A Halogen-Containing Stilbene Derivative from the Leaves of Cajanus cajan that Induces Osteogenic Differentiation of Human Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Elucidation of Compound 1
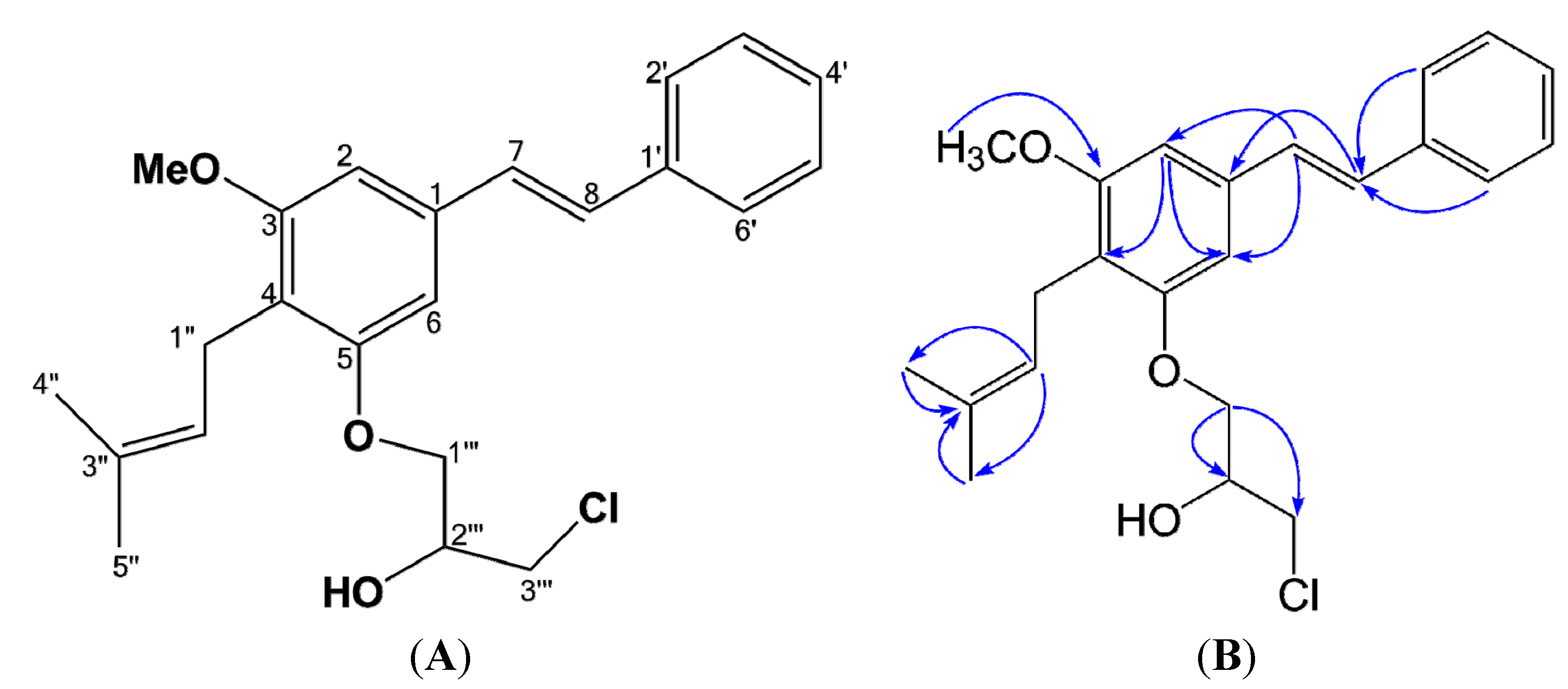
| Position | Cajanstilbene H (1) | ||
|---|---|---|---|
| δC a, type | δH b (J in Hz) | HMBC c | |
| 1 | 136.4 s | 7, 8 | |
| 2 | 103.0 d | 6.68 s | 6, 7, 8 |
| 3 | 156.8 s | 6 | |
| 4 | 118.3 s | 2, 6 | |
| 5 | 158.2 s | 2, OMe | |
| 6 | 102.9 d | 6.71 s | 2, 7, 8 |
| 7 | 128.8 d | 7.04 s | 2, 6, 2′, 3′, 5′, 6′ |
| 8 | 128.8 d | 7.04 s | 2, 6, 2′, 3′, 5′, 6′ |
| 1′ | 137.2 s | 7, 8, 3′, 5′ | |
| 2′, 6′ | 126.6 d | 7.50 d (7.5) | 7, 8, 2′, 4′, 6′ |
| 3′, 5′ | 128.7 d | 7.35 t (7.5) | 2′, 3′, 5′, 6′ |
| 4′ | 127.7 d | 7.24 m | 2′, 6′ |
| 1″ | 22.4 t | 3.34 d (6.8) | |
| 2″ | 123.2 d | 5.11 t (6.8) | |
| 3″ | 131.5 s | 4′′, 5′′ | |
| 4″ | 25.7 q | 1.66 s | 2′′ |
| 5″ | 17.9 q | 1.79 s | 2′′ |
| 5-OMe | 55.8 q | 3.86 s | |
| 3-OH | |||
| 1′′′ | 68.9 t | 4.14 m | 3′′′ |
| 2′′′ | 70.0 t | 4.21 m | 1′′′, 3′′′ |
| 3′′′ | 45.8 t | 3.75 m | 1′′′ |
| 2′′′-OH | 2.70 s | ||
2.2. Osteogenic Differentiation of hMSC
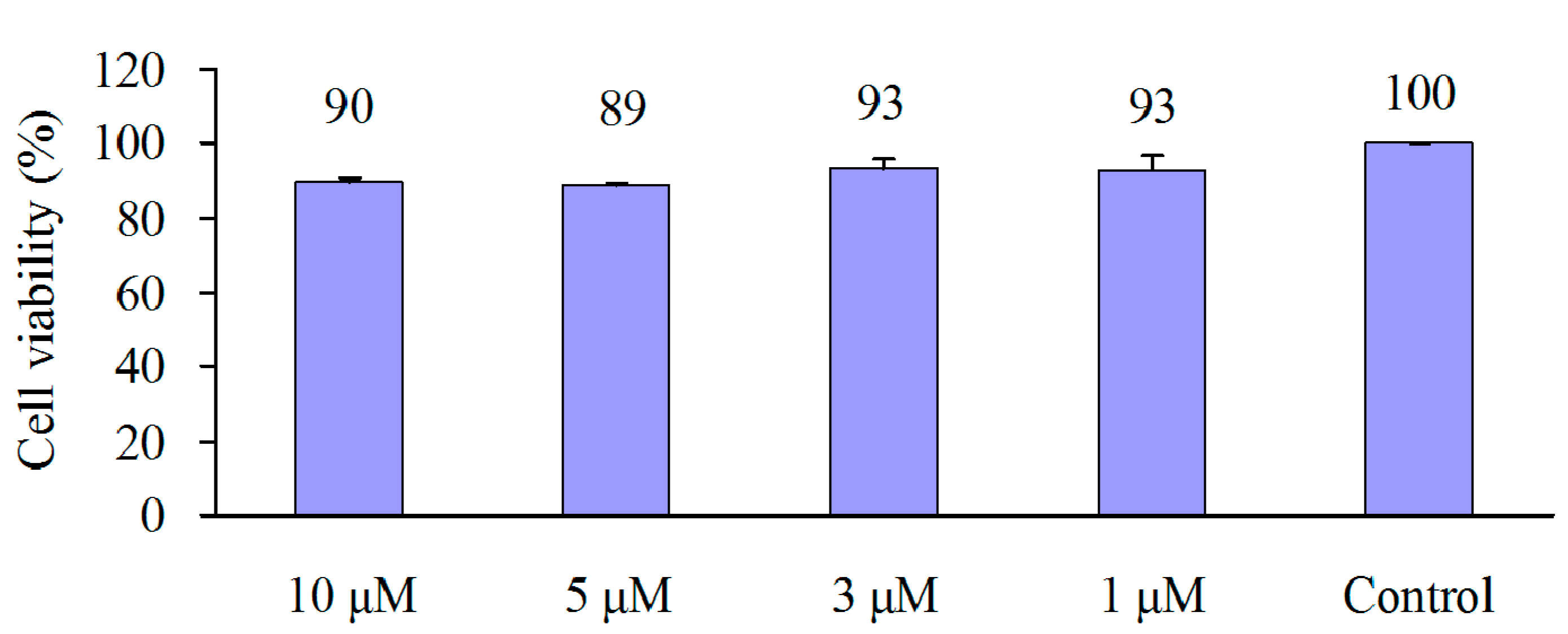

2.3. Cytotoxicity of 1 in Six Human Cancer Cell Lines
| NCI-H460 | PC-3 | MCF-7 | HeLa | HCT-15 | KB-V1 | |
|---|---|---|---|---|---|---|
| 1 | 21.47 ± 3.14 | 25.83 ± 3.64 | 21.42 ± 3.31 | 25.85 ± 2.58 | 24.81 ± 5.20 | 22.29 ± 6.39 |
| Paclitaxel | 0.036 ± 0.004 | 0.048 ± 0.013 | 0.032 ± 0.004 | 0.072 ± 0.018 | 0.021 ± 0.003 | 0.225 ± 0.059 |
3. Experimental Section
3.1. Plant Material and Extraction
3.2. Proliferation and Osteoblast Differentiation in hMSC
3.3. Proliferation in Tumor Cell Lines
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duker-Eshun, G.; Jaroszewski, J.W.; Asomaning, W.A.; Oppong-Boachie, F.; Brøgger Christensen, S. Antiplasmodial constituents of Cajanus cajan. Phytother. Res. 2004, 18, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Ashidi, J.S.; Houghton, P.J.; Hylands, P.J.; Efferth, T. Ethnobotanical survey and cytotoxicity testing of plants of South-western Nigeria used to treat cancer, with isolation of cytotoxic constituents from Cajanus cajan Millsp. leaves. J. Ethnopharmacol. 2010, 128, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Liu, X.; Zu, Y.; Fu, Y.; Zhang, S.; Yao, L.; Efferth, T. Cajanol, a novel anticancer agent from Pigeonpea [Cajanus cajan (L.) Millsp.] roots, induces apoptosis in human breast cancer cells through a ROS-mediated mitochondrial pathway. Chem. Biol. Interact. 2010, 188, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.F.; Sun, L.; Si, J.Y.; Chen, D.H. Hypocholesterolemic effect of stilbenes containing extract-fraction from Cajanus cajan L. on diet-induced hypercholesterolemia in mice. Phytomedicine 2008, 15, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Yang, J.; Chen, D.H.; Sun, L. Effects of the stilbene extracts from Cajanus cajan L. on ovariectomy-induced bone loss in rats. Yao Xue Xue Bao 2007, 42, 562–565. [Google Scholar] [PubMed]
- Zheng, Y.Y.; Yang, J.; Chen, D.H.; Sun, L. Effects of the extracts of Cajanus cajan L. on cell functions in human osteoblast-like TE85 cells and the derivation of osteoclast-like cells. Yao Xue Xue Bao 2007, 42, 386–391. [Google Scholar] [PubMed]
- Zhang, J.C.; Liu, C.L.; Sun, J.; Liu, D.D.; Wang, P. Effects of water extract of Cajanus cajan leaves on the osteogenic and adipogenic differentiation of mouse primary bone marrow stromal cells and the adipocytic trans-differentiation of mouse primary osteoblasts. Pharm. Biol. 2010, 48, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Bhanumati, S.; Chhabra, S.C.; Gupta, S.R.; Krishnamoorthy, V. Cajaflavanone: A new flavanone from Cajanus cajan. Phytochemistry 1978, 17. [Google Scholar] [CrossRef]
- Cooksey, C.J.; Dahiya, J.S.; Garratt, P.J.; Strange, R.N. Two novel stilbene-2-carboxylic acid phytoalexins from Cajanus cajan. Phytochemistry 1982, 21, 2935–2938. [Google Scholar] [CrossRef]
- Chen, D.H.; Li, H.Y.; Lin, H. Studies on the chemical constituents of Cajanus cajan Millsp. Zhong Cao Yao 1985, 16, 434–439. [Google Scholar]
- Lin, L.; Xie, N.; Cheng, Z.H. Flavonoids from Cajanus cajan L. J. Asian. Nat. Prod. Res. 1999, 30, 21–23. [Google Scholar]
- Qiu, S.X.; Shen, X.L. A natural stilbenoidal drug with effects of hypoglycemia and hypolipidemia. China Patent 2008101990126, 9 November 2011. [Google Scholar]
- Zhang, N.L.; Zhu, Y.H.; Huang, R.M.; Fu, M.Q.; Su, Z.W.; Cai, J.Z.; Hu, Y.J.; Qiu, S.X. Two new stilbenoids from Cajanus cajan. Z. Naturforsch. B 2012, 67, 1314–1318. [Google Scholar] [CrossRef]
- Li, X.L.; Zhao, B.X.; Huang, X.J.; Zhang, D.M.; Jiang, R.W.; Li, Y.J.; Jian, Y.Q.; Wang, Y.; Li, Y.L.; Ye, W.C. (+)- and (−)-Cajanusine, a pair of new enantiomeric stilbene dimers with a new skeleton from the leaves of Cajanus cajan. Org. Lett. 2014, 16, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.F.; Wang, L.; Yang, R.Y.; Tian, R.H.; Hu, Y.J.; Shen, X.L. Effects of hydrophobic fraction of Cajanus cajan (L.) Millsp. on bone density and blood lipid level in obese and diabetic mice. Chin. Pharmacol. Bull. 2013, 29, 961–965. [Google Scholar]
- Dubois, M.; Tarres, A.; Goldmann, T.; Empl, A.M.; Donaubauer, A.; Seefelder, W. Comparison of indirect and direct quantification of esters of monochloropropanediol in vegetable oil. J. Chromatogr. A 2012, 1236, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W.; Xiao, M.X.; Li, Y.R.; Shen, X.L.; Lu, Y.Y.; Liu, K.L.; Li, Z.H.; Hu, Y.J. Determination of longistylin A and longistylin C in Cajanus cajan. J. Chin. Mater. Med. 2011, 36, 2680–2683. [Google Scholar]
- Vilmann, H. The in vivo staining of bone with alizarin red S. J. Anat. 1969, 105, 533–545. [Google Scholar] [PubMed]
- Foster, L.J.; Zeemann, P.A.; Li, C.; Mann, M.; Jensen, O.N.; Kassem, M. Differential expression profiling of membrane proteins by quantitative proteomics in a human mesenchymal stemcell line undergoing osteoblast differentiation. Stem Cells 2005, 23, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Halbhuber, K.J.; Krieg, R.; Geidel, O.; Dietz, W. A modified Ce/Mg-BCIP-NBT formazan/indigoblue technique for demonstration of non-specific alkaline phosphatase activity. Cell. Mol. Biol. 2004, 50, 507–514. [Google Scholar]
- Vistica, D.T.; Skehan, P.; Scudiero, D.; Monks, A.; Pittman, A.; Boyd, M.R. Tetrazolium-based assays for cellular viability: A critical examination of selected parameters affecting formazan production. Cancer Res. 1991, 51, 2515–2520. [Google Scholar] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.-Z.; Tang, R.; Ye, G.-F.; Qiu, S.-X.; Zhang, N.-L.; Hu, Y.-J.; Shen, X.-L. A Halogen-Containing Stilbene Derivative from the Leaves of Cajanus cajan that Induces Osteogenic Differentiation of Human Mesenchymal Stem Cells. Molecules 2015, 20, 10839-10847. https://doi.org/10.3390/molecules200610839
Cai J-Z, Tang R, Ye G-F, Qiu S-X, Zhang N-L, Hu Y-J, Shen X-L. A Halogen-Containing Stilbene Derivative from the Leaves of Cajanus cajan that Induces Osteogenic Differentiation of Human Mesenchymal Stem Cells. Molecules. 2015; 20(6):10839-10847. https://doi.org/10.3390/molecules200610839
Chicago/Turabian StyleCai, Jia-Zhong, Rong Tang, Gui-Fu Ye, Sheng-Xiang Qiu, Nen-Ling Zhang, Ying-Jie Hu, and Xiao-Ling Shen. 2015. "A Halogen-Containing Stilbene Derivative from the Leaves of Cajanus cajan that Induces Osteogenic Differentiation of Human Mesenchymal Stem Cells" Molecules 20, no. 6: 10839-10847. https://doi.org/10.3390/molecules200610839
APA StyleCai, J.-Z., Tang, R., Ye, G.-F., Qiu, S.-X., Zhang, N.-L., Hu, Y.-J., & Shen, X.-L. (2015). A Halogen-Containing Stilbene Derivative from the Leaves of Cajanus cajan that Induces Osteogenic Differentiation of Human Mesenchymal Stem Cells. Molecules, 20(6), 10839-10847. https://doi.org/10.3390/molecules200610839






