Abstract
Pincer (Phebox)Ir(mesityl)(OAc) (2) (Phebox = 3,5-dimethylphenyl-2,6-bis(oxazolinyl)) complex, formed by benzylic C-H activation of mesitylene (1,3,5-trimethylbenzene) using (Phebox)Ir(OAc)2OH2 (1), was treated with thionyl chloride to rapidly form 1-(chloromethyl)-3,5-dimethylbenzene in 50% yield at 23 °C. A green species was obtained at the end of reaction, which decomposed during flash column chromatography to form (Phebox)IrCl2OH2 in 87% yield.
1. Introduction
Carbon-chlorine bonds are widespread in pharmaceutically important organic molecules [1]. Organochlorides are also common synthetic precursors for cross-coupling [2], such as the Suzuki-Miyaura reaction [3]. In recent years C-H bond functionalization, involving initial C-H bond activation followed by functionalization of the resulting metal-carbon bond [4,5,6,7], has shown great promise in organic synthesis for constructing C-Cl bonds directly from C-H bonds [1,2,8,9,10,11,12]. These studies have typically employed a Pd catalyst and N-chlorosuccinimide, to convert aryl-H bonds to aryl-Cl bonds.
Besides Pd, Rh and Cu catalysts for carrying out arene C-H chlorination have been reported [2,13,14]. The Shilov Pt system is well-known for effecting the chlorination of methane, but studies on other substrates are rare [4,7]. An alternative method, using a Mn-porphyrin complex [15], can catalyze selective C-H chlorination without proceeding via formation of a metal-carbon bond [6].
Despite these recent advances, C-H bond chlorination is still far from being a robust and versatile synthetic method for constructing C-Cl bond [1]. The substrate scope is largely limited to aromatics and a directing group is needed to ensure regioselectivity. Development of new organometallic reactions relevant to C-H activation and C-Cl bond formation are required to overcome these challenges.
Interest in developing Ir (III) complexes for C-H bond functionalization has been growing rapidly. Crabtree and coworkers showed that Cp*Ir(chelate)X (X = monodentate anionic ligand) catalyzed the selective C-H hydroxylation of alkanes and alkyl groups by NaIO4 [16,17,18]. Chang and coworkers found that [Cp*IrCl2]2 catalyzed directing-group-assisted C-H amidation of arenes [19]. Nishiyama and coworkers reported the C-H activation of n-octane and benzene derivatives by (Phebox)Ir(OAc)2(OH2) (Phebox = 3,5-dimethylphenyl-2,6-bis(oxazolinyl)) [20]. Goldberg and co-workers subsequently found that the resulting (Phebox)Ir n-alkyl derivatives can undergo β-hydrogen elimination to give olefin, and that the resulting hydride can react with O2 to regenerate (Phebox)Ir(OAc)2(OH2) [21,22,23]. Davies and coworkers found that (Phebox)IrCl2(OH2) catalyzed asymmetric carbene C-H insertion [24].
We recently reported the activation and oxidation of the benzylic C-H bond of mesitylene (1,3,5-trimethylbenzene) by (Phebox)Ir complexes [25]. Exclusive benzylic C-H activation (Scheme 1) by (Phebox)Ir(OAc)2(OH2) (1) gave (Phebox)Ir(mesityl)(OAc) (2).
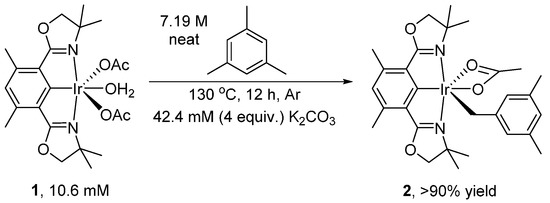
Scheme 1.
Mesitylene C-H activation by (Phebox)Ir(OAc)2(OH2) to form (Phebox)Ir(mesityl)(OAc).
2. Results and Discussion
The reaction of complex 2 (10.6 mM), a red solid, with thionyl chloride (21.2 mM) resulted in the solution rapidly turning dark. The formation of 1-(chloromethyl)-3,5-dimethylbenzene in 50% yield was observed after less than 15 min at 23 °C (Scheme 2; product identity was confirmed by comparing the 1H- and 13C-NMR spectra with those of 1-(chloromethyl)-3,5-dimethylbenzene synthesized independently from purchased (3,5-dimethylphenyl)methanol, as shown in Scheme 3). C6D6 was used as solvent (Scheme 2) for direct 1H{13C} NMR analysis.
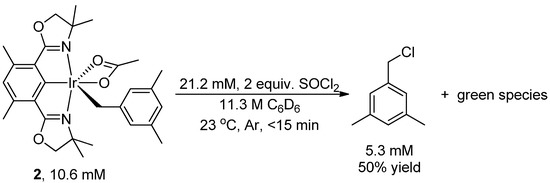
Scheme 2.
Chlorination of (Phebox)Ir(mesityl)(OAc) by thionyl chloride to form 1-(chloromethyl)-3,5-dimethylbenzene.

Scheme 3.
Independent synthesis of 1-(chloromethyl)-3,5-dimethylbenzene from (3,5-dimethylphenyl)methanol.
No significant (<1%) unreacted complex 2 was recovered after 15 min, indicating complete conversion of complex 2. No significant increase (<10%) in the yield of 1-(chloromethyl)-3,5-dimethylbenzene was observed with an additional reaction time of 15 h. Using 10 equivalents of thionyl chloride (106 mM) and under otherwise identical conditions, 1-(chloromethyl)-3,5-dimethylbenzene formed in 71% yield.
Along with 1-(chloromethyl)-3,5-dimethylbenzene, a green species formed at the end of reaction (Scheme 2). We could not characterize this green species from the complex 1H-NMR spectrum of the reaction mixture. The green species turned purple at −196 °C when frozen by liquid nitrogen, and reverted back to green when warming back to room temperature.
After performing silica gel flash column chromatography on the green species using acetone/hexane, we obtained a known complex [26], (Phebox)IrCl2(OH2), in 87% yield (Scheme 4). Before the chromatography no significant amount (<10%) of (Phebox)IrCl2(OH2) was present in the reaction mixture by 1H-NMR analysis. This suggested that the green species decomposed to form (Phebox)IrCl2(OH2) during chromatography. The result is consistent with the Phebox ligand being bound to Ir throughout the chlorination.
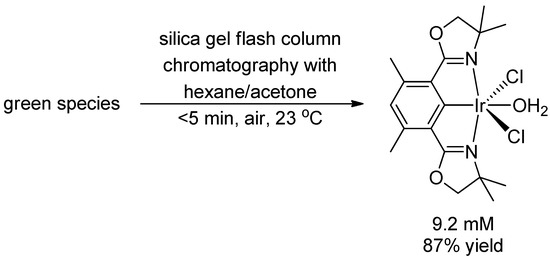
Scheme 4.
Decomposition of the green species to (Phebox)IrCl2(OH2) during silica gel flash column chromatography.
Relevant to this work, oxidative addition of thionyl chloride by Vaska’s complex [27,28,29,30], IrICl(CO)(PPh3)2, gave a mixture of IrIIICl3(CO)(PPh3)2, IrICl(CO)(PPh3)2SO2 and IrIIICl2(SOCl)(CO)(PPh3)2 ((SOCl) = chlorosulfinyl). In light of the products identified in these reports, the green species could consist of a mixture of (Phebox)Ir complexes bearing different monodentate ligands.
Currently we can only speculate on the mechanism of C-Cl bond formation, due to the complex 1H-NMR spectrum of the chlorination reaction mixture. The η2-bound carboxylate ligand in complex 2 may open to η1-bound to give an open coordination site for oxidative addition of thionyl chloride. A high-valent Ir complex [31,32,33,34] may form which could be responsible for C-Cl bond formation. Ison and coworkers recently reported the formation of methanol from methyl-ligated Cp*IrIV(NHC) μ-oxo dimer (Cp* = C5Me5, NHC = N-heterocyclic carbene) by dioxygen [33,35], as an example of carbon-hetero bond formation via high-valent Ir-alkyl complex. Alternatively direct electrophilic attack on the M-C bond by thionyl chloride may occur without formation of a high oxidation state Ir complex.
3. Experimental Section
3.1. General
Solvents and reagents were purchased from VWR or Sigma Aldrich, and used without further purification. A MBraun glove box was used to store complex 2 under argon (<0.1 ppm O2 and <0.1 ppm H2O). 1H- and 13C-NMR analyses were performed on a 300, 400 or 500 MHz Varian spectrometer, using benzene solvent chemical shifts as reference at 7.16 ppm in 1H{13C} NMR spectrum (C6D5H) or 128.6 ppm for 13C{1H} NMR spectrum (C6D6). Silica gel (230–400 mesh) for flash column chromatography was purchased from SiliCycle. J-Young NMR tubes (5 mm outer diameter) were purchased from Sigma Aldrich. Reusable culture tubes (50 mL), equipped with PTFE-faced phenolic caps, were manufactured by Kimax or Pyrex. To ensure high product yield, air was rigorously removed by three cycles of freeze-pump-thaw treatment for all reactions containing Ir. The reaction yield was measured using an internal standard (1,4-bis(trifluoromethyl)benzene in C6D6) added at the end of the reaction, followed by 1H{13C} NMR analysis.
3.2. Chlorination of Complex 2
Complex 2 (3.2 μmol, 10.6 mM, 2.1 mg) was dissolved in C6D6 (200 μL) in a regular 5-mm outer-diameter J-Young NMR tube in a glove box. The tube, with the J-Young valve closed, was then taken out of glove box. The valve was briefly opened under a vigorous flush of argon and an air-free (by 3 cycles of freeze-pump-thaw treatment) solution (100 μL) of thionyl chloride (63.6 mM, 6.4 μmol) in C6D6 was quickly added. The valve was quickly closed upon delivery of thionyl chloride. The red solution turned dark instantly upon addition of thionyl chloride.
After 10 min at 23 °C, the reaction mixture was analyzed directly by 1H-NMR spectroscopy. After obtaining the initial spectrum, an internal standard, (1,4-bis(trifluoromethyl)benzene in C6D6 was added to the reaction mixture under air for quantitation.
3.3. Syntheses and Characterizations
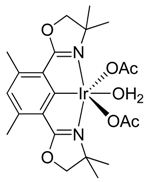
(Phebox)Ir(OCOCH3)2(OH2) was prepared according to literature procedures [26], starting from commercially available 1,5-bis(chloromethyl)-2,4-dimethylbenzene purchased from Sigma-Aldrich.
1H{13C} NMR chemical shifts of (Phebox)Ir(OCOCH3)2(OH2): 6.38 ppm (1H, arene CH, s), 3.91 ppm (4H, OCH2, s), 2.48 (6H, benzylic CH3, s), 1.8 ppm (6H, OCOCH3, s), 1.42 ppm (12H, aliphatic CH3, s).
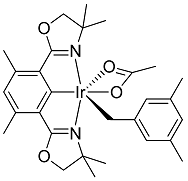
(Phebox)Ir(mesityl)(OCOCH3) (2) was made in one step from complex 1 using mesitylene under argon. Complex 1 (20 mg, 32 μmol, 106 mM), mesitylene (3 mL, 7.19 M) and K2CO3 (4 equivalents, 18 mg, 424 mM) were added to a reusable culture tube (50 mL), equipped with PTFE-faced black phenolic caps and a magnetic stir bar, in a glove box under argon. The tube was then taken out of the glove box and heated in an oil bath at 130 °C for 12 h. At the end of reaction, mesitylene solvent was removed under vacuum.
In a glove box, ether dissolved complex 2 and the solution obtained was filtered through glass wool plug packed in a glass pipette. Complex 2 was obtained in >90% yield by 1H{13C} NMR analysis in C6D6 using MeCN (64 mM) as internal standard. Product can be further purified by recrystallization in ether and pentane, at −32 °C and in a glove box.
Complex 2 in solid state appeared to be stable under argon for at least a week. Preparation in large scale (40 mg complex 2, 64 μmol, 10.6 mM and mesitylene in 6 mL, 7.19 M) was performed in a 50 mL Reusable culture tubes (50 mL), equipped with PTFE-faced black phenolic caps. A magnetic stir bar was used.
1H{13C} NMR chemical shifts of complex 2: 6.67 ppm (1H, ligand Ar-H, s), 6.53 ppm (1H, mesityl Ar-H, s), 6.30 ppm (2H, mesityl Ar-H, s), 3.81 ppm (2H, CH2O, d, J = 8.1 Hz), 3.65 ppm (2H, CH2O, d, J = 8.1 Hz), 2.86 ppm (2H, Ir-CH2-Ar, s), 2.67 ppm (6H, phebox ligand benzylic CH3, s), 2.16 ppm (6H, mesityl benzylic CH3, s), 2.04 ppm (3H, OCOCH3, s), 1.29 ppm (6H, two aliphatic CH3, s), 1.20 ppm (6H, two aliphatic CH3, s).
13C{1H} NMR chemical shifts of complex 2: −1.43 ppm (s, Ir-CH2), 19.50 ppm (s), 21.83 ppm (s), 25.93 ppm (s), 26.44 ppm (s), 28.06 ppm (s), 66.45 ppm (s), 82.52 ppm (s), 123.6 ppm (s), 125.1 ppm (s), 127.1 ppm (s), 127.3 ppm (s), 136.4 ppm (s), 140.0 ppm (s), 150.5 ppm (s), 177.6 ppm (s), 182.4 ppm (s), 185.9 ppm (s).
X-ray crystal structure determination of complex 2 was reported previously [25].
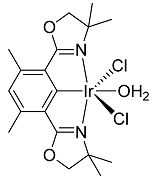
(Phebox)IrCl2(OH2) was prepared according to literature procedures [26].
1H{13C} NMR chemical shifts of (Phebox)IrCl2(OH2): 6.39 ppm (1H, arene CH, s), 3.94 ppm (4H, OCH2, s), 2.55 ppm (6H, benzylic CH3, s), 1.41 ppm (12H, aliphatic CH3, s).
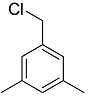
1-(chloromethyl)-3,5-dimethylbenzene was prepared from (3,5-dimethylphenyl)methanol and thionyl chloride: thionyl chloride (300 μL, 4.13 mmol, 11.8 M) and (3,5-dimethylphenyl)methanol (50 μL, 0.39 mmol, 1.1 M) were added to a NMR tube, capped. Teflon wrapping around the cap of nmr tube was used to prevent reaction mixture from leaking out. The NMR tube was heated at reflux under air (100 °C) for 10 min. After cooling to 23 °C, the nmr cap was replaced by a septum and volatiles were removed under vacuum via a needle through the septum. A solution (300 μL) of (1,4-bis(trifluoromethyl)benzene in C6D6 was added to the product mixture for 1H{13C} NMR analysis. All (3,5-dimethylphenyl)methanol was converted at the end of reaction.
1H{13C} NMR chemical shifts of 1-(chloromethyl)-3,5-dimethylbenzene: 6.75 ppm (2H, arene CH, s), 6.68 ppm (1H, arene CH, s), 4.15 ppm (2H, CH2Cl, s), 2.04 ppm (6H, benzylic CH3, s).
13C{1H} NMR chemical shifts of 1-(chloromethyl)-3,5-dimethylbenzene: 138.3 (s), 138.8 ppm (s), 130.7 ppm (s), 127.4 ppm (s), 46.97 ppm, 21.65 ppm (s).
4. Conclusions
Pincer Ir-mesityl complex 2, formed by C-H activation of mesitylene, underwent facile chlorination by thionyl chloride to form 1-(chloromethyl)-3,5-dimethylbenzene. (Phebox)IrCl2(OH2) was obtained after purification of reaction mixture, indicating that the Phebox ligand remained bound to Ir throughout the course of the reaction.
Acknowledgments
We thank Chevron Corporation and NSF, through the CCI Center for Enabling New Technologies through Catalysis (CENTC), Phase II Renewal, CHE-1205189, for funding.
Author Contributions
ASG and MZ designed research and wrote the paper. MZ performed the experiments and analyzed the data. Both authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sun, X.; Shan, G.; Sun, Y.; Rao, Y. Regio- and Chemoselective C-H Chlorination/Bromination of Electron-Deficient Arenes by Weak Coordination and Study of Relative Directing-Group Abilities. Angew. Chem. Int. Ed. 2013, 52, 4440–4444. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, R.; Qiao, X.; Shen, Z. Copper-Catalyzed Aromatic C-H Bond Halogenation Using Lithium Halides as Halogenating Reagents. Synlett 2011, 2011, 1038–1042. [Google Scholar] [CrossRef]
- Molander, G.A.; Elia, M.D. Suzuki-Miyaura Cross-Coupling Reactions of Benzyl Halides with Potassium Aryltrifluoroborates. J. Org. Chem. 2006, 71, 9198–9202. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, R.H. The Organometallic Chemistry of Alkanes. Chem. Rev. 1985, 85, 245–269. [Google Scholar] [CrossRef]
- Crabtree, R.H. Organometallic Alkane CH Activation. J. Organomet. Chem. 2004, 689, 4083–4091. [Google Scholar] [CrossRef]
- Bergman, R.G. Organometallic chemistry—C-H activation. Nature 2007, 446, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.R.; Sanford, M.S. Transition Metal Catalyzed Oxidative Functionalization of Carbon–hydrogen Bonds. Tetrahedron 2006, 62, 2439–2463. [Google Scholar] [CrossRef]
- Stowers, K.J.; Sanford, M.S. Mechanistic Comparison between Pd-Catalyzed Ligand-Directed C-H Chlorination and C-H Acetoxylation. Org. Lett. 2009, 11, 4584–4587. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, S.R.; Sanford, M.S. Reactivity of Pd(II) Complexes with Electrophilic Chlorinating Reagents: Isolation of Pd(IV) Products and Observation of C-Cl Bond-Forming Reductive Elimination. J. Am. Chem. Soc. 2007, 129, 15142–15143. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, P.; Alla, S.K.; Punniyamurthy, T. Pd(II)-Catalyzed Aminotetrazole-Directed Ortho-Selective Halogenation of Arenes. J. Org. Chem. 2013, 78, 6104–6111. [Google Scholar] [CrossRef] [PubMed]
- Bedford, R.B.; Haddow, M.F.; Mitchell, C.J.; Webster, R.L. Mild C-H Halogenation of Anilides and the Isolation of an Unusual Palladium(I)–Palladium(II) Species. Angew. Chem. Int. Ed. 2011, 50, 5524–5527. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Jiang, X.; Sun, P. Palladium-Catalyzed Highly Selective ortho-Halogenation (I, Br, Cl) of Arylnitriles via sp2 C-H Bond Activation Using Cyano as Directing Group. J. Org. Chem. 2013, 78, 2786–2791. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, N.; Schröder, N.; Glorius, F. Rh(III)-Catalyzed Halogenation of Vinylic C-H Bonds: Rapid and General Access to Z-Halo Acrylamides. Org. Lett. 2013, 15, 3860–3863. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zhou, X.; Pu, S.; Cao, B. Rhodium-catalyzed ortho-selective C-H halogenation of 2-arylbenzo[d]thiazoles using N-halosuccinimides as halogen sources. Tetrahedron 2015, 71, 2376–2381. [Google Scholar] [CrossRef]
- Liu, W.; Groves, J.T. Manganese Porphyrins Catalyze Selective C-H Bond Halogenations. J. Am. Chem. Soc. 2010, 132, 12847–12849. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Balcells, D.; Parent, A.R.; Crabtree, R.H.; Eisenstein, O. Cp* Iridium Precatalysts for Selective C-H Oxidation via Direct Oxygen Insertion: A Joint Experimental/Computational Study. ACS Catal. 2011, 2, 208–218. [Google Scholar] [CrossRef]
- Zhou, M.; Hintermair, U.; Hashiguchi, B.G.; Parent, A.R.; Hashmi, S.M.; Elimelech, M.; Periana, R.A.; Brudvig, G.W.; Crabtree, R.H. Cp* Iridium Precatalysts for Selective C-H Oxidation with Sodium Periodate as the Terminal Oxidant. Organometallics 2013, 32, 957–965. [Google Scholar] [CrossRef]
- Zhou, M.; Schley, N.D.; Crabtree, R.H. Cp* Iridium Complexes Give Catalytic Alkane Hydroxylation with Retention of Stereochemistry. J. Am. Chem. Soc. 2010, 132, 12550–12551. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Kwak, J.; Shin, K.; Lee, D.; Chang, S. Ir(III)-Catalyzed Mild C-H Amidation of Arenes and Alkenes: An Efficient Usage of Acyl Azides as the Nitrogen Source. J. Am. Chem. Soc. 2013, 135, 12861–12868. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.-I.; Kaneda, T.; Nishiyama, H. Intermolecular C-H Bond Activation of Alkanes and Arenes by NCN Pincer Iridium(III) Acetate Complexes Containing Bis(oxazolinyl)phenyl Ligands. Organometallics 2012, 31, 4442–4449. [Google Scholar] [CrossRef]
- Allen, K.E.; Heinekey, D.M.; Goldman, A.S.; Goldberg, K.I. Regeneration of an Iridium(III) Complex Active for Alkane Dehydrogenation Using Molecular Oxygen. Organometallics 2014, 33, 1337–1340. [Google Scholar] [CrossRef]
- Pahls, D.R.; Allen, K.E.; Goldberg, K.I.; Cundari, T.R. Understanding the Effect of Ancillary Ligands on Concerted Metalation-Deprotonation by (dmPhebox)Ir(OAc)2(H2O) Complexes: A DFT Study. Organometallics 2014, 33, 6413–6419. [Google Scholar] [CrossRef]
- Allen, K.E.; Heinekey, D.M.; Goldman, A.S.; Goldberg, K.I. Alkane Dehydrogenation by C-H Activation at Iridium(III). Organometallics 2013, 32, 1579–1582. [Google Scholar] [CrossRef]
- Owens, C.P.; Varela-Alvarez, A.; Boyarskikh, V.; Musaev, D.G.; Davies, H.M.L.; Blakey, S.B. Iridium(iii)-bis(oxazolinyl)phenyl Catalysts for Enantioselective C-H Functionalization. Chem. Sci. 2013, 4, 2590–2596. [Google Scholar] [CrossRef]
- Zhou, M.; Johnson, S.I.; Yang, G.; Emge, T.J.; Nielsen, R.J.; Goddard, W.A., III; Goldman, A.S. Activation and Oxidation of Mesitylene C-H Bonds by (Phebox)Ir(III) Complexes. Organometallics 2015. [Google Scholar] [CrossRef]
- Ito, J.-I.; Shiomi, T.; Nishiyama, H. Efficient Preparation of New Rhodium- and Iridium-[Bis(oxazolinyl)-3,5-dimethylphenyl] Complexes by C-H Bond Activation: Applications in Asymmetric Synthesis. Adv. Synth. Catal. 2006, 348, 1235–1240. [Google Scholar] [CrossRef]
- Schmid, G.; Ritter, G. Versuche zur Oxydativen Addition von Thionylhalogeniden an Übergangsmetallkomplexe. Z. Anorg. Allg. Chem. 1975, 415, 97–103. [Google Scholar] [CrossRef]
- Markham, S.J.; Chung, Y.L.; Branum, G.D.; Blake, D.M. Reactions of Iridium(I) Compounds with Oxidized Derivatives of Organic Disulfides and with Thionyl Chloride. J. Organomet. Chem. 1976, 107, 121–127. [Google Scholar] [CrossRef]
- Cartwright, J.; Hill, A.F. Oxidative Addition of Seleninyl Chloride to Vaska’s Complex. Polyhedron 1996, 15, 157–159. [Google Scholar] [CrossRef]
- Vanderpool, R.A.; Abrahamson, H.B. Reaction of Vaska’s complex with thionyl chloride. Inorg. Chem. 1985, 24, 2985–2989. [Google Scholar] [CrossRef]
- Brewster, T.P.; Blakemore, J.D.; Schley, N.D.; Incarvito, C.D.; Hazari, N.; Brudvig, G.W.; Crabtree, R.H. An Iridium(IV) Species, [Cp*Ir(NHC)Cl]+, Related to a Water-Oxidation Catalyst. Organometallics 2011, 30, 965–973. [Google Scholar] [CrossRef]
- Isobe, K.; Bailey, P.M.; Maitlis, P.M. An Iridium(V) Organometallic Compound; Synthesis and X-ray Crystal Structure of Tetramethyl([small eta]5-pentamethylcyclo-pentadienyl)iridium. J. Chem. Soc. Chem. Commun. 1981, 808–809. [Google Scholar] [CrossRef]
- Lehman, M.C.; Pahls, D.R.; Meredith, J.M.; Sommer, R.D.; Heinekey, D.M.; Cundari, T.R.; Ison, E.A. Oxyfunctionalization with Cp*IrIII(NHC)(Me)(Cl) with O2: Identification of a Rare Bimetallic IrIV μ-Oxo Intermediate. J. Am. Chem. Soc. 2015, 137, 3574–3584. [Google Scholar] [CrossRef] [PubMed]
- Hay-Motherwell, R.S.; Wilkinson, G.; Hussain-Bates, B.; Hursthouse, M.B. Synthesis and X-ray Crystal Structure of Oxotrimesityliridium(V). Polyhedron 1993, 12, 2009–2012. [Google Scholar] [CrossRef]
- Lehman, M.C.; Boyle, P.D.; Sommer, R.D.; Ison, E.A. Oxyfunctionalization with Cp*IrIII(NHC)(Me)L Complexes. Organometallics 2014, 33, 5081–5084. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).