| Glycan No. | Structure | RFU | SD |
|---|
| 237 | Neu5Acα2-3GalNAcα-Sp8 | 60561 | 2397 |
| 4 | GalNAcα-Sp8 | 25418 | 2052 |
| 17 | GlcNAcβ-Sp8 | 24386 | 857 |
Table 2.
Binding affinity of SSR1 towards substituted form of Tn antigen.
Table 2.
Binding affinity of SSR1 towards substituted form of Tn antigen.
| Glycan No. | Structure | RFU | SD |
|---|
| 193 | GlcNAcβ1-6GalNAcα-Sp8 | 42247 | 886 |
| 180 | GlcNAcβ1-3GalNAcα-Sp8 | 42113 | 1009 |
| 194 | GlcNAcβ1-6GalNAcα-Sp14 | 41801 | 10768 |
| 95 | GalNAcβ1-3GalNAcα-Sp8 | 40990 | 1513 |
| 181 | GlcNAcβ1-3GalNAcα-Sp14 | 36016 | 6177 |
Table 3.
Binding affinity of SSR1 towards different anomeric forms of TF disaccharides.
Table 3.
Binding affinity of SSR1 towards different anomeric forms of TF disaccharides.
| Glycan No. | Structure | RFU | SD |
|---|
| 143 | Galβ1-3GalNAcβ-Sp8 | (ββ) | 39767 | 1105 |
| 140 | Galβ1-3GalNAcα-Sp8 | (βα) | 37094 | 1287 |
| 111 | Galα1-3GalNAcα-Sp8 | (αα) | 17943 | 2523 |
| 113 | Galα1-3GalNAcβ-Sp8 | (αβ) | 55 | 34 |
Another recombinant variant, SSR2 also recognizes the TF disaccharide and its derivatives with high affinity similar to SRL. Among all the O-glycans tested, SSR2 shows very high affinity towards sulphated TF disaccharide (G#29) with RFU of 60407 (
Table 4), on the other hand it showed comparatively lower affinity (RFU of 38107) for unsubstituted TF disaccharide (
Table 5). Strikingly, it has highest affinity for βα among all anomers of TF, making it more selective than native. Further, various substitutions such as SO
4, Neu5Ac or GlcNAc at C3 of galactose in TF disaccharide significantly enhances the affinity of SSR2. It is important to note that though substitution of Neu5Ac or GlcNAc on galactose favors the binding of SSR2, affinity of these glycans decrease sharply if substitutions occurs on GalNAc of TF disaccharide (G#134, 135, 136 and 137 in
Table 1).
Table 4.
Binding affinity of SSR1 towards TF disaccharide and its substituted forms.
Table 4.
Binding affinity of SSR1 towards TF disaccharide and its substituted forms.
| Glycan No. | Structure | RFU | SD |
|---|
| 29 | (3S)Galβ1-3GalNAcα-Sp8 | 60407 | 578 |
| 243 | Neu5Acα2-3Galβ1-3(6S)GalNAcα-Sp8 | 59832 | 1388 |
| 224 | Neu5Acα2-3Galβ1-3GalNAcα-Sp8 | 56636 | 2693 |
| 89 | GlcNAcβ1-3Galβ1-3GalNAcα-Sp8 | 55053 | 6128 |
| 167 | Galβ1-4GlcNAcβ1-6(Galβ1-3)GalNAcα-Sp8 | 40694 | 1329 |
| 595 | GlcNAcβ1-3Galβ1-4GlcNAcβ1-6(Galβ1-3)GalNAcα-Sp14 | 38968 | 2788 |
| 562 | GlcNAcβ1-3Galβ1-4GlcNAcβ1-6(GlcNAcβ1-3Galβ1-3)GalNAcα-Sp14 | 38043 | 1942 |
| 134 | GlcNAcβ1-6(Galβ1-3)GalNAcα-Sp8 | 37860 | 2198 |
| 567 | GlcNAβ1-3Galβ1-3GalNAc-Sp14 | 32913 | 2103 |
| 600 | Galβ1-4GlcNAcβ1-3Galβ1-3GalNAcα-Sp14 | 31899 | 1067 |
| 289 | Neu5Acα2-3Galβ1-4GlcNAcβ1-6(Galβ1-3)GalNAcα-Sp14 | 30392 | 1803 |
| 135 | GlcNAcβ1-6(Galβ1-3)GalNAcα-Sp14 | 29926 | 1115 |
| 168 | Galβ1-4GlcNAcβ1-6(Galβ1-3)GalNAc-Sp14 | 29704 | 1107 |
| 568 | Galβ1-3GlcNAcβ1-6(Galβ1-3)GalNAc-Sp14 | 29288 | 1294 |
| 603 | Neu5Acα2-6Galβ1-4GlcNAcβ1-6(Galβ1-3)GalNAcα-Sp14 | 28023 | 3613 |
| 605 | GlcNAcβ1-6(Neu5Acα2-3Galβ1-3)GalNAcα-Sp14 | 27706 | 943 |
| 345 | GlcNAcα1-4Galβ1-3GalNAc-Sp14 | 27443 | 1913 |
| 360 | KDNa2-3Galβ1-3GalNAcα-Sp14 | 25053 | 2863 |
| 601 | Neu5Acα2-3Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-6(Galβ1-3)GalNAcα-Sp14 | 21093 | 1523 |
| 225 | Neu5Acα2-3Galβ1-3GalNAcα-Sp14 | 20448 | 1701 |
| 591 | Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-6(Galβ1-3)GalNAcα-Sp14 | 19429 | 826 |
| 244 | Neu5Acα2-6(Neu5Acα2-3Galβ1-3)GalNAcα-Sp8 | 19265 | 1501 |
| 137 | Neu5Acα2-6(Galβ1-3)GalNAcα-Sp14 | 13550 | 617 |
| 602 | Neu5Acα2-6Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-6(Galβ1-3)GalNAcα-Sp14 | 9887 | 957 |
| 318 | Neu5Acα2-3Galβ1-4GlcNAcβ1-6(Neu5Acα2-3Galβ1-3)GalNAcα-Sp14 | 9348 | 724 |
| 138 | Neu5Acβ2-6(Galβ1-3)GalNAcα-Sp8 | 3150 | 1564 |
| 245 | Neu5Acα2-6(Neu5Acα2-3Galβ1-3)GalNAcα-Sp14 | 3015 | 157 |
| 136 | Neu5Acα2-6(Galβ1-3)GalNAcα-Sp8 | 374 | 199 |
| 63 | Fuca1-2Galβ1-3GalNAcα-Sp14 | 287 | 95 |
In continuation of these results, fucosylated TF antigen is completely ineffective against SSR2 especially when it is attached to galactose through C2 carbon (
Table 4) mimicking the binding property of SRL and SSR1 for this particular glycan.
Like SRL, this variant also showed higher affinity for βα anomer of TF (Galβ1-3GalNAcα) among all the anomers analyzed. This result is in sharp contrast to SSR1 which showed slightly higher affinity for β anomer (ββ) while SSR2 completely fails to recognize β anomer of TF disaccharide (
Table 1). Furthermore, unlike SSR1, SSR2 doesn’t recognize any monosaccharides and Tn antigen as well as its substituted forms. This is an interesting and specific property of SSR2 which resembles SRL that also did not bind to any monosaccharides present in the array [
9]. However, it is important to note that other glycan-lectin interaction studies such as surface plasmon resonance (SPR) [
16] and isothermal calorimetry (ITC) [
17] are essential to provide detailed subtle binding differences between SSR1 and SSR2. Apart from recognizing O-linked glycans, SSR2 also recognize many N-linked glycans present in the array (supplementary file
S2) which is very similar property exhibited by both SSR1 and SSR2 unlike SRL.
Table 5.
Binding affinity of SSR2 towards different anomeric forms of TF disaccharides.
Table 5.
Binding affinity of SSR2 towards different anomeric forms of TF disaccharides.
| Glycan No. | Structure | RFU | SD |
|---|
| 140 | Galβ1-3GalNAcα-Sp8 | 38017 | 1244 |
| 111 | Galα1-3GalNAcα-Sp8 | 545 | 48 |
| 143 | Galβ1-3GalNAcβ-Sp8 | 155 | 23 |
| 113 | Galα1-3GalNAcβ-Sp8 | 30 | 14 |
2.5. Structural Studies
The crystal structures of SSR1 and SSR2 lectins in their free form were determined at 1.7 Å and 1.6 Å resolution, respectively. Like SRL [
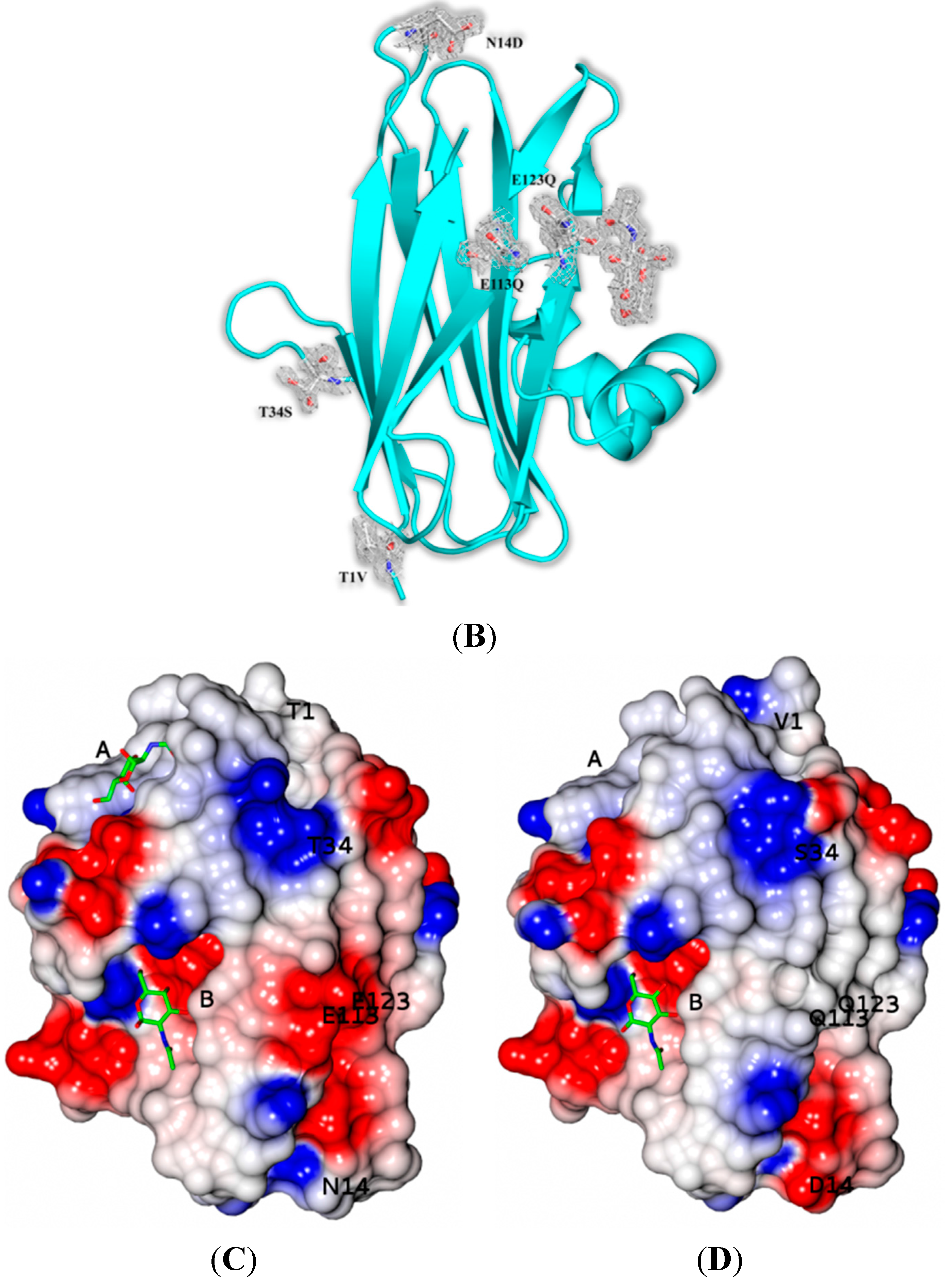
7] there are two protein molecules (named mol A and mol B) in the crystallographic asymmetric unit of both variants related by a non-crystallographic two-fold symmetry. The two molecules pack antiparallel with their β-sheets inclined ~45° while the α-helices are located at the same side of the dimer. The mutations of SSR1 (N14D, E113Q, E123Q) and SSR2 (T1V, N14D, T34S, E113Q, E123Q) were all identified and all atoms of the mutated residues were well defined within the electron density map (
Figure 2). A bound MPD molecule was found in both variants at the same place like in SRL [
7] while in the SSR2 structure two additional molecules form the crystallization medium were found bound (MPD and a 2-amino-2-hydroxymethyl-propane-1,3 diol). Overall the crystal structures of SSR1 and SSR2 are similar to the SRL structure [
7] and the introduction of the mutations did not seem to affect the overall structure of the protein. The rms displacement for all atoms between the SRL structure and those of SSR1 and SSR2 is 0.59 Å and 0.38 Å, respectively. In both structures of SSR1 and SSR2 the mutated residues, Asp14, Gln113, and Gln123 are located in a loop connecting strands S1 and F1, and in strands S4 and S5 respectively. In the structure of SSR2 the mutated residues Val1 and Ser34 are located in strands S1 and S2, respectively. The side chains of all mutated residues have the same conformation as their homologs in the SRL structure and do not induce any significant conformational change in their immediate environment. Only, the mutation N14D seems to cause a minor disturbance in the loop region of residues 12–14 in both variants (rms displacement of all atoms 0.18 Å and 0.26 Å from the SRL structure, respectively). In the SSR2 structure the T1V mutation leads to a shift of the residue by 0.6–1.0 Å (depending on the atoms) while the T34S mutation does not cause any conformational change on this residue. The surface potential of the two variants is reduced by ~10% both for the monomer and the dimers with respect to that of SRL.
In both the SSR1-GlcNAc and the SSR2-GlcNAc complexes, determined at 1.9 Å and 1.7 Å respectively, GlcNAc was found bound only in the secondary carbohydrate binding site of SRL in agreement with the complex of GlcNAc with SRL [
7]. In the SSR1-GlcNAc structure an MPD molecule was found bound in the primary carbohydrate binding site similarly to the SRL-GlcNAc complex structure [
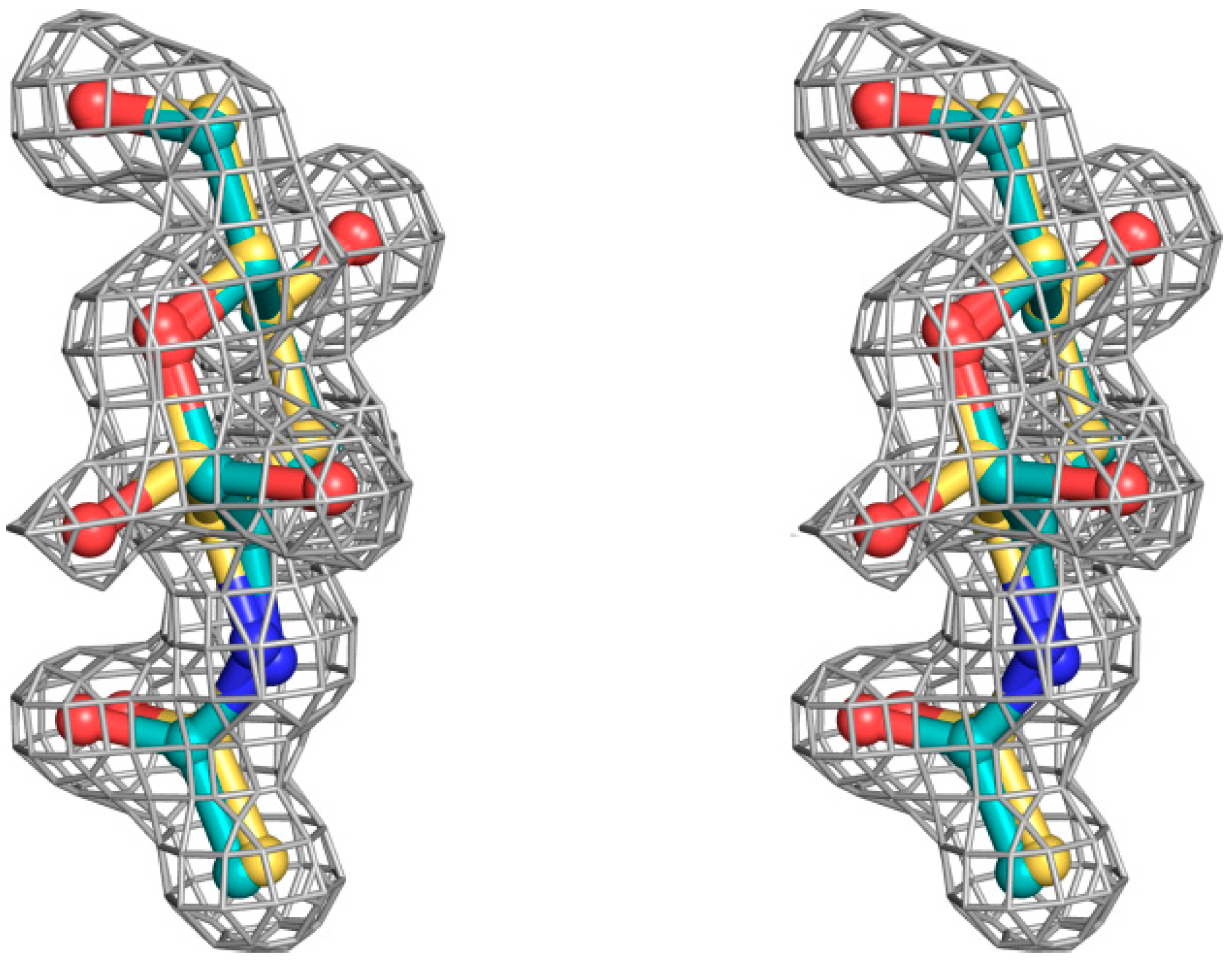
7]. In the SSR2-GlcNAc complex the primary binding site is occupied by water molecules. All atoms of the ligand are well defined in both variant complexes, within the electron density map which has shown that GlcNAc binds to this site with two alternative conformations with respect to the hydroxyl group O1, α and β (
Figure 3) that have equal occupancies. This finding is consistent with the ambiguity in the location of O1 hydroxyl group of both GlcNAc and GalNAc observed upon binding at the secondary binding site of SRL [
7]. The binding of GlcNAc is almost identical in both molecules of the non-crystallographic dimer of both variant structures and thus we will only discuss the binding of this ligand in mol A. There are not any significant conformations upon binding of the ligand in each of the two variants. The rms displacement values for all atoms upon superposition of the SSR1-GlcNAc and the SSR2-GlcNAc structures onto the SRL-GlcNAc structure are 0.5 Å and 0.4 Å, respectively.
Figure 3.
Stereoview of sigmaA 2|Fo|–|Fc| electron density map, contoured at the 1.0 σ level, for the N-acetyl β-d-glucosamine and N-acetyl α-d-glucosamine molecules bound at the secondary SSR1 carbohydrate-binding site before the incorporation of the carbohydrate ligand model.
Figure 3.
Stereoview of sigmaA 2|Fo|–|Fc| electron density map, contoured at the 1.0 σ level, for the N-acetyl β-d-glucosamine and N-acetyl α-d-glucosamine molecules bound at the secondary SSR1 carbohydrate-binding site before the incorporation of the carbohydrate ligand model.
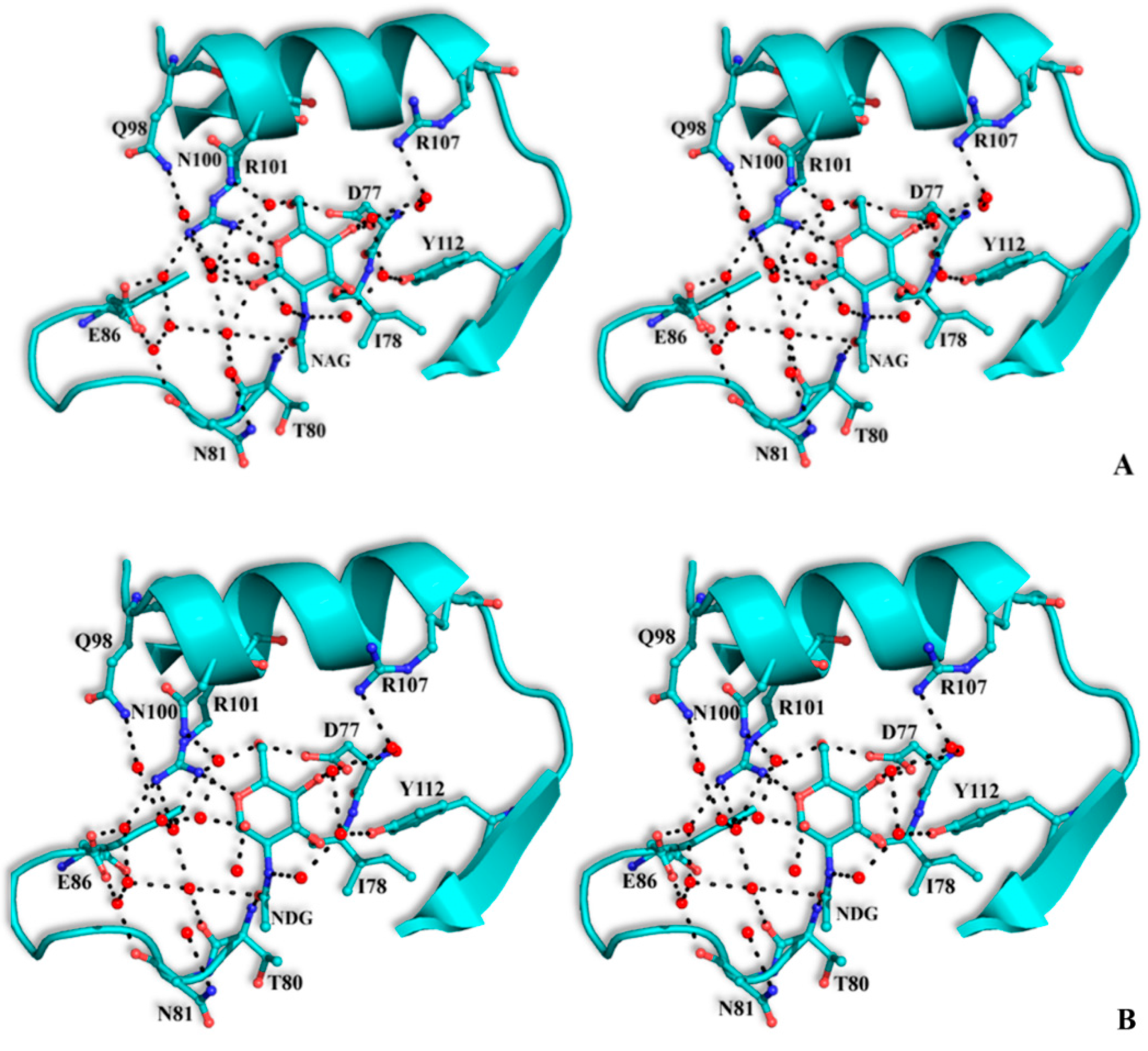
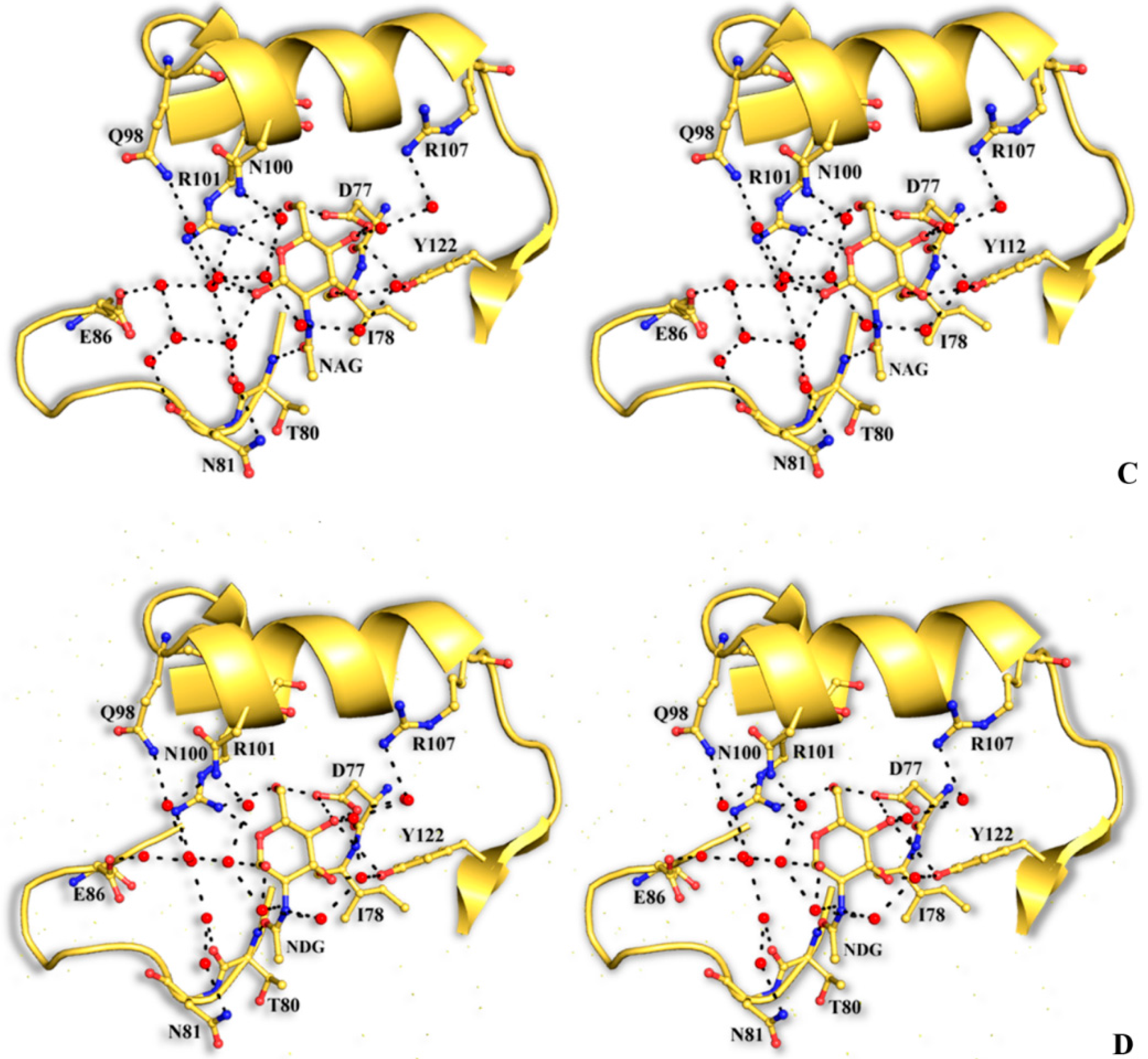
The structural mode of binding of GlcNAc does not differ significantly in the variants and the SRL structure. Briefly, GlcNAc is involved in hydrogen bond interactions with residues Asp77, Ile78, Thr80, Arg101, and Tyr112 (
Table 6,
Figure 4). All hydroxyl groups of GlcNAc except hydroxyl group O1, are involved in hydrogen bond interactions with protein residues, a fact that offers an explanation for the alternative conformations of this group. In both alternative conformations, the hydroxyl group O1, in the ligand complexes, is involved in hydrogen bond interactions only with water molecules. The existence of alternative conformations as well as the lack of protein interactions of hydroxyl group O1 is in agreement with the fact that this group is used to link GlcNAc with other sugar molecules in polysaccharides such as Neu5Acα2-3GalNAcα-Sp8 (G#237), a sialylated-Tn antigen with highest affinity of RFU (
Table 1). GlcNAc atoms and 7 SRL residues participate in 19 (mol A) and 20 (mol B) van der Waals interactions at the secondary binding site.
Table 6.
Potential hydrogen bonds of GlcNAc with SRL (data from [
7]) and SRL variants in the crystal. In the SSR1 and SSR2 complexes the distance for the α and β conformations of GlcNAc is shown.
Table 6.
Potential hydrogen bonds of GlcNAc with SRL (data from [7]) and SRL variants in the crystal. In the SSR1 and SSR2 complexes the distance for the α and β conformations of GlcNAc is shown.
| GlcNAc Atom | Protein Atom | Distance (Å) |
|---|
| SRL | SSR1 | SSR2 |
|---|
| O1 | Water | 2.8 | 3.2/2.9 | 2.7/2.8 |
| O1 | Water | 2.8 | 2.6/2.7 | 3.2/2.7 |
| O1 | Water | - | 3.3/- | 3.1/- |
| O3 | Ile78 O | 2.7 | 2.6/2.6 | 2.6/2.6 |
| O3 | Tyr112 Oη | 2.6 | 2.8/2.6 | 2.7/2.6 |
| O4 | Asp77 Oδ1 | 2.6 | 2.4/2.4 | 2.6/2.4 |
| O4 | Asp77 Oδ2 | | 3.3/3.3 | -/3.3 |
| O4 | Tyr112 Oη | 3.3 | 3.2/3.2 | 3.3/3.3 |
| O4 | Water | 2.6 | 2.7/2.7 | 2.5/2.7 |
| O5 | Arg101 Nη1 | 2.9 | 3.0/3.0 | 3.0/3.0 |
| O6 | Asp77 Oδ2 | 2.7 | 2.7/2.7 | 2.7/2.7 |
| O6 | Arg101 Nη1 | 2.9 | 3.0/2.9 | 3.0/2.9 |
| O7 | Thr80 N | 2.9 | 2.7/2.7 | 2.7/2.7 |
| O7 | Thr80 Oγ1 | 2.9 | 3.2/3.0 | 3.2/2.9 |
| O7 | Water | - | 3.1/3.3 | 3.2/- |
| N2 | Water | 2.9 | 2.9/3.0 | 2.8/2.9 |
Figure 4.
Stereoview of the carbohydrate interactions with the residues of SRL variants. SSR1 with N-acetyl β-d-glucosamine (A) and N-acetyl α-d-glucosamine (B); SSR2 with N-acetyl β-d-glucosamine (C) and N-acetyl α-d-glucosamine (D). The side-chains of protein residues are shown as ball-and-stick models. Bound water molecules are shown as blue spheres. Hydrogen bond interactions are represented by broken lines.
Figure 4.
Stereoview of the carbohydrate interactions with the residues of SRL variants. SSR1 with N-acetyl β-d-glucosamine (A) and N-acetyl α-d-glucosamine (B); SSR2 with N-acetyl β-d-glucosamine (C) and N-acetyl α-d-glucosamine (D). The side-chains of protein residues are shown as ball-and-stick models. Bound water molecules are shown as blue spheres. Hydrogen bond interactions are represented by broken lines.
Superposition of the ABL-T antigen (Galβ1–3GalNAc-α-
O-Ser) complex structure (PDB entry: 1Y2X [
18]) onto the SSR1 and SSR2 structures reveals that the GalNAc moiety of the T glycan structure can be accommodated in the SRR1 or SSR2 primary binding site without any steric conflicts by displacing six or seven water molecules, respectively. All SSR1 and SSR2 residues in this site adopt conformations very close to their structural equivalents in ABL favoring potential hydrogen bond interactions with the hydroxyl groups O2, O3, and O4 of the galactose moiety analogous to those observed in the ABL-T-antigen complex [
18]. The serine moiety in SSR1, like in the ABL complex, could form van der Waals interaction with His70 and Arg105 but not with Tyr72 (the structural equivalent of Tyr74 in ABL). The shape complementarity between the surfaces of T-antigen and SSR1 and SSR2 in the primary binding site is 0.776 and 0.772 respectively (for SRL is 0.792 [
7]). Therefore, all structural data show that both SSR1 and SSR2 can bind both the TF and the Tn antigen like SRL in disagreement with carbohydrate binding data.
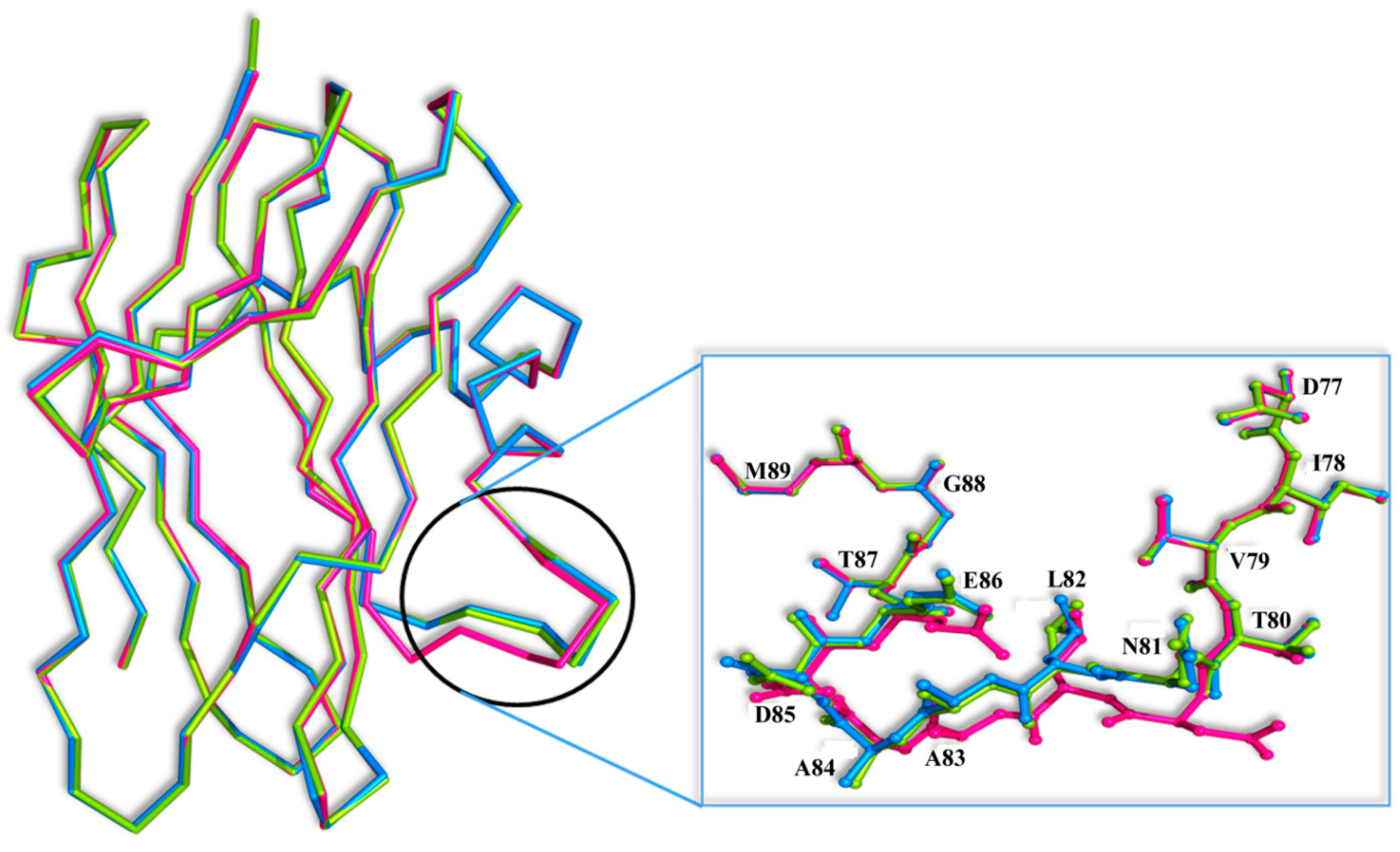
Superposition of the SRL-GalNAc complex [
7] onto the SSR1 and SSR2 structures reveals that GalNAc can easily bind at both the primary and secondary binding site by displacing five and three water molecules, respectively. However, there is a significant conformational change at the secondary binding site of the loop composed by residues 81–86 between the three structures (
Figure 5). The most profound conformational difference is on SSR1 and especially on the side chain of Asn81 which moves away by 5 Å from its position in the SRL structure towards the secondary carbohydrate binding site. At this position the side chain atoms of Asn81 are not at a hydrogen bonding distance from the
N-acetyl group of GalNAc. However, they are close enough (3.5 Å) to assume that upon binding they will make small shifts and they will be able to form hydrogen bond interactions with the ligand. The adoption of this conformation of Asn81 in SSR1 is made possible by a shift of the entire loop encompassing residues 81–86 by 3.0 Å on average, from their positions in the SRL structure. This shift is less profound in the SSR2 structure (1.0 Å on average) and does not lead to a change in the conformation of the side chain of Asn81 which maintains a similar conformation to that of the SRL structure. It is not clear whether this conformational change is the result of the mutated residues of SSR1. Examining the hydrogen bonding pattern of the mutated residues in the two variants it seems that mutations N14D, E113Q and E123Q (common in the two variants) do not alter the hydrogen bond and van der Waals interactions to neighboring protein residues. Similarly mutations T1V and T34S (SSR2) do not cause any change in the polar or non-polar interactions of these two residues in comparison to those observed in the SRL structure.
Figure 5.
Superposition of the Cα traces of the SRL (magenta), SSR1 (blue) and SSR2 (green) monomers. Inset: residues 81–86 are shown as ball-and-stick models.
Figure 5.
Superposition of the Cα traces of the SRL (magenta), SSR1 (blue) and SSR2 (green) monomers. Inset: residues 81–86 are shown as ball-and-stick models.