γ-Tocotrienol and 6-Gingerol in Combination Synergistically Induce Cytotoxicity and Apoptosis in HT-29 and SW837 Human Colorectal Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
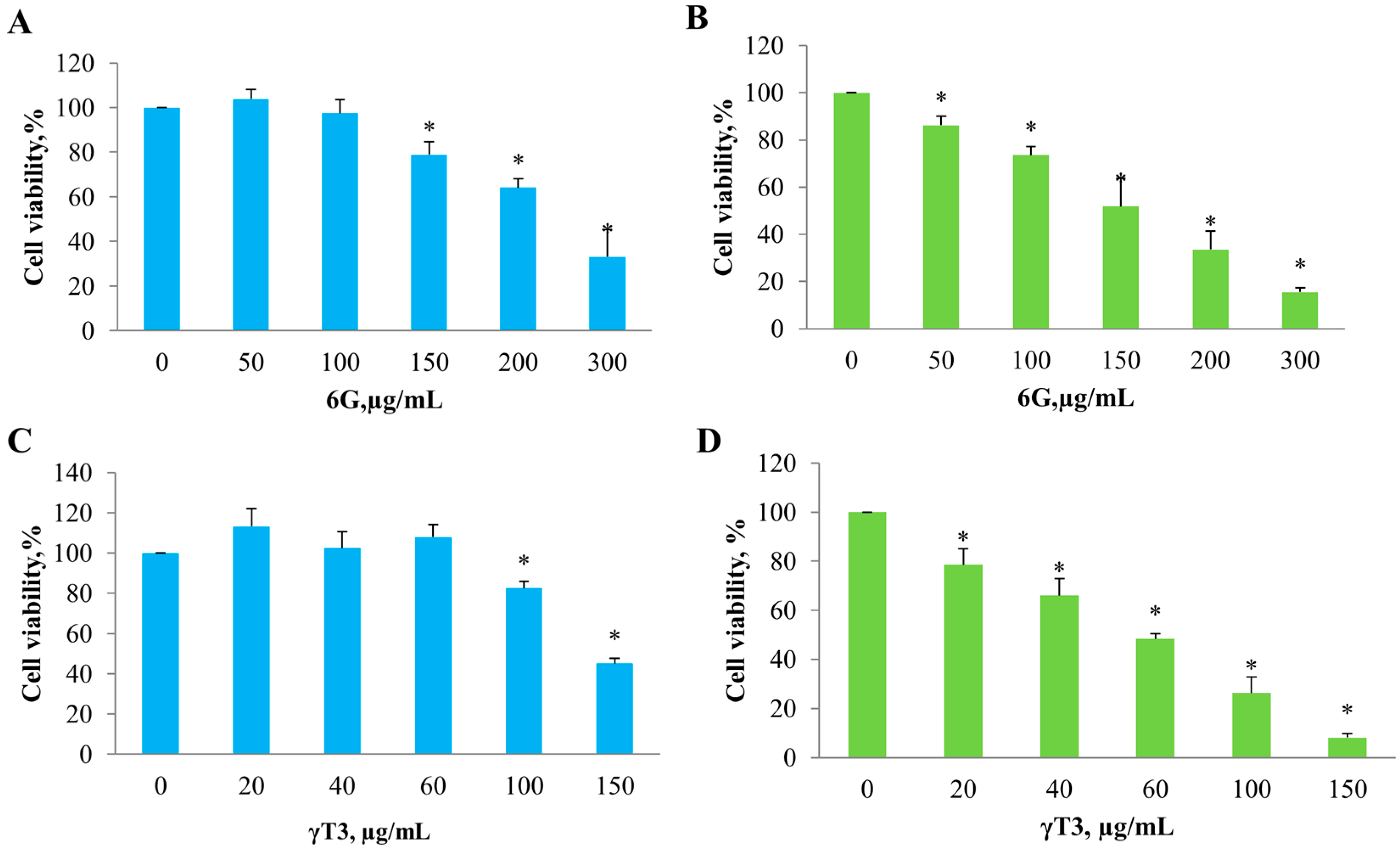
2.1. Effect of Individual 6G and γT3 and in Combination on Cell Viability
| Cell Lines | Bioactive Compound | IC50 Value (µg/mL) | * Cell Viability, % |
|---|---|---|---|
| HT-29 | 6-Gingerol (6G) | 254.0 ± 42.0 | 40.1 ± 18.0 |
| γ-Tocotrienol (γ-T3) | 138.9 ± 8.7 | 41.1 ± 8.4 | |
| SW837 | 6-Gingerol (6G) | 158.4 ± 20.5 | 13.4 ± 2.8 |
| γ-Tocotrienol (γ-T3) | 57.7 ± 5.8 | 8.0 ± 1.9 |
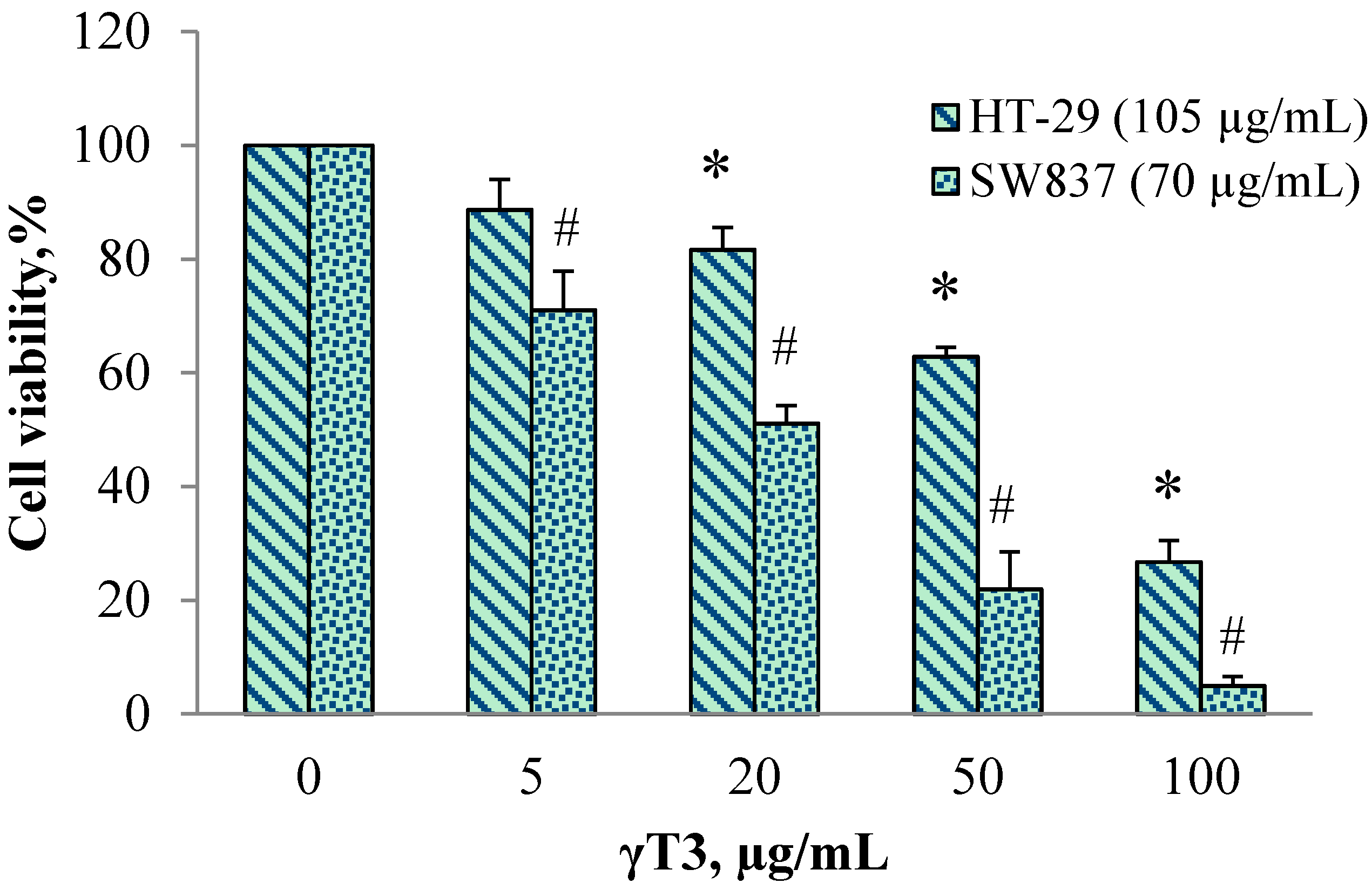
| Cell Lines | γT3 (µg/mL) | Combination Index, C.I | |||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 20 | 50 | 100 | IC50 Value | ||
| HT-29 (105 µg/mL 6G) | 100 | 88.6 ± 9.2 | 81.6 ± 6.9 | 62.8 ± 8 | 26.7 ±6.5 | 67.0 ± 3.0 | 0.89 |
| SW837 (70 µg/mL 6G) | 100 | 70.9 ± 9 | 51.1 ± 9.7 | 21.9 ± 10.9 | 4.9 ± 3.9 | 20.1 ± 8.8 | 0.79 |
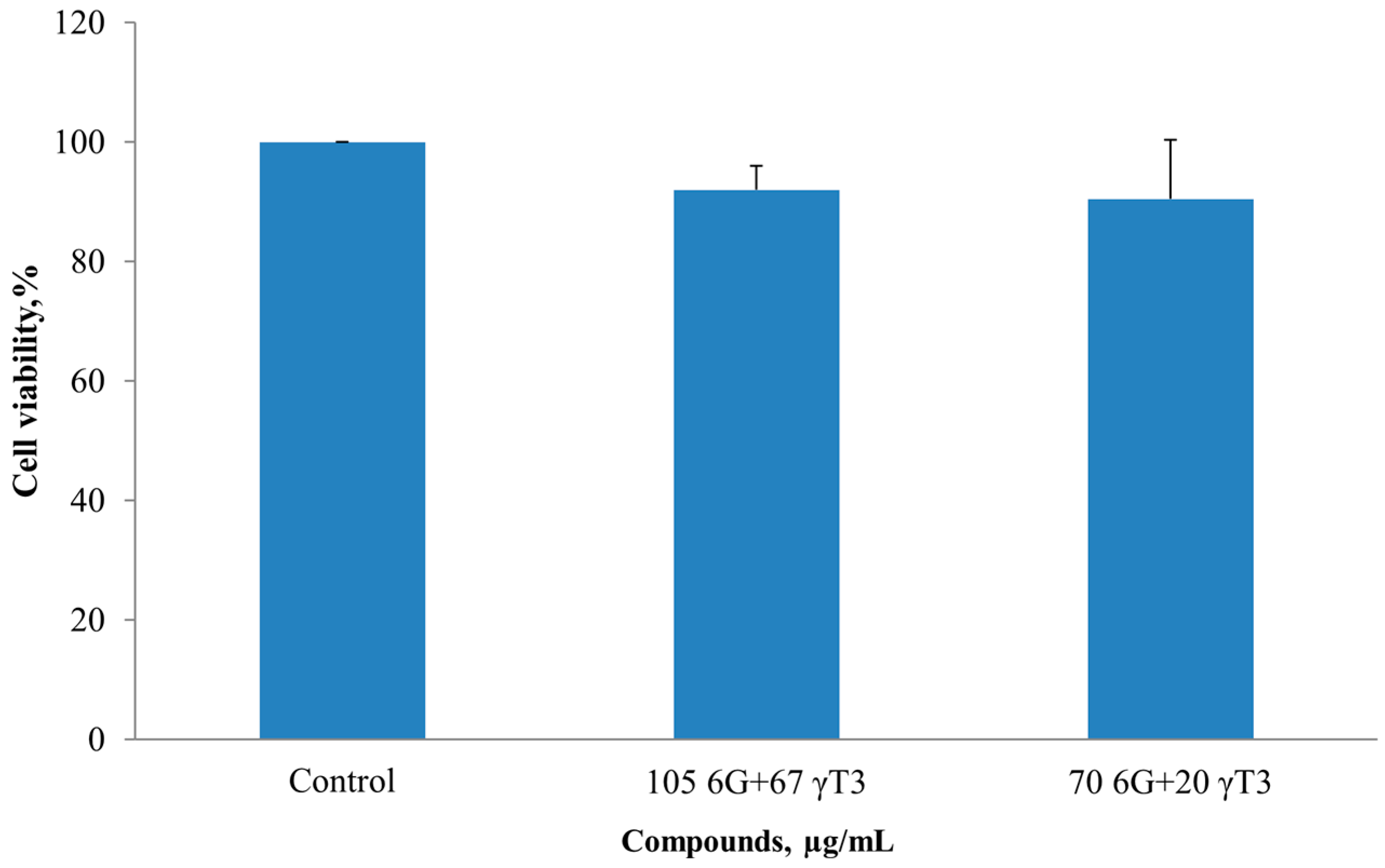
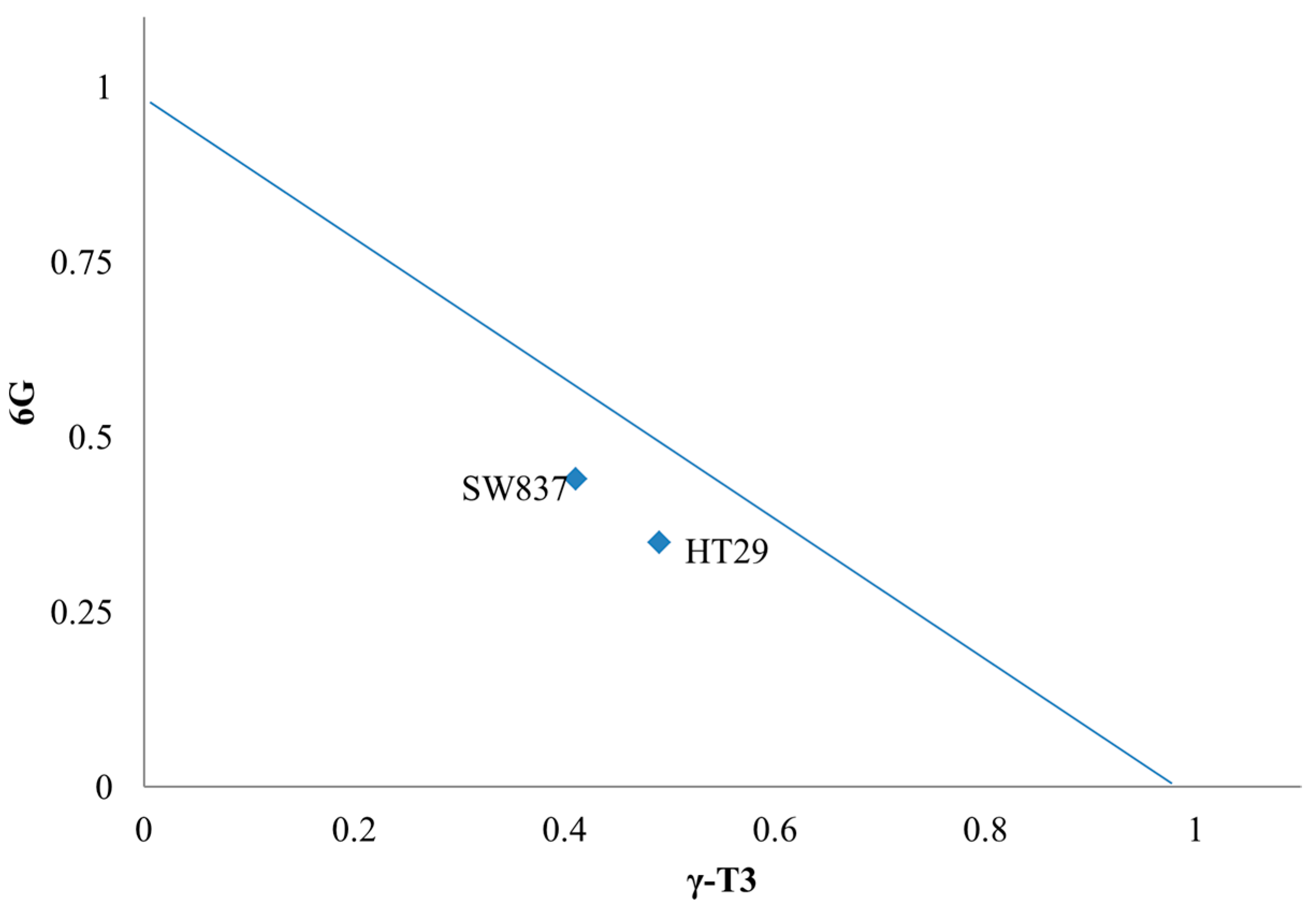
2.2. Isobologram Analysis of 6G+γT3 of HT-29 and SW837 Cells
2.3. Morphological Changes of Combined Treatment 6G with γ-T3 on HT-29 and SW837 Cells
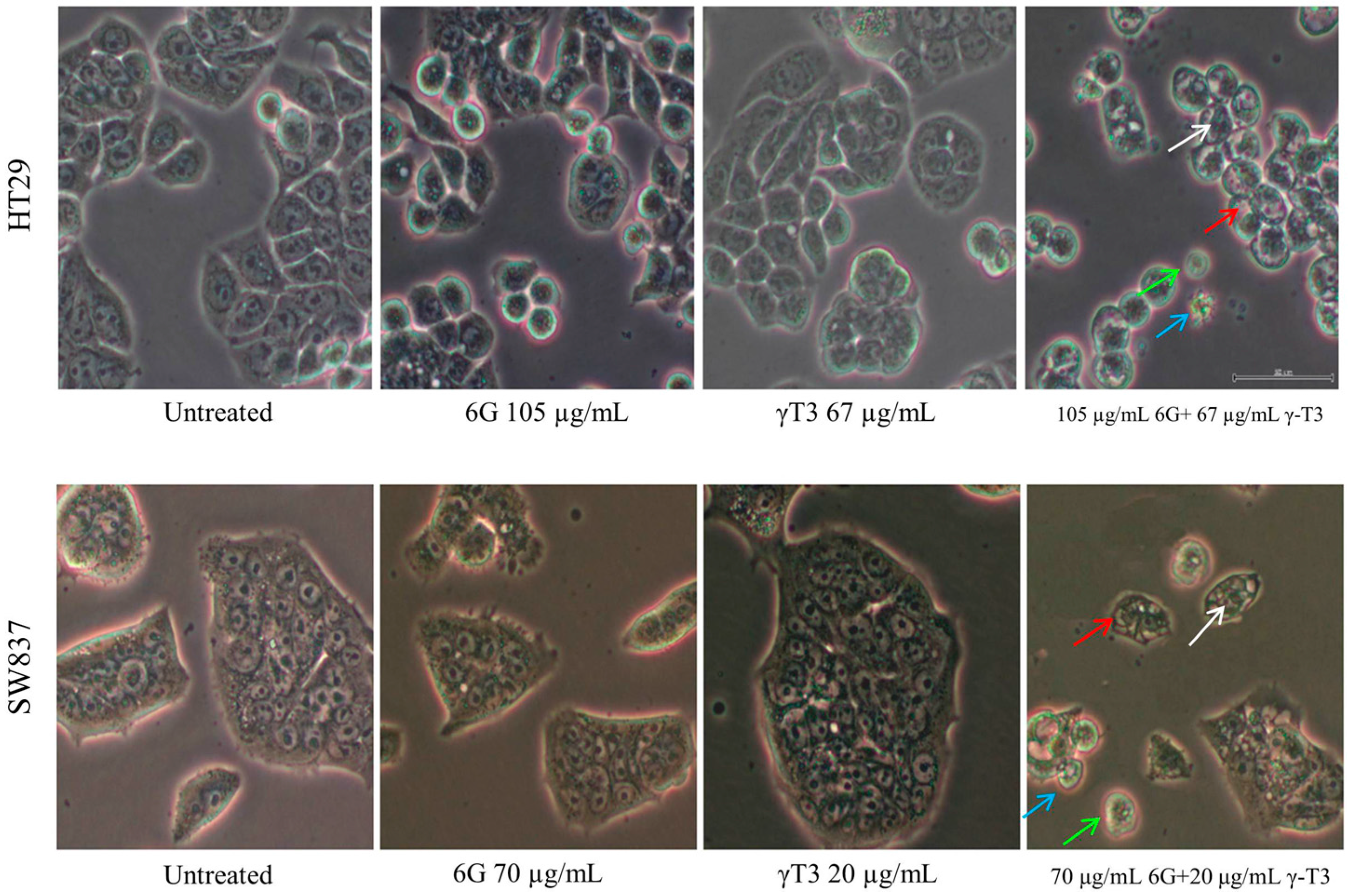
2.4. Effects of Combined 6G+γ-T3 on Apoptosis of HT-29 and SW837 Cells
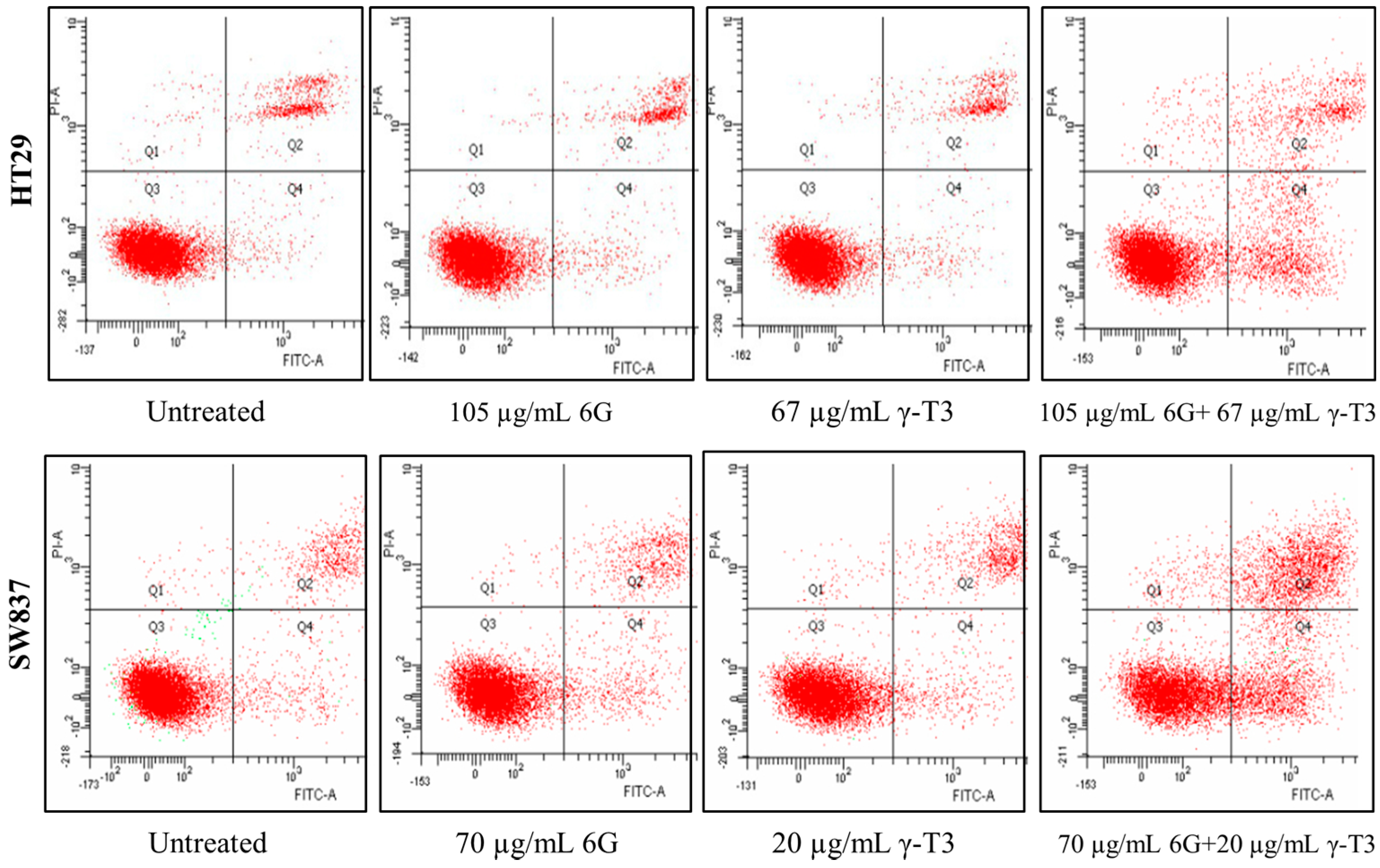
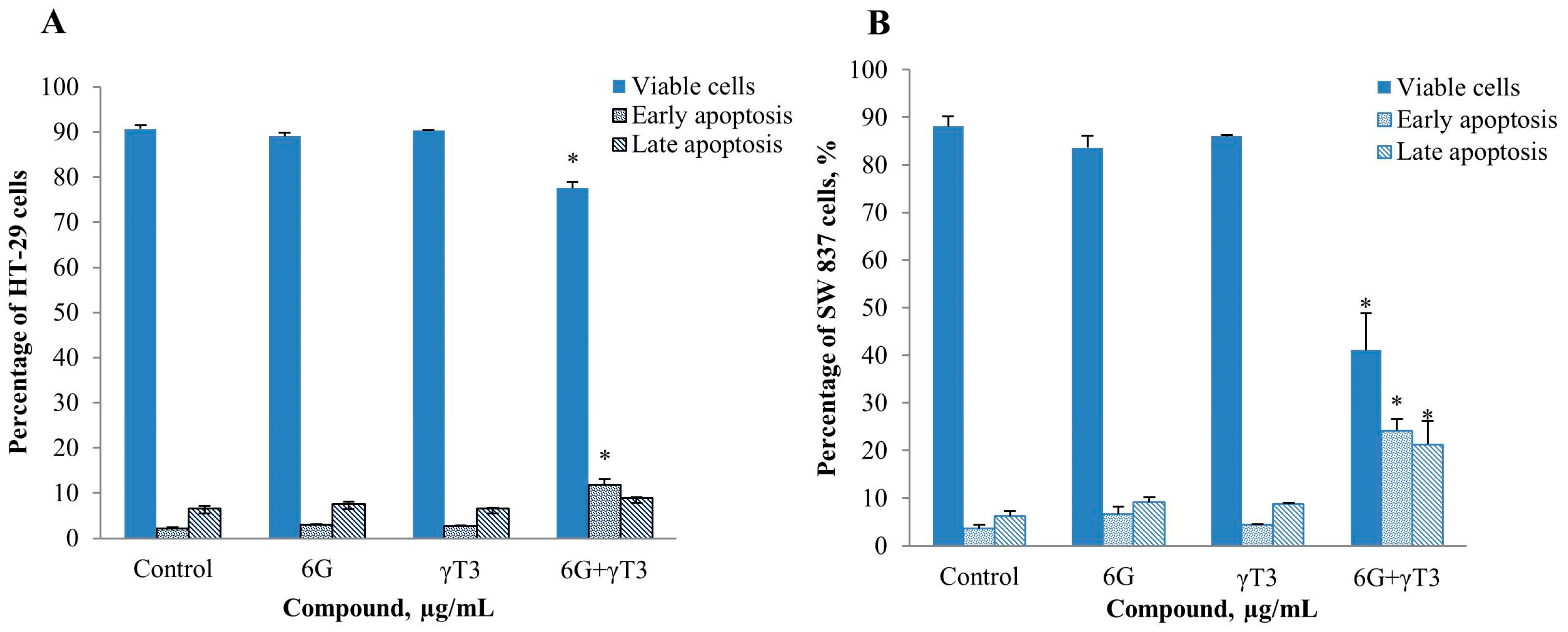

3. Experimental Section
3.1. Cell Culture
3.2. Chemicals and Materials
3.3. Cell Viability Assay
3.4. Isobologram and Combination Index Analysis
3.5. Apoptosis Analysis by Annexin V FITC Binding Assay
3.6. Active Caspase-3 Assay
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 2009, 59, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.G.; Murilo, G.; Nathani, R.; Peng, X. Cancer chemoprevention by natural produtcs: How far have we come? Pharmacol. Res. 2010, 27, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Thomasset, S.C.; Berry, D.P.; Garcea, G.; Marczylo, T.; Steward, W.P.; Gescher, A.J. Dietary phenolis phytochemicals-promising cancer chemopreventive agents in humans? A review of their clinical properties. Int. J. Cancer 2006, 120, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin nanoformulations: A future nanomedicine for cancer. Drug Discov. Today 2012, 17, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Bishayee, K.; Ghosh, S.; Biawas, R.; Mandal, S.K.; Buksh, A.R.K. 6-gingerol induces caspase 3 dependent apoptosis and autophagy in cancer cells: Drug DNA interaction and expression of certain signals genes in HeLa cells. Eur. J. Pharmacol. 2012, 694, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Cekanova, M.; Baek, S.J. Multiple mechanisms are involved in 6-gingerol induced cell growth arrest and apoptosis in human colorectal cancer cells. Mol. Carcinog. 2008, 47, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, P.W.; Kaddoumi, A.; Nazzal, S.; El Sayed, K.A. The value of tocotrienols in the prevention and treatment of cancer. J. Am. Coll. Nutr. 2010, 29, 324s–333s. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, C.; Hyatt, J.A.; Vraka, P.S.; Papas, A.; Papas, K.A.; Neophytou, C.; Hadjivassiliou, V.; Constantinou, A.I. Induction of caspase-independent programmed cell death by vitamin E natural homologs and synthetic derivatives. Nutr. Cancer 2009, 61, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Ng, L.T. Tocotrienols inhibited growth and induced apoptosis in human HeLa cells through the cell cycle signalling pathway. Integr. Cancer Ther. 2010, 9, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Liu, J.R.; Li, Y.; Wang, Q.; Liu, H.K.; Yan, Y.C.; Chen, B.Q.; Sun, W.G. Gamma tocotrienol modulates paracrine section of VEGF induced cobalt (II) chloride via ERK signalling pathway in gastric adenocarcinoma SGC-7901 cell line. Toxicology 2010, 274, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, Q.; Chen, B.; Liu, J.; Liu, H.K.; Xu, W.L. Gamma tocotorienol induced apoptosis in human gastric cancer SGC-7901 cells is associated with a suppression in mitogen-activated protein kinase signalling. Brit. J. Nutr. 2008, 99, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.K.; Wang, Q.; Li, Y.; Sun, W.G.; Liu, J.R.; Yang, Y.M.; Xu, W.L.; Sun, X.R.; Chen, B.Q. Inhibitory effects of gamma tocotrienol on invasion and metastatis of human gastric adenocarcinoma SGC-7901 cells. J. Nutr. Biochem. 2010, 21, 206–213. [Google Scholar] [CrossRef] [PubMed]
- De Flora, S.; Izzoti, A.; D’Agostini, F.; Balansky, R.M. Mechanisms of N-acetylcystine in the prevention of DNA damage and cancer, with special reference to smoking-related end points. Carcinogenesis 2001, 22, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Matsumoto, K.; Koshimizu, K.; Ohigashi, H. Effects of selected food factors with chemopreventive properties on combined lipopolysaccharide-interferon gamma induced IkappaB degradation is RAW264.7 macrophages. Cancer Lett. 2003, 195, 17–25. [Google Scholar] [CrossRef]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2003, 134, 3479s–3485s. [Google Scholar]
- D’Incalci, M.; Steward, W.P.; Gescher, A.J. Use of cancer chemopreventive phytochemicals as antineoplastic agents. Lancet 2005, 6, 899–904. [Google Scholar] [CrossRef]
- Forman, M.; Hursting, S.; Umar, A.; Barrett, J. Nutrtion and cancer prevention: A multidisciplinary perspective on human trials. Annu. Rev. Nutr. 2004, 24, 223–254. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Merels, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for clinical trials: more pitfalls than promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Cheng, L.G.; Lee, L.T.; Yang, L.L. Effects of 6-gingerol, an antioxidant from ginger, on inducing apoptosis in human leukemic HL-60 cells. In Vivo 2003, 17, 641–645. [Google Scholar] [PubMed]
- Young, H.Y.; Luo, Y.L.; Cheng, H.Y.; Hsieh, W.C.; Liao, J.C.; Peng, W.H. Analgesic and anti-inflammatory activities of 6-gingerol. J. Ethnopharmacol. 2005, 96, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Beg, T.; Siddiqie, Y.S.; Ara, G.; Gupta, J.; Afzal, M. Antigenotoxic effect of genistein and gingerol on genotoxicity induced by norethandrolone and oxadrolone in cultures human lymphocytes. Int. J. Pharmacol. 2008, 4, 177–183. [Google Scholar]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Timmermann, B.N. Comparative effects of two-gingerol-containing Zingiber officinale extracts on experimental rheumatoid arthritis. J. Nat. Prod. 2009, 72, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, N.; Nakamura, Y.; Ueda, Y.; Abe, M.; Ozawa, Y.; Uchida, K.; Osawa, T. Dietary ginger constituents, ganalals A and B, are potent apoptosis inducers in human T lymphoma Jurkat cells. Cancer Lett. 2003, 199, 113–119. [Google Scholar] [CrossRef]
- Weng, C.J.; Chou, C.P.; Ho, C.T.; Yen, G.C. Molecular mechanism inhibiting human hepatocarcinoma cellinvasion by 6-shagaol and 6-gingerol. Mol. Nutr. Food Res. 2012, 56, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Wen, J.; Bang, S.; Park, S.W.; Song, S.Y. 6-gingerol induces cell cycle arrest and cell death of mutant p53-expressing pancreatic cells. Yonsei Med. J. 2006, 47, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, M.; Gundala, S.R.; Asif, G.; Shamal, S.A.; Aneja, R. Ginger phytochemicals exhibit synergy to inhibit prostate cancer cell proliferation. Nutr. Cancer 2013, 65, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, E.K.; Bava, S.V.; Narayanan, S.S.; Nath, L.R.; Thulasidasan, A.K.T.; Soniya, E.V.; Anto, R.J. 6-gingerol induces caspase-dependent apoptosis and prevents PMA-induced proliferation in colon cancer cells by inhibiting MAPK/AP-1signaling. PLoS ONE 2014, 9, e104401. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, M.; Kiefer, D.; Farrel, K.; Kemper, K. A review of 12 commonly used medicinal herbs. Arch. Fam. Med. 1998, 7, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Z.; Wang, N.S.; Mi, S.Q. Plasma pharmacokinetics and tissue distribution of 6-gingerol in rats. Biopharm. Drug Dispos. 2008, 29, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Djuric, Z.; Ruffin, M.T.; Litzinger, A.J.; Normolle, D.P.; Alrawi, S.; Feng, M.R.; Brenner, D.E. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.L.; Liu, J.R.; Liu, H.K.; Qi, G.Y.; Sun, X.R.; Sun, W.G.; et al. Inhibition of proliferation and induction of apoptosis by γ-tocotrienol in human colon carcinoma HT-29 cells. Nutrition 2009, 25, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.L.; Ming, D.; Zhao, Y.; Wang, Q.; Sun, W.; Chen, B. γ-tocotrienol inhibits cell viability through suppresiion of β-catenin/Tcf signalling in human coloncarcinoma HT 29 cells. J. Nutr. Biochem. 2012, 23, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Li, D.M.; Ma, Y.; He, N.; Gu, Q.; Wang, F.S.; Jiang, S.Q.; Chen, B.Q.; Liu, J.R. γ-tocotrienol induces paraptosis-like cell death in human colon carcinoma SW620 cells. PLoS ONE 2013, 8, e57779. [Google Scholar] [CrossRef] [PubMed]
- Yap, W.N.; Chang, P.N.; Han, H.Y.; Lee, D.T.W.; Ling, M.T.; Wong, Y.C.; Yap, Y.L. γ-tocotrienol suppresses prostate cancer cell proliferation and invasion through multiple-signalling pathways. Br. J. Cancer 2008, 99, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Luk, S.U.; Yap, W.N.; Chiu, Y.T.; Lee, D.T.; Ma, S.; Lee, T.K.; Vasireddy, R.S.; Wong, Y.C.; Ching, Y.P.; Nelson, C.; et al. Gamma-tocotrienol as an effective agent in targeting prostate cancer stem cell-like population. Int. J. Cancer 2011, 128, 2182–2191. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.M.; Bachawal, S.V.; Sylvester, P.W. Gamma tocotrienol inhibits HGF-dependent mitogenesis and Met activation in highly mammary tumour cells. Cell Prolif. 2011, 44, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.V.; Parajuli, P.; Sylvester, P.W. γ-tocotrienol-induced autophagy in malignant mammary cancer cells. Exp. Biol. Med. 2014, 239, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Okabe, M.; Yamasaki, M.; Tachibana, H.; Yamada, K. Induction of apoptosis by tocotrienol in rat hepatoma dRLh-84 cells. Anticancer Res. 2004, 24, 1683–1688. [Google Scholar] [PubMed]
- Chang, P.N.; Yap, W.N.; Lee, D.T.; Ling, M.T.; Wong, Y.C.; Yap, Y.L. Evidence of gamma-tocotrienol as an apoptosis-inducing, invasion-suppreessing, and chemotherapy drug-sensitizing agent in human melanoma cells. Nutr. Cancer 2009, 61, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Bienz, M.; Clevers, H. Linking colorectal cancer to Wnt signalling. Cell Press 2000, 103, 311–320. [Google Scholar]
- Lin, G.B.; Lin, C.C.; Tsay, G.J. 6-gingerol inhibits growth of colon cancer cell LoVo via induction of G2/M arrest. Evid. Based Complement. Altern. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.F.; Yang, J.M.; Vassil, A.; Yang, J.; Babula, J.B.; Hait, W.N. Regulation of expression of the multidrug resistance protein MRP1 by p53 in human prostate cancer cells. J. Clin. Investig. 2000, 105, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Samant, G.V.; Wali, V.B.; Sylvester, P.W. Anti-proliferative effects of gamma-tocotrienol on mammary tumour cells are associated with suppression of cell cycle progression. Cell Prolif. 2009, 43, 516–526. [Google Scholar]
- Zimmermann, G.R.; Lehar, J.; Keith, C.T. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Kitano, H. A robustness-based approach to systems oriented drug design. Nat. Rev. Drug Discov. 2007, 6, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.A.; Hammond, D.L.; Proudfit, H.K. Synergistic interactions between two α2-adrenoceptor agonists, dexmedetomidine and ST-91, in two substrains of Sprague-Dawley rats. Pain 2000, 85, 135–143. [Google Scholar] [CrossRef]
- Nakamuta, Y.; Yogosawa, S.; Izutani, Y.; Watanabe, H.; Otsuji, E.; Sakai, T. A combination of indole-3-carbinoland genistein synergistically induces apoptosis in human colon cancer HT-29 cells by inhibiting Akt phosphorylation and progression of autophagy. Mol. Cancer Res. 2009, 8, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Priyani, A.; Sadrieh, L.; Brahmbhatt, K.; Ahmed, M.; Sharma, C. Concurrent sulforaphane and eugenol induces differential effects on human cervical cancer cells. Integr. Cancer Ther. 2012, 11, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Akl, M.R.; Ayoub, N.M.; Abuasal, B.S.; Kaddoumi, A.; Sylvester, P.W. Sesamin synergistically potentiates the anticancer effects of γ-tocotrienol in mammary cancer cell lines. Fitoterapia 2013, 84, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Wilankar, C.; Khan, N.M.; Checker, R.; Sharma, D.; Patwardhan, R.; Gota, V.; Sandur, S.K.; Devasagayam, T.P.A. γ-tocotrienol induces apoptosis in human T cell lymphoma through activation of both intrinsic and extrinsic pathways. Curr. Pharm. Des. 2011, 17, 2176–2189. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A.; Becceneri, A.B.; Mutti, H.S.; Martin, A.C.B.M.; Silva, M.F.G.F.; Fernandes, J.B.; Viera, P.C.; Cominetti, M.R. Purification and differential biological effects of ginger-derived substances on normal and tumor cell lines. J. Chromatogr. B 2012, 903, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Wu, J.M. Suppression of cell proliferation and gene expression by combinatorial synergy of EGCG, resveratrol, and γ-tocotrienol in estrogen receptor-positive MCF-7 breast cancer cells. Int. J. Oncol. 2008, 33, 851–859. [Google Scholar] [PubMed]
- Tuerdi, G.; Ichinomiya, S.; Sato, H.; Siddiq, S.; Suwa, E.; Iwata, H.; Yano, T.; Ueno, K. Synergistic effect of combined treatment with gamma-toctrienol and statin on human malignant mesothelioma cells. Cancer Lett. 2013, 339, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Shirode, A.B.; Sylvester, P.W. Synergistic anticancer effects of combined gamma-tocotrienol and celecoxib treatment are associated with suppression in Akt and NF-κB signalling. Biomed. Pharmacother. 2010, 64, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Bachawal, S.V.; Wali, V.B.; Sylvester, P.W. Combined gamma-tocotrienol and erlotinib/gefitinib treatment suppresses STAT and Akt signalling in murine mammary tumor cells. Anticancer Res. 2010, 30, 429–437. [Google Scholar] [PubMed]
- Lantz, R.C.; Chen, G.J.; Sarihan, M.; Solyom, A.M.; Jolad, S.D.; Timmermann, B.N. The effect of ectracts from ginger rhizome on inflammatory mediator production. Phytomedicine 2007, 14, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; McGrath, K.C.Y.; Tran, V.H.; Li, Y.M.; Duke, C.C.; Roufgalis, B.D.; Heather, A.K. Attenuation of proinflammatory responses by S-6-gingerol via inhibition of ROS/NF-kappa B/COX2 activation in HuH7 cells. Evid. Based Complement. Altern. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Shibata, A.K.; Nakagawa, K.; Tsuzuki, T. Anti-angiogenic function of tocotrienol. Asia Pac. J. Clin. 2008, 17, 253–256. [Google Scholar]
- Kim, H.W.; Oh, D.H.; Jung, C.; Kwon, D.D.; Lim, Y.C. Apoptotic effects of 6-gingerol in LNCaP human prostate cancer cells. Soonchungyang Med. Sci. 2011, 17, 75–79. [Google Scholar] [CrossRef]
- Silva, M.T. Secondary necrosis: The natural outcome of the complete apoptotic program. FEBS Lett. 2010, 584, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Don, M.M.; Alblett, G.; Bishop, C.J.; Bundesen, P.G.; Donald, K.J.; Searie, J.; Kerr, J.F. Death of cells by apoptosis following attachment of specifically allergized lymphocytes in vitro. Aust. J. Exp. Biol. Med. Sci. 1977, 55, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, B.; Chen, X.; Singh, K.; Washington, M.K.; Datta, P.K. Loss of SMAD4 in colorectal cancer induces resistance to 5-fulorouracil through activating Akt pathway. Br. J. Cancer 2014, 110, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Papegeorgis, P.; Cheng, K.; Ozturk, S.; Gong, Y.; Lambert, A.W.; Abdolmaleky, H.M.; Zhou, J.R.; Thiagalingam, S. SMAD4 inactivation promotes malignancy and drug resistance of colon cancer. Cancer Res. 2011, 71, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.; Fan, F.; Dang, L.H.; Xia, L.; Gaur, P.; Ellis, L.M. Intracrine vascular endothelial growth factor signaling in survival and chemoresistance of human colorectal cancer cells. Oncogene 2011, 30, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Alazzouzi, H.; Alhopuro, P.; Salovaara, R.; Sammalkorpi, H.; Jarvinen, H.; Mecklin, J.P.; Hemminki, A.; Schwartz, S., Jr.; Aaltonen, L.A.; Arango, D. SMAD4 as a prognostic marker in colorectal cancer. Clin. Cancer Res. 2005, 11, 2606–2611. [Google Scholar] [CrossRef] [PubMed]
- Alhopuro, P.; Alazzouzi, H.; Sammalkorpi, H.; Davalos, V.; Salovaara, R.; Hemminki, A.; Jarvinen, H.; Mecklin, J.P.; Schwartz, S., Jr.; Aaltonen, L.A.; et al. SMAD4 levels and response to 5-fluorouracil in colorectal cancer. Clin. Cancer Res. 2005, 11, 6311–6316. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Quintavalle, C.; Zanca, C.; De Rienzo, A.; Romano, G.; Acunzo, M.; Puca, L.; Incoronato, M.; Croce, C.M.; Condorelli, G. Akt regulates drug induced cell death through Bcl-w downregulation. PLoS ONE 2008, 3, e4070. [Google Scholar] [CrossRef] [PubMed]
- Shukla, Y.; Prasad, S.; Tripathi, C.; Singh, M.; George, J.; Kaira, N. In vitro and in vivo modulation of testosterone mediated alterations in apoptosis related proteins by 6-gingerol. Mol. Nutr. Food Res. 2007, 51, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Loo, G. Disruption ofmitochondria during tocotrienol-induced apoptosis in MDA-MB-321 human breast cancer cells. Biochem. Pharm. 2004, 67, 315–324. [Google Scholar]
- Unal-Cevik, I.; Kilinc, M.; Can, A.; Gursoy-Ozdemir, Y.; Dalkara, T. Apoptotic and necrotic death mechanisms are concomitantly activated in same cell after cerebral ischemia. Stroke 2004, 35, 2189–2194. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitaive analysis of concentration effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Md Yusof, K.; Makpol, S.; Jamal, R.; Harun, R.; Mokhtar, N.; Wan Ngah, W.Z. γ-Tocotrienol and 6-Gingerol in Combination Synergistically Induce Cytotoxicity and Apoptosis in HT-29 and SW837 Human Colorectal Cancer Cells. Molecules 2015, 20, 10280-10297. https://doi.org/10.3390/molecules200610280
Md Yusof K, Makpol S, Jamal R, Harun R, Mokhtar N, Wan Ngah WZ. γ-Tocotrienol and 6-Gingerol in Combination Synergistically Induce Cytotoxicity and Apoptosis in HT-29 and SW837 Human Colorectal Cancer Cells. Molecules. 2015; 20(6):10280-10297. https://doi.org/10.3390/molecules200610280
Chicago/Turabian StyleMd Yusof, Khairunnisa', Suzana Makpol, Rahman Jamal, Roslan Harun, Norfilza Mokhtar, and Wan Zurinah Wan Ngah. 2015. "γ-Tocotrienol and 6-Gingerol in Combination Synergistically Induce Cytotoxicity and Apoptosis in HT-29 and SW837 Human Colorectal Cancer Cells" Molecules 20, no. 6: 10280-10297. https://doi.org/10.3390/molecules200610280
APA StyleMd Yusof, K., Makpol, S., Jamal, R., Harun, R., Mokhtar, N., & Wan Ngah, W. Z. (2015). γ-Tocotrienol and 6-Gingerol in Combination Synergistically Induce Cytotoxicity and Apoptosis in HT-29 and SW837 Human Colorectal Cancer Cells. Molecules, 20(6), 10280-10297. https://doi.org/10.3390/molecules200610280





