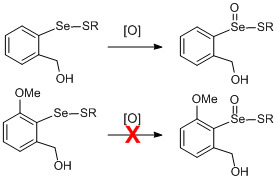
Effect of Methoxy Substituents on the Activation Barriers of the Glutathione Peroxidase-Like Mechanism of an Aromatic Cyclic Seleninate
Abstract
:1. Introduction
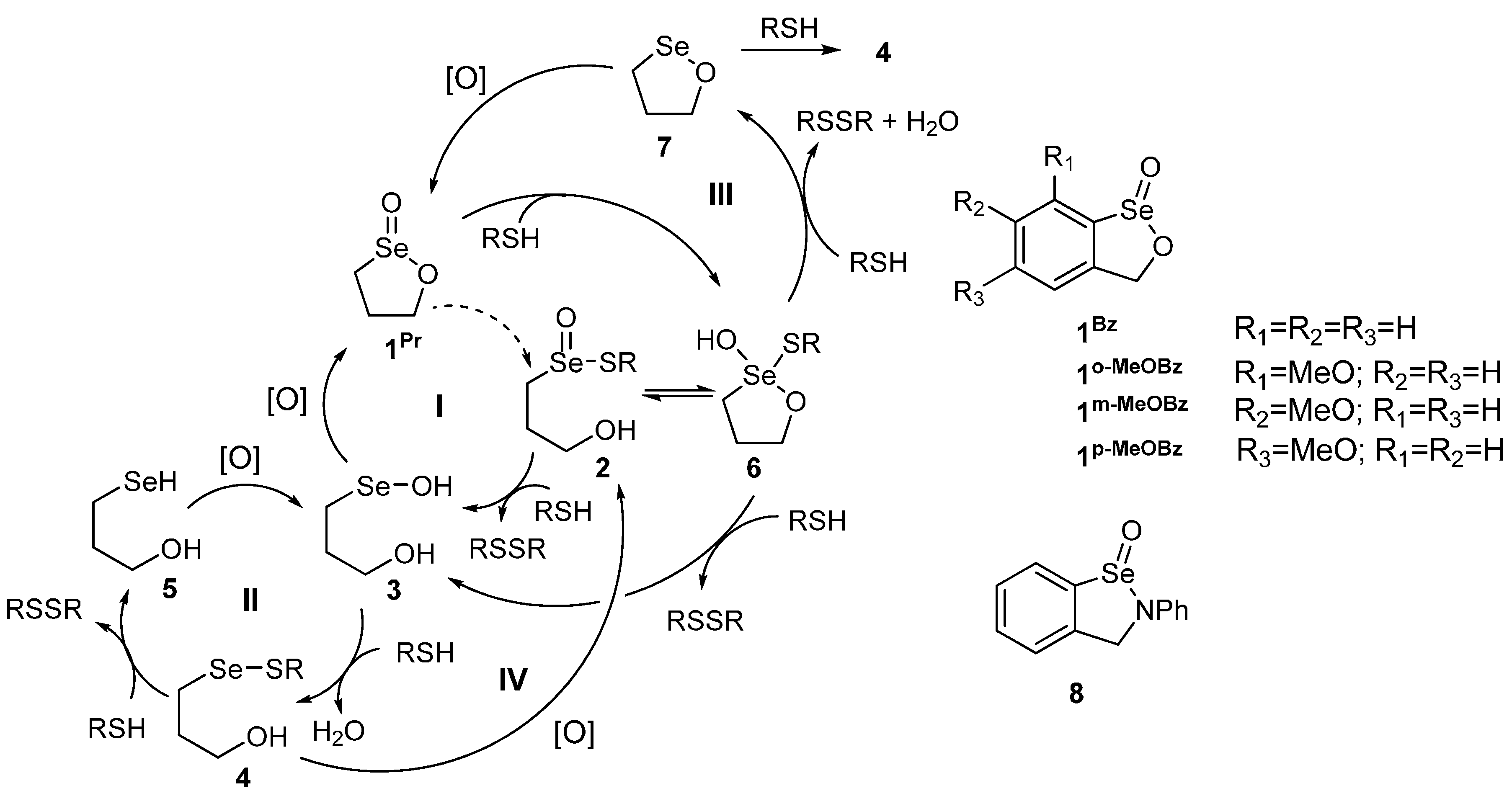
2. Results and Discussion
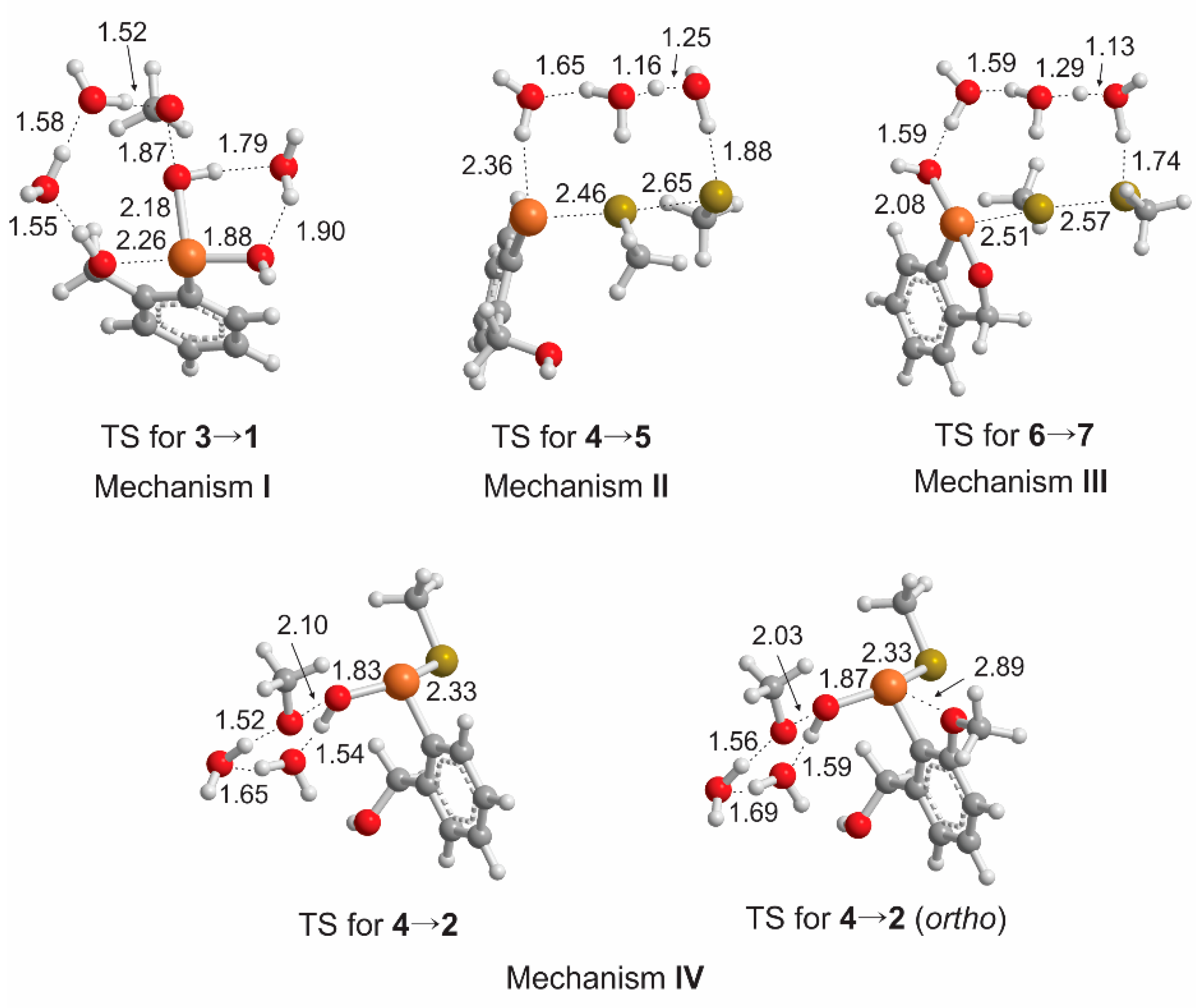
| Step | ∆G‡ + ∆Gsolv, kcal/mol | ∆G + ∆Gsolv, kcal/mol |
|---|---|---|
| 2→3 | 16.2 (16.9) | −14.4 (−7.8) |
| 3→1 | 18.9 (19.7) | −44.0 (−47.5) |
| 3→4 | 8.1 (8.4) | −29.9 (−34.5) |
| 4→5 | 25.1 (25.2) | 4.6 (9.7) |
| 5→3 | 20.0 (24.5) | −59.4 (−59.4) |
| 1→6 | 9.2 (8.4) | −5.8 (−1.0) |
| 6→3 | 16.3 (14.1) | −15.8 (−24.9) |
| 6→7 | 12.0 (16.9) | −21.6 (−22.4) |
| 7→1 | 11.1 (12.2) | −48.6 (−55.6) |
| 7→4 | 4.4 (3.6) | −20.8 (−23.7) |
| 4→2 | 23.1 (22.3) | −33.2 (−36.1) |
| ∆G‡ + ∆Gsolv, kcal/mol | ∆G + ∆Gsolv, kcal/mol | ||
|---|---|---|---|
| 3→1 | para | 20.1 | −45.8 |
| meta | 20.6 | −44.6 | |
| ortho | 20.5 | −47.2 | |
| 5→3 | para | 21.0 | −58.4 |
| meta | 21.0 | −58.9 | |
| ortho | 21.0 | −54.1 | |
| 7→1 | para | 8.2 | −52.4 |
| meta | 9.4 | −51.4 | |
| ortho | 14.6 | −52.5 | |
| 4→2 | para | 21.8 | −34.9 |
| meta | 23.2 | −35.2 | |
| ortho | 25.0 | −32.5 |
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mugesh, G.; Singh, H.B. Synthetic organoselenium compounds as antioxidants: Glutathione peroxidase activity. Chem. Soc. Rev. 2000, 29, 347–357. [Google Scholar] [CrossRef]
- Mugesh, G.; Du Mont, W.W.; Sies, H. Chemistry of Biologically Important Synthetic Organoselenium Compounds. Chem. Rev. 2001, 101, 2125–2180. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, D.L.; Berry, M.L.; Gladyshev, V.N. Selenium. Its Molecular Biology and Role in Human Health, 2nd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Bhabak, K.P.; Mugesh, G. Functional Mimics of Glutathione Peroxidase: Bioinspired Synthetic Antioxidants. Acc. Chem. Res. 2010, 43, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Bayse, C.A.; Brumaghim, J.L. Biochalcogen Chemistry: The Biological Chemistry of Sulfur, Selenium and Tellurium; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2013; Volume 1152. [Google Scholar]
- Orian, L.; Toppo, S. Organochalcogen peroxidase mimetics as potential drugs: A long story of a promise still unfulfilled. Free Radic. Biol. Med. 2014, 66, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T. Glutathione Peroxidase-like Activities of Oxygen-Containing Diselenides. Molecules 1998, 3, 164–166. [Google Scholar] [CrossRef]
- Back, T.G.; Moussa, Z. Remarkable Activity of a Novel Cyclic Seleninate Ester as a Glutathione Peroxidase Mimetic and Its Facile in Situ Generation from Allyl 3-Hydroxypropyl Selenide. J. Am. Chem. Soc. 2002, 124, 12104–12105. [Google Scholar] [CrossRef] [PubMed]
- Back, T.G.; Moussa, Z. Diselenides and Allyl Selenides as Glutathione Peroxidase Mimetics. Remarkable Activity of Cyclic Seleninates Produced in Situ by the Oxidation of Allyl ω-Hydroxyalkyl Selenides. J. Am. Chem. Soc. 2003, 125, 13455–13460. [Google Scholar] [CrossRef] [PubMed]
- Press, D.J.; Mercier, E.A.; Kuzma, D.; Back, T.G. Substituent Effects upon the Catalytic Activity of Aromatic Cyclic Seleninate Esters and Spirodioxyselenuranes That Act as Glutathione Peroxidase Mimetics. J. Org. Chem. 2008, 73, 4252–4255. [Google Scholar] [CrossRef] [PubMed]
- Back, T.G. Investigations of new types of glutathione peroxidase mimetics. In Biochalcogen Chemistry: The Biological Chemistry of Sulfur, Selenium, and Tellurium; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2013; Volume 1152, pp. 143–162. [Google Scholar]
- Press, D.J.; McNeil, N.M.R.; Hambrook, M.; Back, T.G. Effects of Methoxy Substituents on the Glutathione Peroxidase-like Activity of Cyclic Seleninate Esters. J. Org. Chem. 2014, 79, 9394–9401. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.K.; Sharma, S.; Singh, H.B.; Butcher, R.J. 2-Phenoxyethanol derived diselenide and related compounds; synthesis of a seven-membered seleninate ester. Org. Biomol. Chem. 2010, 9, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Singh, H.B.; Butcher, R.J. Synthesis of intramolecularly coordinated cyclic selenenate/thioselenenate esters and their glutathione peroxidase-like activity. Indian J. Chem. 2011, 50, 1263–1272. [Google Scholar]
- Bayse, C.A.; Antony, S. Molecular modeling of bioactive selenium compounds. Main Group Chem. 2007, 6, 185–200. [Google Scholar] [CrossRef]
- Bayse, C.A. Modeling of Mechanisms of Selenium Bioactivity Using Density Functional Theory. In Biochalcogen Chemistry: The Biological Chemistry of Sulfur, Selenium, and Tellurium; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2013; Volume 1152, pp. 179–200. [Google Scholar]
- Bayse, C.A. DFT Study of the Glutathione Peroxidase-Like Activity of Phenylselenol Incorporating Solvent-Assisted Proton Exchange. J. Phys. Chem. A 2007, 111, 9070–9075. [Google Scholar] [CrossRef] [PubMed]
- Antony, S.; Bayse, C.A. Modeling the Mechanism of the Glutathione Peroxidase Mimic Ebselen. Inorg. Chem. 2011, 50, 12075–12084. [Google Scholar] [CrossRef] [PubMed]
- Bayse, C.A. Transition states for cysteine redox processes modeled by DFT and solvent-assisted proton exchange. Org. Biomol. Chem. 2011, 9, 4748–4751. [Google Scholar] [CrossRef] [PubMed]
- Bayse, C.A.; Pavlou, A. Tuning the activity of glutathione peroxidise mimics through intramolecular Se···N,O interactions: A DFT study incorporating solvent-assisted proton exchange (SAPE). Org. Biomol. Chem. 2011, 9, 8006–8015. [Google Scholar] [CrossRef] [PubMed]
- Bayse, C.A.; Ortwine, K.N. Modeling the Glutathione Peroxidase-Like Activity of a Cyclic Seleninate by DFT and Solvent-Assisted Proton Exchange. Eur. J. Inorg. Chem. 2013, 2013, 3680–3688. [Google Scholar] [CrossRef]
- Sarma, B.K.; Mugesh, G. Glutathione Peroxidase (GPx)-like Antioxidant Activity of the Organoselenium Drug Ebselen: Unexpected Complications with Thiol Exchange Reactions. J. Am. Chem. Soc. 2005, 127, 11477–11485. [Google Scholar] [CrossRef] [PubMed]
- Bhabak, K.P.; Mugesh, G. A Simple and Efficient Strategy to Enhance the Antioxidant Activities of Amino-Substituted Glutathione Peroxidase Mimics. Chem. Eur. J. 2008, 14, 8640–8651. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.K.; Boyd, R.J. Effect of Substituents on the GPx-like Activity of Ebselen: Steric versus Electronic. J. Phys. Chem. A 2008, 112, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Bayse, C.A. Model mechanisms of sulfhydryl oxidation by methyl- and benzeneseleninic acid, inhibitors of zinc-finger transcription factors. J. Inorg. Biochem. 2010, 104, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Additional computational data are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayse, C.A.; Shoaf, A.L. Effect of Methoxy Substituents on the Activation Barriers of the Glutathione Peroxidase-Like Mechanism of an Aromatic Cyclic Seleninate. Molecules 2015, 20, 10244-10252. https://doi.org/10.3390/molecules200610244
Bayse CA, Shoaf AL. Effect of Methoxy Substituents on the Activation Barriers of the Glutathione Peroxidase-Like Mechanism of an Aromatic Cyclic Seleninate. Molecules. 2015; 20(6):10244-10252. https://doi.org/10.3390/molecules200610244
Chicago/Turabian StyleBayse, Craig A., and Ashley L. Shoaf. 2015. "Effect of Methoxy Substituents on the Activation Barriers of the Glutathione Peroxidase-Like Mechanism of an Aromatic Cyclic Seleninate" Molecules 20, no. 6: 10244-10252. https://doi.org/10.3390/molecules200610244