Kinetics of the Reaction of Pyrogallol Red, a Polyphenolic Dye, with Nitrous Acid: Role of •NO and •NO2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Studies of the PGR-HONO Reaction
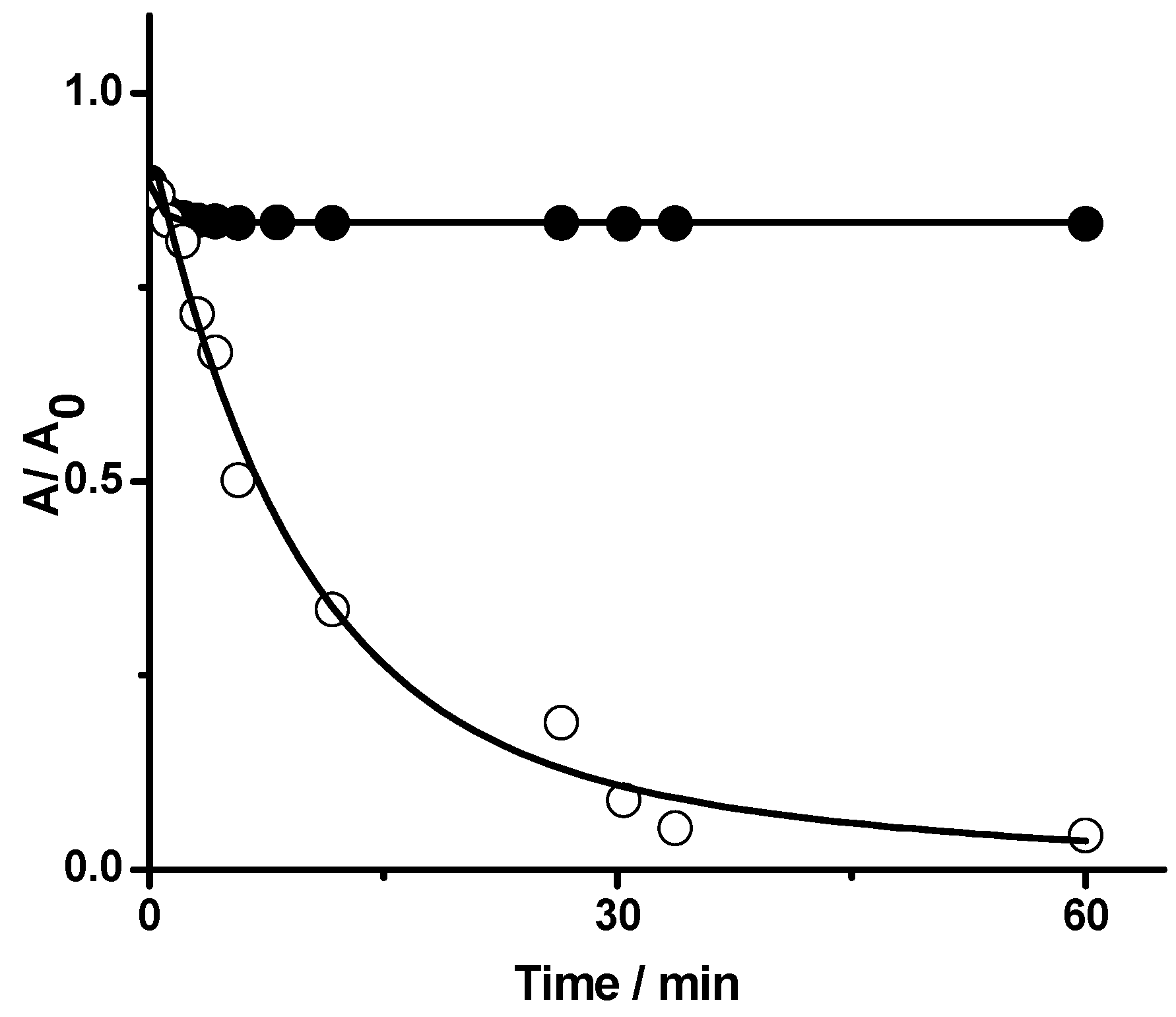
2.1.1. Kinetics of PGR Removal by HONO
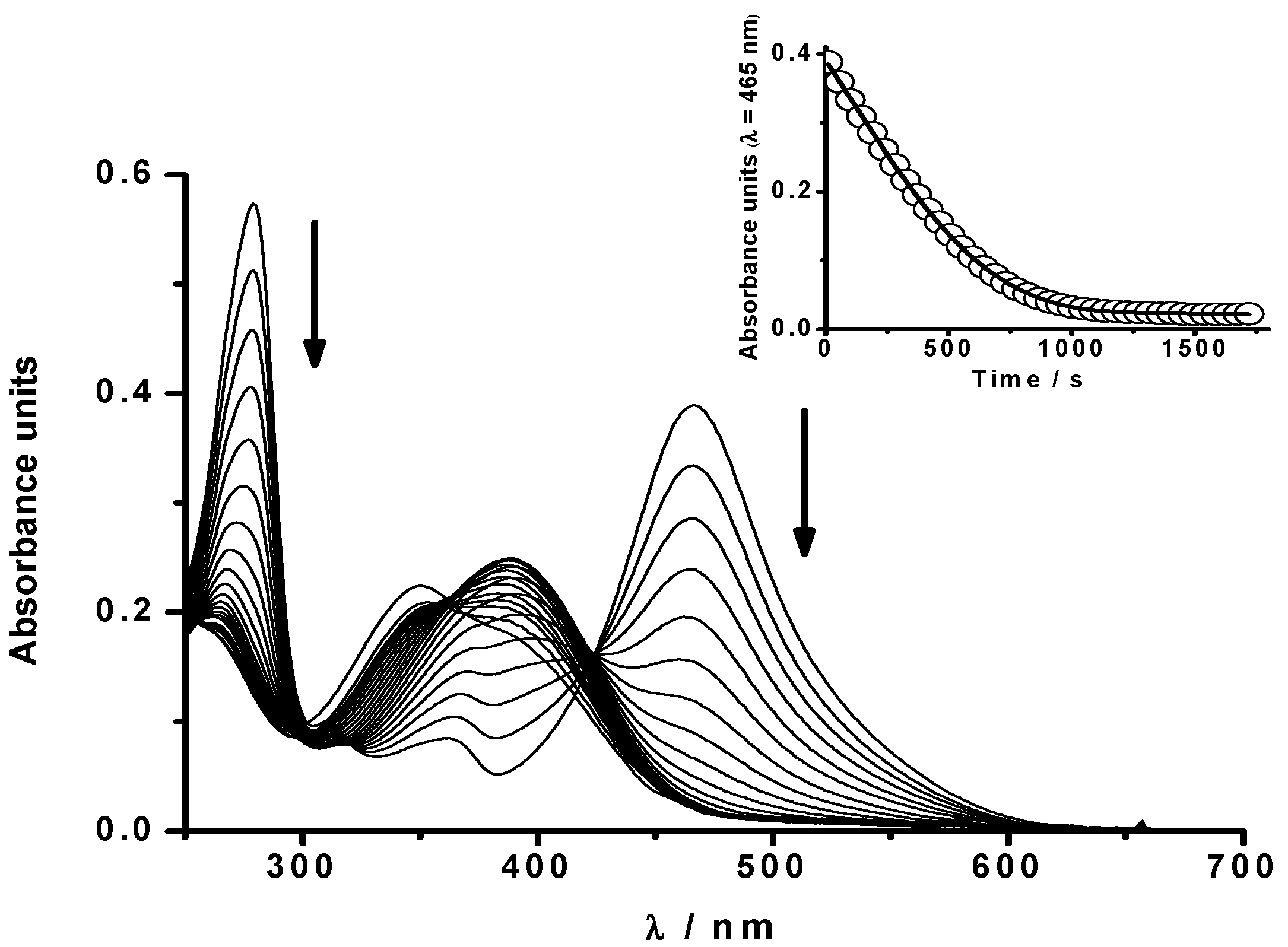
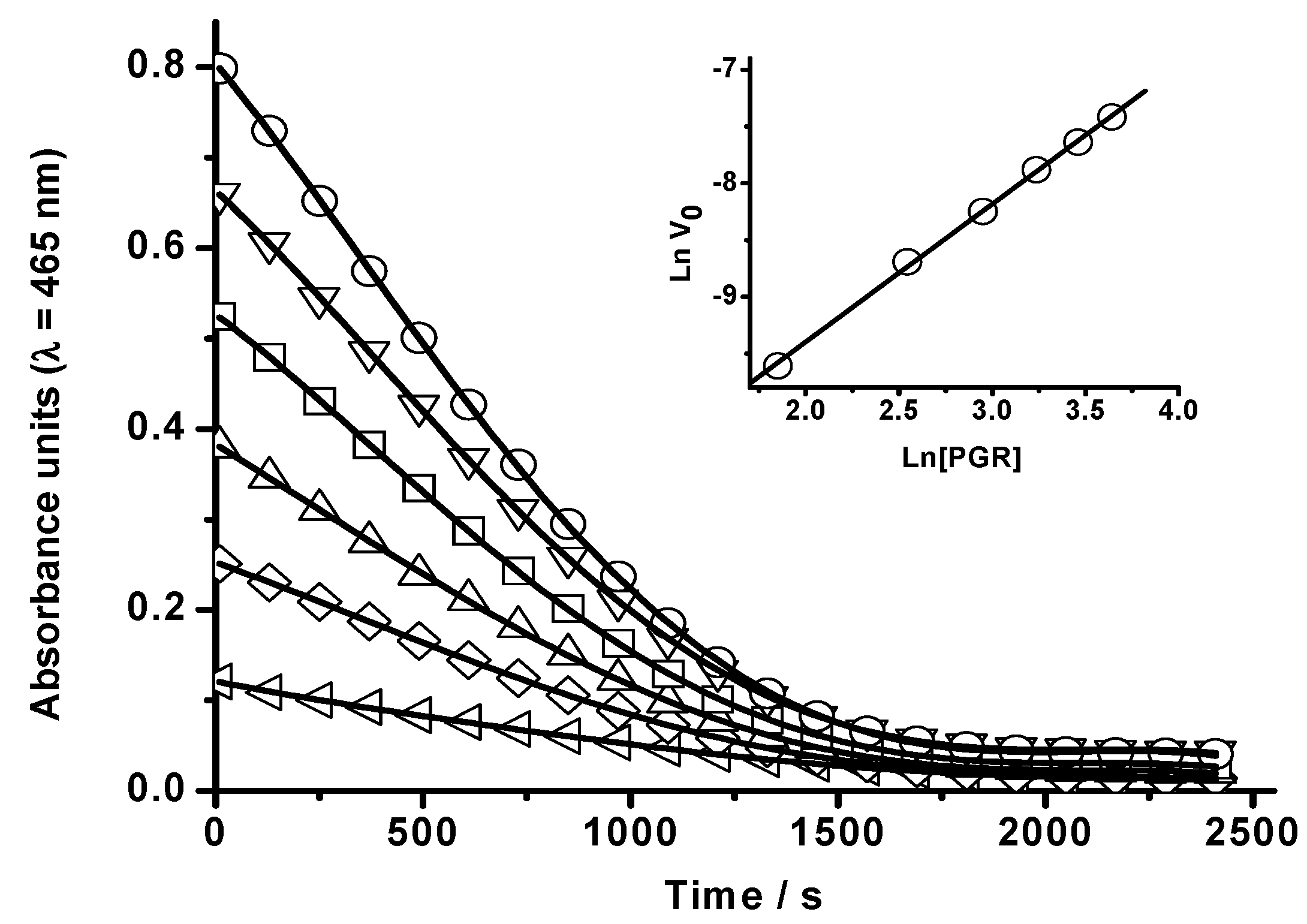
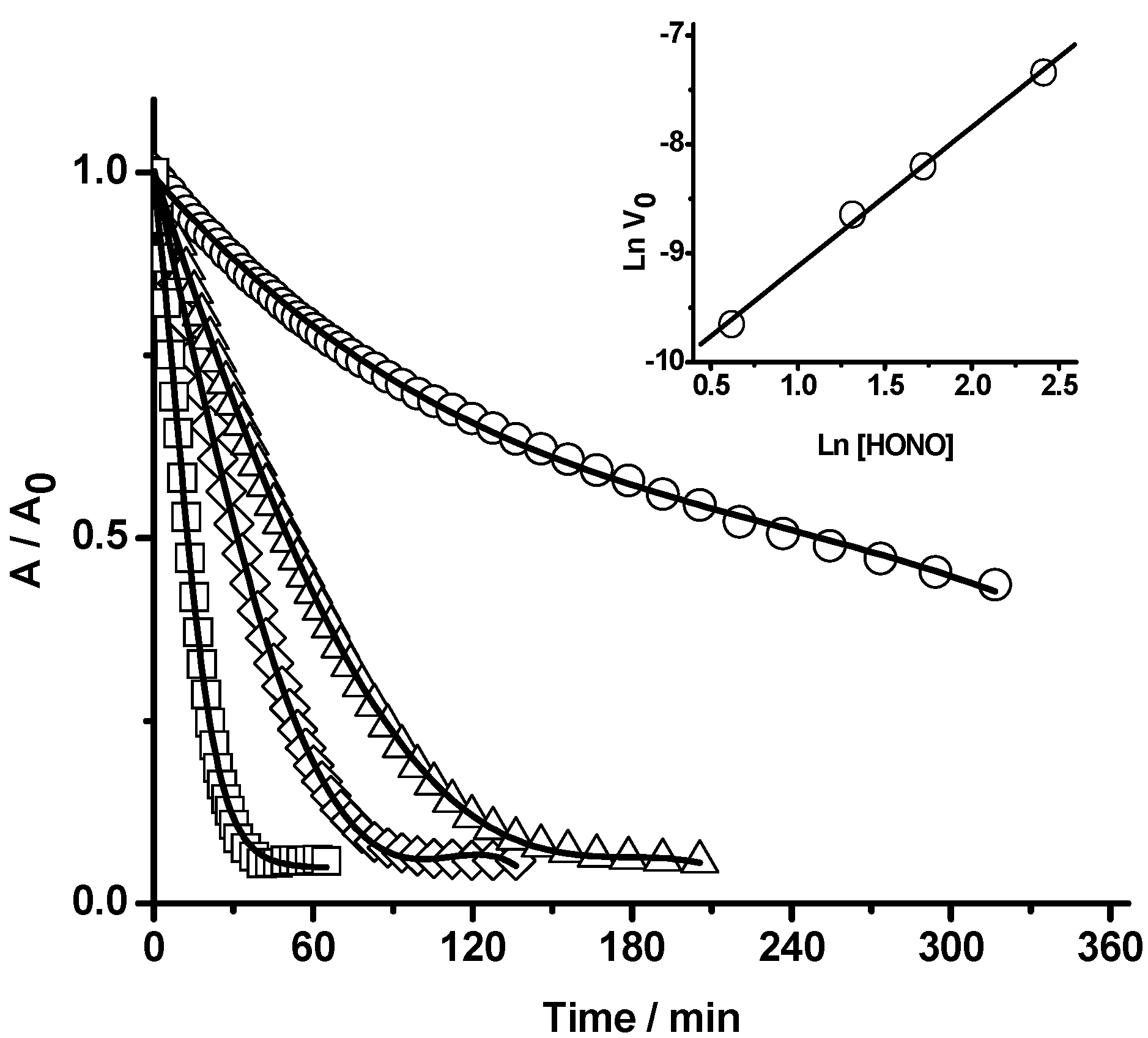
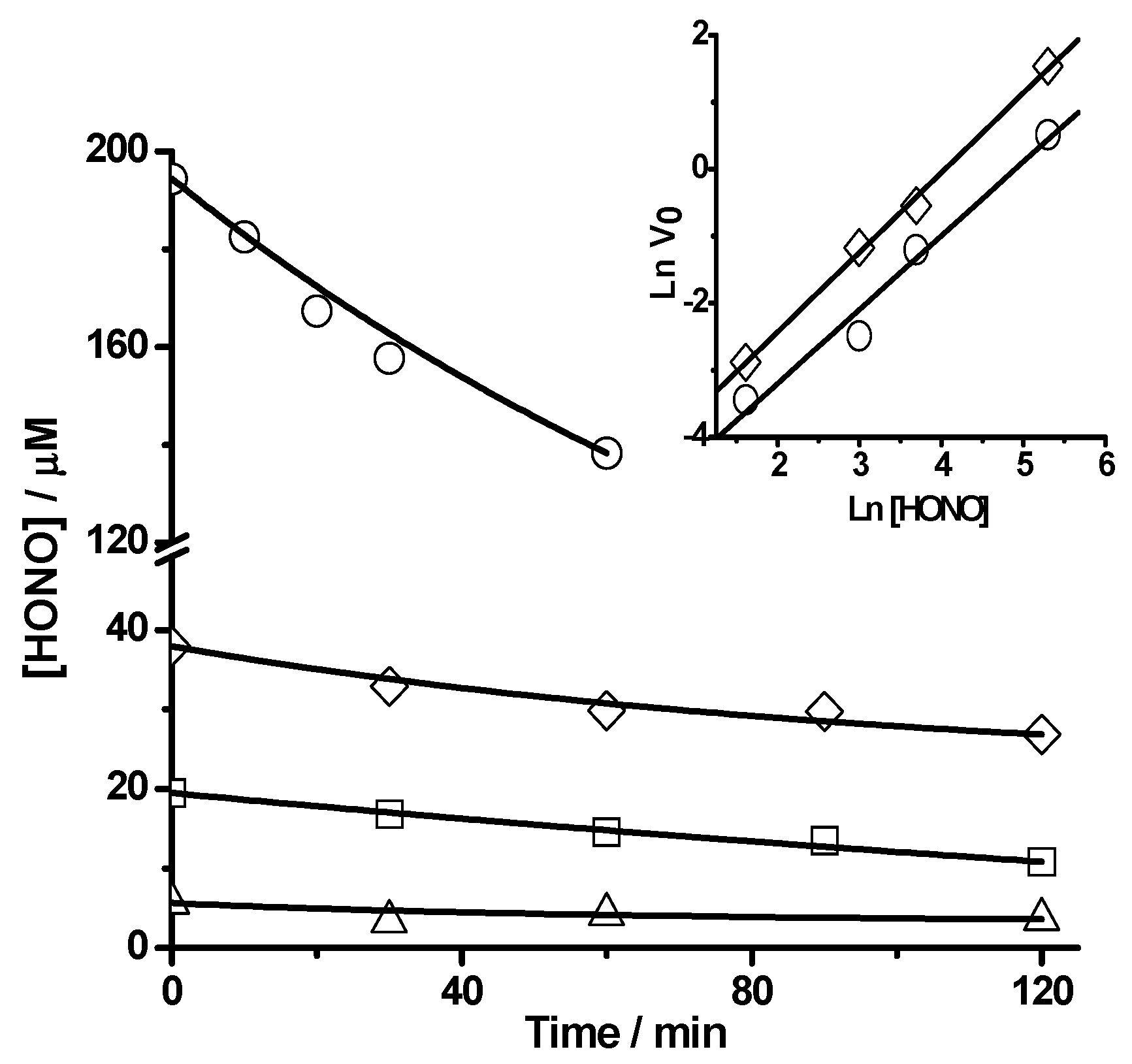
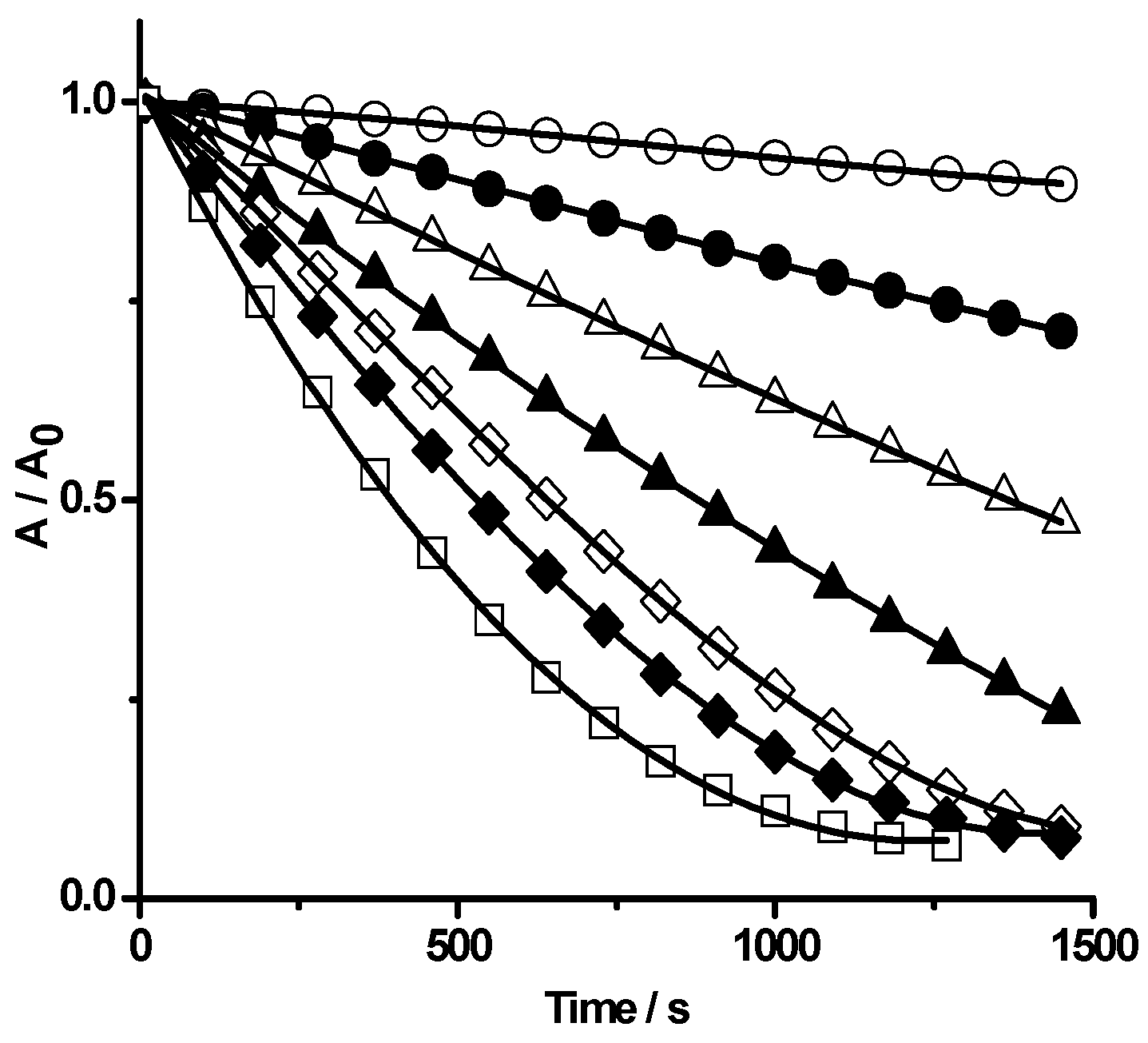
2.1.2 Nitric Oxide (•NO) Formation
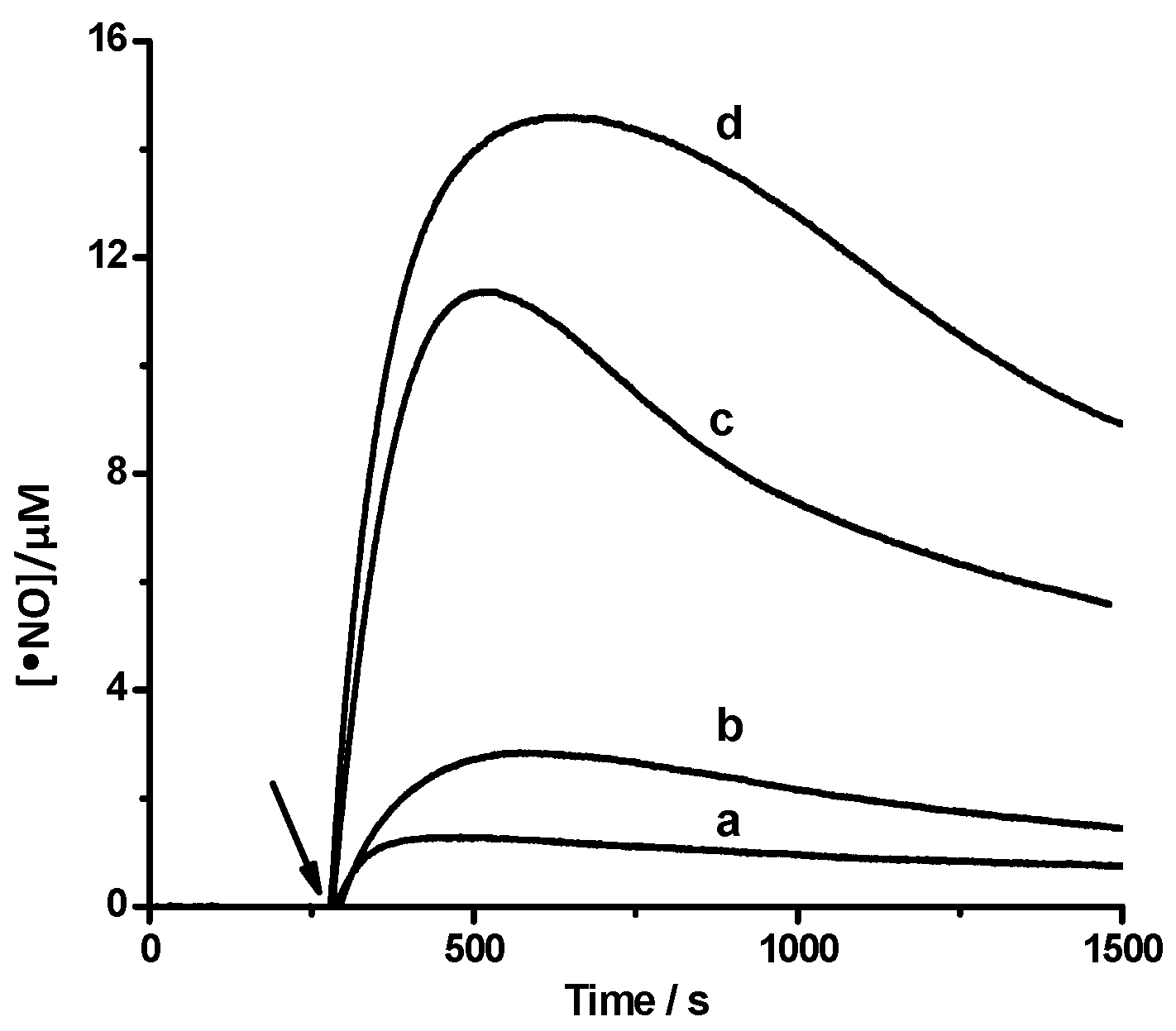
2.1.3. Studies by HPLC-DAD Technique
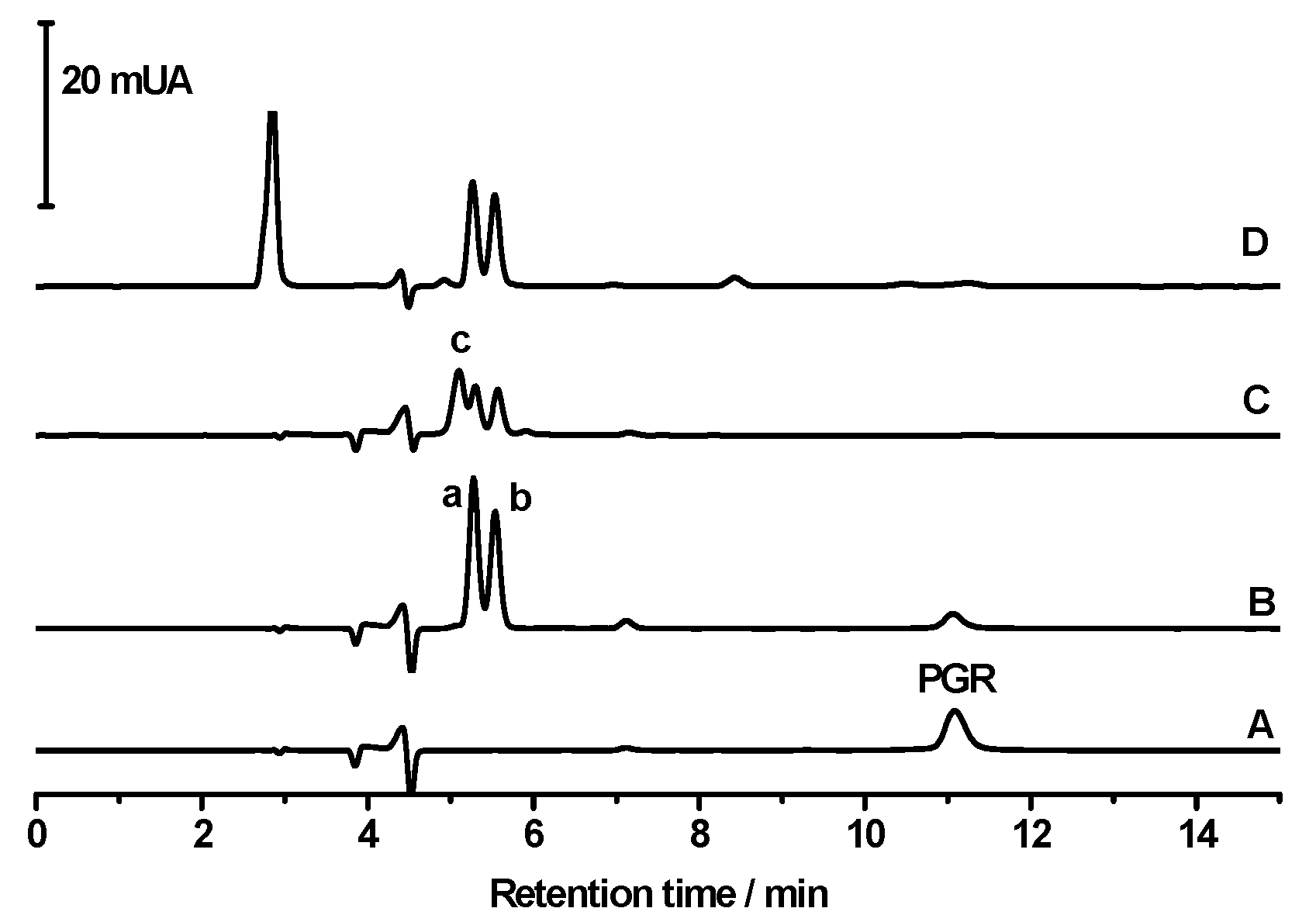
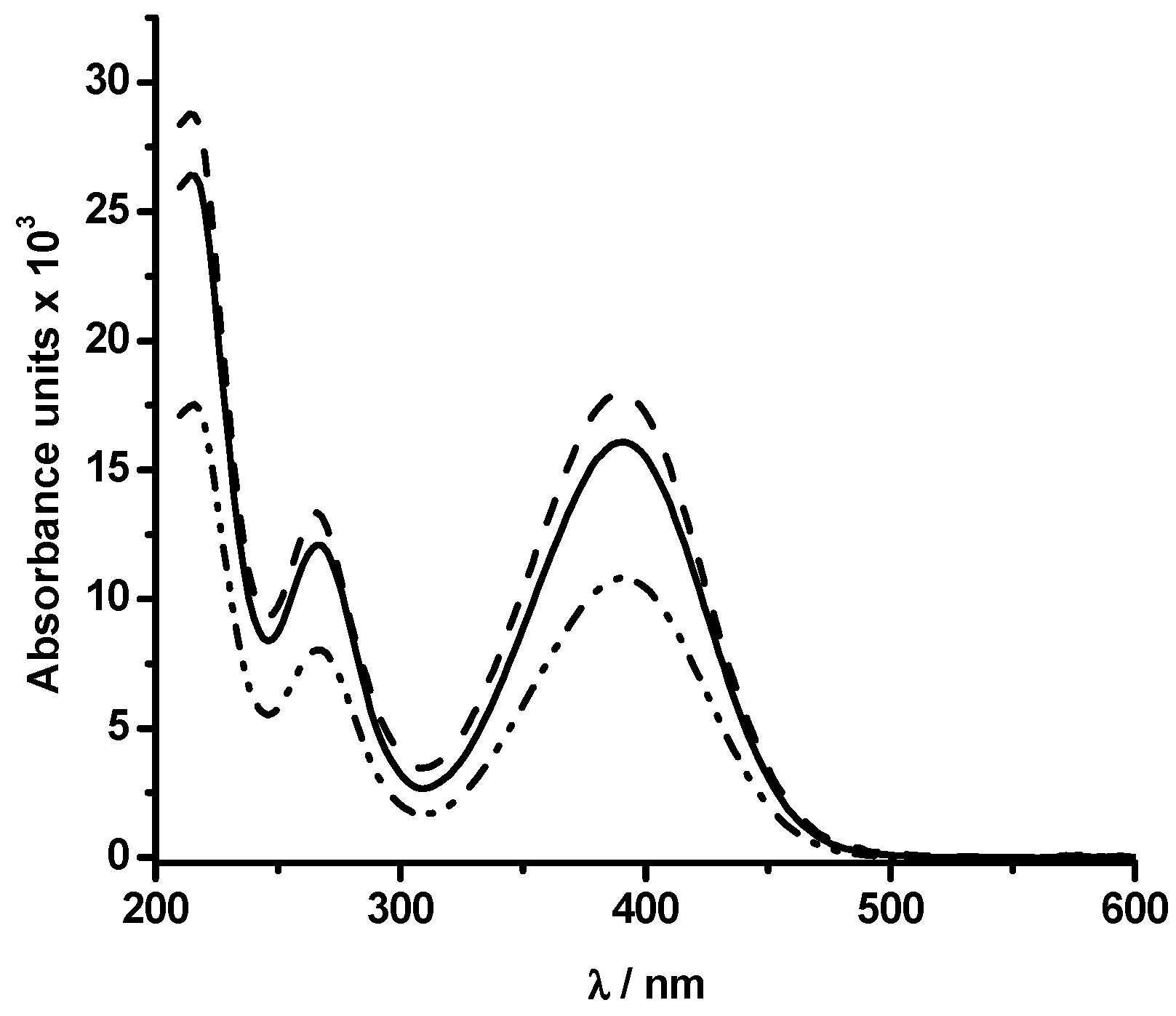
2.2. Reaction Mechanism
Consumption of PGR by Chain Reactions and/or HONO Recycling Processes
3. Experimental Section
3.1. Chemicals and Solutions
3.2. UV-Visible Studies
3.3. Electrochemical Detection of •NO
3.4. High Performance Liquid Chromatography (HPLC) Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- D’Ischia, M.; Napolitano, A.; Manini, P.; Panzella, L. Secondary targets of nitrite-derived reactive nitrogen species: Nitrosation/nitration pathways, antioxidant defense mechanisms and toxicological implications. Chem. Res. Toxicol. 2011, 24, 2071–2092. [Google Scholar] [CrossRef] [PubMed]
- Gago, B.; Lundberg, J.O.; Barbosa, R.M.; Laranjinha, J. Red wine-dependent reduction of nitrite to nitric oxide in the stomach. Free Radic. Biol. Med. 2007, 43, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.S.; Nunes, C.; Pereira, C.; Barbosa, R.M.; Laranjinha, J. A shortcut to wide-ranging biological actions of dietary polyphenols: Modulation of the nitrate-nitrite-nitric oxide pathway in the gut. Food Funct. 2014, 5, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Gillatt, P.N.; Palmer, R.C.; Smith, P.L.; Walters, C.L.; Reed, P.I. Susceptibilities of drugs to nitrosation under simulated gastric conditions. Food Chem. Toxicol. 1985, 23, 849–855. [Google Scholar] [CrossRef]
- Lee, S.Y.; Munerol, B.; Pollard, S.; Youdim, K.A.; Pannala, A.S.; Kuhnle, G.G.; Debnam, E.S.; Rice-Evans, C.; Spencer, J.P. The reaction of flavanols with nitrous acid protects against N-nitrosamine formation and leads to the formation of nitroso derivatives which inhibit cancer cell growth. Free Radic. Biol. Med. 2006, 40, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Peri, L.; Pietraforte, D.; Scorza, G.; Napolitano, A.; Fogliano, V.; Minetti, M. Apples increase nitric oxide production by human saliva at the acidic pH of the stomach: A new biological function for polyphenols with a catechol group? Free Radic. Biol. Med. 2005, 39, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Weitzberg, E.; Lundberg, J.O. Nonenzymatic nitric oxide production in humans. Nitric Oxide 1998, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hirota, S.; Takayama, U. Reaction of apple fruit polyphenol with nitrite under conditions of the gastric lumen: Generation of nitric oxide and formation of nitroso catechins. Food Sci. Technol. Res. 2014, 20, 439–447. [Google Scholar] [CrossRef]
- Pereira, C.; Ferreira, N.; Rocha, B.; Barbosa, R.M.; Laranjinha, J. The redox interplay between nitrite and oxide nitric: From the gut to the brain. Redox Biol. 2013, 1, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Robaszkiewicz, A.; Bartosz, G. Estimation of antioxidant capacity against pathophysiologically relevant oxidants using Pyrogallol Red. Biochem. Biophys. Res. Commun. 2009, 390, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Balavoine, G.G.; Geletii, Y.V. Peroxynitrite scavenging by different antioxidants. Part I: Convenient assay. Nitric Oxide 1999, 3, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Faundez, M.; Rojas, M.; Bohle, P.; Reyes, C.; Letelier, M.E.; Aliaga, M.E.; Speisky, H.; Lissi, E.; Lopez-Alarcon, C. Pyrogallol red oxidation induced by superoxide radicals: application to evaluate redox cycling of nitro compounds. Anal. Biochem. 2011, 419, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Alarcon, C.; Lissi, E. Interaction of pyrogallol red with peroxyl radicals. A basis for a simple methodology for the evaluation of antioxidant capabilities. Free Radic. Res. 2005, 39, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cruz, F.; Cortes, C.; Atala, E.; Bohle, P.; Valenzuela, F.; Olea-Azar, C.; Speisky, H.; Aspee, A.; Lissi, E.; Lopez-Alarcon, C.; et al. Use of pyrogallol red and pyranine as probes to evaluate antioxidant capacities towards hypochlorite. Molecules 2013, 18, 1638–1652. [Google Scholar] [CrossRef] [PubMed]
- Atala, E.; Velasquez, G.; Vergara, C.; Mardones, C.; Reyes, J.; Tapia, R.A.; Quina, F.; Mendes, M.A.; Speisky, H.; Lissi, E.; et al. Mechanism of Pyrogallol Red Oxidation Induced by Free Radicals and Reactive Oxidant Species. A Kinetic and Spectroelectrochemistry Study. J. Phys. Chem. B 2013, 117, 4870–4879. [Google Scholar] [CrossRef] [PubMed]
- Vione, D.; Belmondo, S.; Carnino, L. A kinetic study of phenol nitration and nitrosation with nitrous acid in the dark. Environ. Chem. Lett. 2004, 2, 135–139. [Google Scholar] [CrossRef]
- Augusto, O.; Bonini, M.G.; Amanso, A.M.; Linares, E.; Santos, C.C.X.; De Menezes, S.L. Nitrogen dioxide and carbonate radical anion: Two emerging radicals in biology. Free Radic. Biol. Med. 2002, 32, 841–859. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Moreno, J.J.; Pryor, W.A.; Ischiropoulos, H.; Beckman, J.S. Peroxynitrite, A Cloaked Oxidant Formed by Nitric-Oxide and Superoxide. Chem. Res. Toxicol. 1992, 5, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Bou, R.; Codony, R.; Tres, A.; Decker, E.A.; Guardicila, F. Determination of hydroperoxides in foods and biological samples by the ferrous oxidation-xylenol orange method: A review of the factors that influence the method’s performance. Anal. Biochem. 2008, 377, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Finlayson-Pitts, B.J.; Pitts, J. Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Williams, D.H.L. Nitrosation Reactions and the Chemistry of Nitric Oxide; Elsevier: Amsterdam, The Nertherlands, 2004. [Google Scholar]
- Sample Availability: Samples of PGR are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hugo, E.; Reyes, J.; Montupil, E.; Bridi, R.; Lissi, E.; Denicola, A.; Rubio, M.A.; López-Alarcón, C. Kinetics of the Reaction of Pyrogallol Red, a Polyphenolic Dye, with Nitrous Acid: Role of •NO and •NO2. Molecules 2015, 20, 10582-10593. https://doi.org/10.3390/molecules200610582
Hugo E, Reyes J, Montupil E, Bridi R, Lissi E, Denicola A, Rubio MA, López-Alarcón C. Kinetics of the Reaction of Pyrogallol Red, a Polyphenolic Dye, with Nitrous Acid: Role of •NO and •NO2. Molecules. 2015; 20(6):10582-10593. https://doi.org/10.3390/molecules200610582
Chicago/Turabian StyleHugo, Estefania, Jael Reyes, Elisa Montupil, Raquel Bridi, Eduardo Lissi, Ana Denicola, María Angélica Rubio, and Camilo López-Alarcón. 2015. "Kinetics of the Reaction of Pyrogallol Red, a Polyphenolic Dye, with Nitrous Acid: Role of •NO and •NO2" Molecules 20, no. 6: 10582-10593. https://doi.org/10.3390/molecules200610582
APA StyleHugo, E., Reyes, J., Montupil, E., Bridi, R., Lissi, E., Denicola, A., Rubio, M. A., & López-Alarcón, C. (2015). Kinetics of the Reaction of Pyrogallol Red, a Polyphenolic Dye, with Nitrous Acid: Role of •NO and •NO2. Molecules, 20(6), 10582-10593. https://doi.org/10.3390/molecules200610582






